Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Phyllobilins – the abundant bilin-type tetrapyrrolic catabolites of the green plant pigment chlorophyll†‡
Bernhard
Kräutler
Institute of Organic Chemistry and Centre of Molecular Biosciences, University of Innsbruck, Innrain 80/82, A-6020 Innsbruck, Austria. E-mail: bernhard.kraeutler@uibk.ac.at
First published on 5th June 2014
Abstract
The seasonal disappearance of the green plant pigment chlorophyll in the leaves of deciduous trees has long been a fascinating biological puzzle. In the course of the last two and a half decades, important aspects of the previously enigmatic breakdown of chlorophyll in higher plants were elucidated. Crucial advances in this field were achieved by the discovery and structure elucidation of tetrapyrrolic chlorophyll catabolites, as well as by complementary biochemical and plant biological studies. Phyllobilins, tetrapyrrolic, bilin-type chlorophyll degradation products, are abundant chlorophyll catabolites, which occur in fall leaves and in ripe fruit. This tutorial review outlines ‘how’ chlorophyll is degraded in higher plants, and gives suggestions as to ‘why’ the plants dispose of their valuable green pigments during senescence and ripening. Insights into chlorophyll breakdown help satisfy basic human curiosity and enlighten school teaching. They contribute to fundamental questions in plant biology and may have practical consequences in agriculture and horticulture.
Key learning points(1) Phyllobilins are linear tetrapyrroles from chlorophyll breakdown (chlorophyll catabolites), which accumulate in de-greened leaves and vegetables, as well as in ripening fruit.(2) Chlorophyll breakdown involves an amount of about 1000 million tons each year, globally. About 25 years ago, it was still a striking biological enigma. (3) In higher plants, chlorophyll breakdown follows a largely common, regulated path, named the ‘phyllobilin/PaO’ pathway. (4) Phyllobilins are related to the tetrapyrrolic heme-catabolites (called bilins), which play important biological roles. (5) Phyllobilins are suspected to have relevant physiological functions in plants; they occur in our nutrition, and may also play a role in human metabolism. |
1. Introduction
Have you never wondered what happens to chlorophyll, when the leaves of deciduous plants de-green and display their fall colours, or when fruit ripen and turn to an appealing yellow, red or blue (Fig. 1)? The quest of finding remains of the green plant pigment has, indeed, engaged natural scientists' interest for a considerable time. Generally, their search was guided by the idea that the breakdown products were assumed to be coloured.1 Their failure in actually identifying chlorophyll degradation products was puzzling, as chlorophyll could not seriously be considered to disappear ‘without leaving a trace’. In fact, the global formation of chlorophyll breakdown products has been estimated to amount to about 1000 million tons, each year,2 and the seasonal disappearance of chlorophyll can be observed (and can be studied) from outer space.3 | ||
| Fig. 1 Characteristic colour changes observed in fall leaves and ripening fruit are a visual sign of chlorophyll breakdown (reproduced from Chem. Biol., 2008, 3, B79). | ||
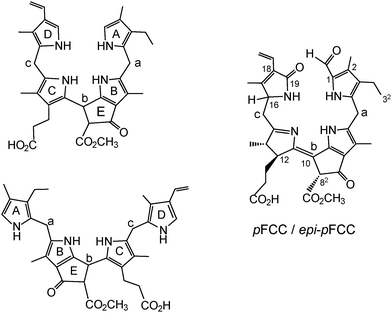
About twenty-five years ago, in de-greening leaves, a colourless tetrapyrrole was identified as the first non-green catabolite of chlorophyll (Chl).4 This colourless compound, now named Hv-NCC-1, was called a ‘rusty pigment’, originally, as it easily oxidized with formation of coloured product mixtures.4,5 When its structure could be established as a genuine Chl-breakdown product, it was classified as a ‘nonfluorescent’ Chl-catabolite (NCC), as it displayed no fluorescence (or other apparent photo-activity, see Fig. 2).4 Later studies have confirmed the broad relevance of the tetrapyrrolic NCCs as products of Chl-breakdown in higher plants.3,6 The original source of NCCs in specific plants was incorporated into their provisional names, e.g. Hv-NCC-1 for the most polar NCC from barley (Hordeum vulgare, see Fig. 2). Nowadays, over a dozen NCCs with different chemical structures are known, which are linear tetrapyrroles derived from Chl (for a list, see ESI,‡ Table S1).6 As will be delineated further below, NCCs are now classified as 1-formyl-19-oxo-phyllobilanes, as they are remarkably similar to bilanes,6 a well known class of natural linear tetrapyrroles.7
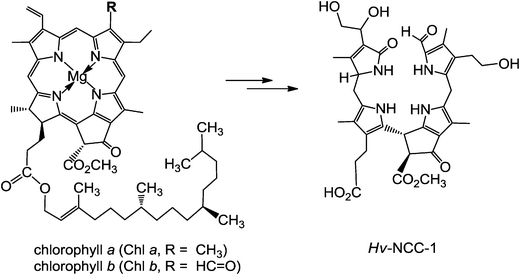 | ||
| Fig. 2 ‘Rusty pigment’ from senescent leaves of barley (Hordeum vulgare), later named Hv-NCC-1, was identified as the first non-green Chl-catabolite.4,6 | ||
Breakdown of Chl is the visible sign of senescence and cell death in leaves and vegetables, as well as of ripening of fruit.8 As we now know, this process degrades Chl to a variety of linear tetrapyrroles, classified as phyllobilins.6 These Chl-catabolites, in turn, are ‘biomarkers’ for senescence in higher plants. In this review, most representative formulas of the phyllobilins are drawn in a pseudo-cyclic fashion (see Fig. 2 and 3), which helps to make visual structure–correlations with the macro-cyclic precursors (Chls, pheophorbide a). However, the saturated linkages between the 5-membered heterocyclic rings show high conformational flexibility: there are three saturated linkages in phyllobilanes (see Fig. 3), two in phyllobilenes, etc. In the cases of unsaturated linkages the E/Z-geometry is defined in the formulas of the corresponding Chl-catabolites, as shown below (see e.g.Fig. 8 and ESI,‡ Fig. S2).
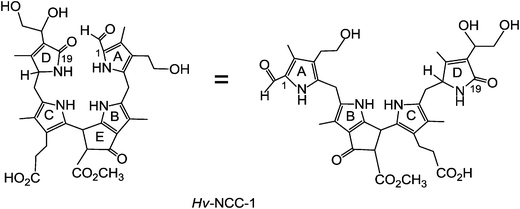 | ||
| Fig. 3 Constitutional formula of Hv-NCC-1 in ‘pseudo-cyclic’ (left) and ‘extended’ versions (right). | ||
Considering the massive amount of Chl broken down each year on Earth, phyllobilins are also an interesting class of natural products from ecological and phytochemical points of view.9 Systematic isolation from a variety of plant sources, and subsequent structural work, have revealed the basic chemical nature of phyllobilins.6 They are structurally related to the much better known and biologically important bilins,7 which originate from heme-breakdown.10 In spite of their importance in higher plants, the linear tetrapyrroles derived from Chl-breakdown are remarkable newcomers to the area of the heterocyclic natural products.9
2. Phyllobilins – a long overlooked class of bilin-type tetrapyrrolic heterocycles
2.1. Phyllobilins and key enzymes in the common ‘early’ phase of chlorophyll breakdown
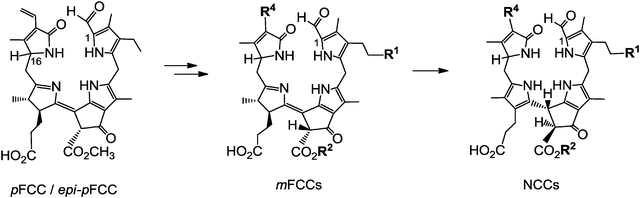
Structure elucidation of phyllobilins has provided a solid basis for detailed studies of the biochemical processes that constitute the Chl-breakdown pathway, which undergoes its ‘early’ phase in senescent chloroplasts.11 In these plastids, the Chls are de-greened rapidly in a strictly regulated process to colourless, blue fluorescent Chl-catabolites (FCCs), which do not accumulate in senescent leaves but exist only fleetingly (see below). Along this path, the green plant pigments, Chls a and b, are first degraded to pheophorbide a (Pheo a). The macrocycle of the green Pheo a is cut open at the ‘northern’ meso-position by pheophorbide a oxygenase (PaO), an oxygen-dependent mono-oxygenase, which is highly active in senescent leaves.12 PaO furnishes (an enzyme bound form of) the red Chl-catabolite (RCC), a linear tetrapyrrole. This 1-formyl-19-oxophyllobilin is the progenitor of all phyllobilins formed ‘later’ during Chl-breakdown. Thus, PaO is considered the key enzyme of the largely common path of Chl-breakdown in higher plants,12 named the ‘phyllobilin/PaO’ pathway.6 However, since RCC, the product of the PaO reaction, remained bound to PaO, its detection in senescent plants was futile, at first. In fact, RCC was first prepared by chemical synthesis from Pheo a, and then it was also identified as breakdown intermediate in senescent leaves.11In a type of metabolic channeling PaO-bound RCC is reduced directly at its C15-meso-position by RCC-reductase (RCCR), yielding a fluorescent Chl-catabolite (FCC). RCCR of Arabidopsis thaliana was expressed in a functional form13 and its crystal structure (with RCC bound) was elucidated.14 RCCR occurs in two stereo-divergent lines, which produce either the ‘primary’ FCC (pFCC) or its C16-epimer, epi-pFCC (see Fig. 4). It is a ferredoxin-dependent enzyme, homologous to the ‘ferredoxin-dependent bilin reductases’, which catalyze a range of reductive modifications of heme-derived bilins.15 RCCR is presumed to reduce RCC via single electron/proton transfer steps,14 for which electrochemical investigations with RCC provided a mechanistic model.16 pFCC was first identified from an enzyme extract from senescent leaves of oil seed rape,17epi-pFCC from a related system using leaves of sweet pepper.6 So far, the critical absolute configuration at C16 of FCCs remains unassigned. pFCC and related FCCs are colourless tetrapyrroles that luminesce blue (at 450 nm) when excited with UV-light (at about 360 nm), making the detection of minute amounts of FCCs possible.3
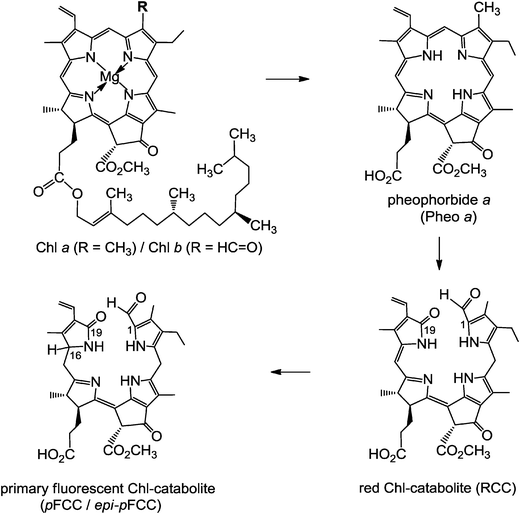 | ||
| Fig. 4 Chlorophyll a/b is degraded, in senescent chloroplasts, via pheophorbide a (Pheo a) and red Chl-catabolite (RCC) to the ‘primary’ fluorescent Chl-catabolite (pFCC/epi-pFCC), following the ‘phyllobilin/PaO’ path.6 | ||
2.2. Phyllobilins and key enzymes in divergent ‘later’ phases of chlorophyll breakdown
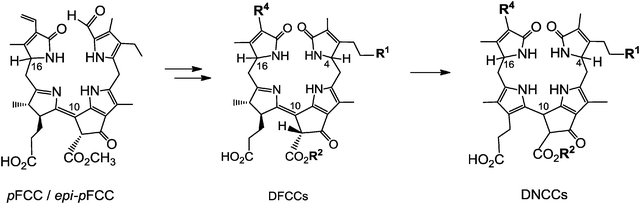
Typical FCCs are only short-lived metabolic precursors of the colourless, ‘nonfluorescent’ Chl-catabolites (NCCs). Only the ‘hypermodified’ FCCs (hmFCCs) are persistent, in which the propionic acid side chain carries an ester function.16 hmFCCs are generated by still uncharacterized enzymes and accumulate in specific plant tissues (e.g. in ripe bananas, see below).18 FCCs and NCCs are 1-formyl-19-oxophyllobilins, which are also classified here as type-I phyllobilins. FCCs are also precursors of dioxobilin-type NCCs (DNCCs),19 which lack the 1-formyl group.20,21 DNCCs (and their possible descendants, see below) are dioxobilin-type Chl-catabolites, or type-II phyllobilins (see Fig. 5).6 Interestingly, DNCCs carry the same type of oxo-groups at their 1- and 19-positions as the heme-derived (dioxo)-bilins.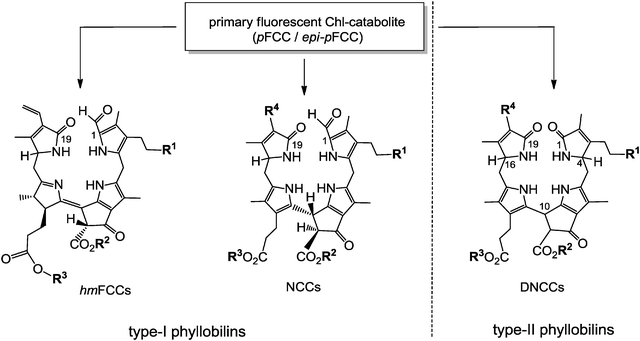 | ||
| Fig. 5 pFCC/epi-pFCC represent a three-way branching point of Chl-breakdown (see Fig. 4 for formula of pFCC/epi-pFCC). Further transformations lead to other type-I phyllobilins, such as NCCs (e.g. Hv-NCC-1: R1 = OH, R2 = CH3, R3 = H, R4 = CH(OH)–CH2OH) and ‘hypermodified’ FCCs (hmFCCs, e.g. Mc-FCC-56: R1 = OH, R2 = CH3, R3 = daucic acid), or to type-II phyllobilins, such as DNCCs (e.g.: Ap-DNCC: R1 = OH, R2 = CH3, R3 = H, R4 = CH(OH)–CH2OH). | ||
NCCs were found to accumulate in a variety of leaves, where they localized to the vacuoles of senescent cells.3 Spontaneous, acid-induced isomerization of FCCs to the corresponding NCCs has been proposed to take place in these acidic ‘storage’ vessels. The free carboxylic acid-function at the propionate side chain of typical FCCs proved to be the amazing prerequisite for achieving a significant rate and high stereo-selectivity of this isomerization at the pH typically found in vacuoles.22 Based on a detailed stereo-chemical analysis of the FCC/NCC-isomerization, the newly formed asymmetric centre at C10 was deduced to have (R)-configuration. Interestingly, all structurally characterized NCCs were inferred to have the same configuration at this position, based on their similar circular dichroism (CD)-spectra.9,16
Over a dozen constitutionally different (colourless) NCCs have been identified, so far (see ESI,‡ Table S1).6 They were decorated with one or several new functional groups (R1 to R4, see Fig. 5), when compared to the structures of the ‘earlier’ phyllobilins (RCC or ‘primary’ FCCs): a hydroxyl group at C32, which may carry a glucopyranosyl or a malonyl group, a free carboxylic acid function at C82, a 1,2-dihydroxyethyl group at C18, and malonylation at the sugar unit bound to O33.
In general, the new polar functions of the NCCs appear to increase their hydrophilicity. However, to be consistent with the general relevance of the previously delineated FCC/NCC-isomerization step, these NCCs require the existence of the correspondingly ‘modified’ FCCs (mFCCs). This, in turn, demands the activities of enzymes catalyzing the suggested modifications at the three FCC-side chains concerned (see Fig. 6 and Table S2, ESI‡). Thus, the structures of NCCs identified, so far, indicate the broad relevance of an FCC-hydroxylation at C32, and a possible subsequent glycosylation, or attachment of a malonyl group at the newly introduced O33, the hydrolysis of the methyl ester function at C82, a dihydroxylation of the vinyl group at C18, and malonylation at a sugar unit bound to O33. A few such enzymes are now known:11 a methyl esterase was found in Arabidopsis thaliana and was shown to be localized to the cytosol, which hydrolyzes the methyl ester function bound at C82 of pFCC. A malonyl transferase attaches the malonyl unit to O33 (as was actually shown with several NCCs).11
When dioxobilin-type NCCs (DNCCs) were discovered in barley leaves, they were considered to be formed from NCCs by an oxidative deformylation reaction.6,20 However, the structure of Ap-DNCC from senescent leaves of Norway maple (see Fig. 5) suggested an alternative path for the formation of DNCCs.21 Indeed, in studies with A. thaliana, a cytochrome P450 enzyme (cyp89A9) was identified that catalyzed the oxidative deformylation of pFCC to the corresponding DpFCC (two stereo-isomers).19 In weakly acidic solution, the latter then isomerized to DNCCs of the type also found in senescent leaves of A. thaliana (see Fig. 7). Cyp89A9 was localized in the endoplasmatic reticulum, consistent with inferred transport of the FCC–substrate from the chloroplast into the cytosol.19 In line with this finding and with the observation of NCCs in the vacuole, transport of FCCs through the chloroplast envelope into the cytosol, and (eventually) from this compartment into the vacuoles is a relevant and integral part of Chl-breakdown.11 Modified DFCCs would, likewise, be expected to be formed in the cytosol, and to be transported into the vacuole, with subsequent rapid isomerization to the corresponding DNCCs.19
Colourless phyllobilins, such as NCCs and DNCCs, accumulate only temporarily in senescent leaves. Indeed, besides NCCs a (small) variety of DNCCs have meanwhile been identified in extracts of senescent leaves and characterized (see ESI,‡ Table S3). Together with the remarkably persistent ‘hypermodified’ FCCs (hmFCCs), three major lines of bilin-type Chl-catabolites have thus been discovered, which arise from a diversity of the breakdown pathway in its ‘later’ phases (see Fig. 5).
In addition, yellow and pink coloured phyllobilins, named yellow Chl-catabolites (YCCs) and pink Chl-catabolites (PiCCs), were found in some fully senescent, yellow leaves as oxidation products of NCCs.6,23 Part of the yellow and red colours of fall leaves may thus be due to these coloured pigments derived from Chl. YCCs and PiCCs are also available from NCCs by chemical oxidation and they are formed, as well, when NCCs turn ‘rusty’. The first PiCC to be characterized by NMR-spectroscopy and mass spectrometry, (10E,15Z)-1-formyl-32-hydroxy-19-oxophyllobiladiene-b,c, turned out to possess an extended conjugated system, including a new C10![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C11 double bond in E-configuration (see Fig. 8).23 In spite of this structural feature (which was confirmed by crystallography), this PiCC has an excellent capacity for binding (divalent) transition metal ions in a three-dentate fashion, requiring the C10
C11 double bond in E-configuration (see Fig. 8).23 In spite of this structural feature (which was confirmed by crystallography), this PiCC has an excellent capacity for binding (divalent) transition metal ions in a three-dentate fashion, requiring the C10![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C11 and C15
C11 and C15![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C16 double bonds to be Z in the resulting metal complexes.24
C16 double bonds to be Z in the resulting metal complexes.24
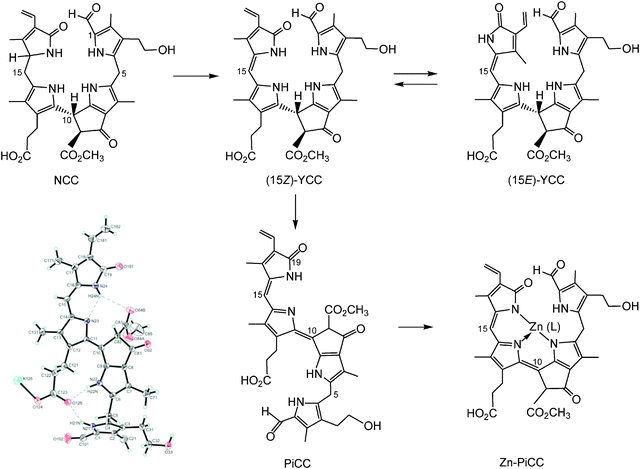 | ||
Fig. 8 Oxidation of the NCC 32-hydroxy-1-formyl-19-oxophyllobilane leads to the corresponding (15Z)-YCC, which isomerizes to its 15E-isomer photo-chemically. Oxidation of the (15Z)-YCC (15Z)-32-hydroxy-1-formyl-19-oxophyllobilene-c occurs readily and yields the corresponding PiCC, whose C10![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C11 double bond displays E-configuration (see formula and structural model taken from a X-ray crystal structure, bottom left). Binding of divalent transition metal ions by PiCC gives metallo-PiCCs, such as Zn-PiCC (bottom, right), in which both of the double bonds, at C10/C11 and at C15/C16, have been derived to have Z-configuration.24 C11 double bond displays E-configuration (see formula and structural model taken from a X-ray crystal structure, bottom left). Binding of divalent transition metal ions by PiCC gives metallo-PiCCs, such as Zn-PiCC (bottom, right), in which both of the double bonds, at C10/C11 and at C15/C16, have been derived to have Z-configuration.24 | ||
It is not known, at this stage, where in the senescent plant cell these coloured phyllobilins are formed and where they are accumulating, or whether the types of metal complexes of the PiCC, as prepared by chemical synthesis, would also exist in the plants. Likewise, the role of these coloured phyllobilins in Chl-breakdown and their further fate are still puzzling.
2.3. Chemical properties and spectroscopy of phyllobilins
Phyllobilins are amphiphilic heterocyclic compounds that differ in their polarity by the nature of their peripheral modifications. High performance liquid chromatography (HPLC) is an efficient method of their analysis and separation in small scale. Phyllobilins can be classified on the basis of their UV/Vis-spectra (and of their associated optical properties, see Fig. 9 and 10).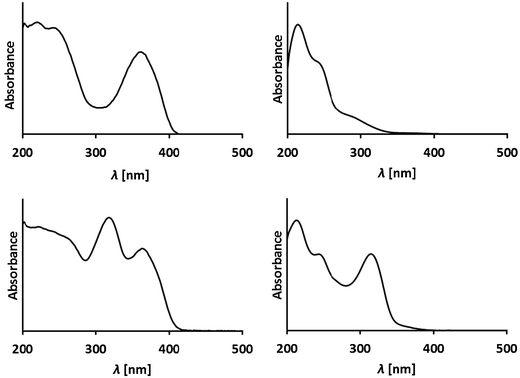 | ||
| Fig. 9 Representative UV-spectra of phyllobilins from A. thaliana. Top: UV-spectra of a DFCC and a DNCC, two type-II phyllobilins;25 bottom: UV-spectra of an FCC and an NCC show an (additional) absorption maximum near 320 nm, due to the α-formyl pyrrole of type-I phyllobilins.26 | ||
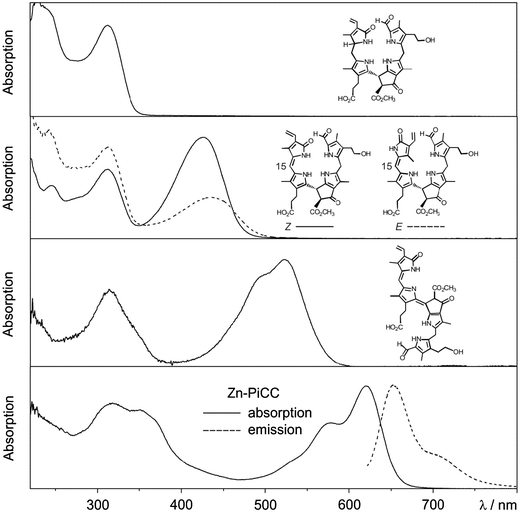 | ||
| Fig. 10 UV/Vis-spectra of an NCC, E/Z-isomeric YCCs, a PiCC and its Zn(II)-complex Zn-PiCC, together with an emission spectrum of Zn-PiCC (formulas are shown here and/or in Fig. 8). | ||
Type-I phyllobilins feature an absorbance maximum near 315 to 320 nm (of the 1-formyl-pyrrole unit at ring A), as well as other absorption bands of their chromophore(s) at rings B to D, which depend upon the eventual conjugation between these ring sections (see Fig. 10). Type-II phyllobilins lack the formyl group and, thus, the corresponding band near 320 nm (see Fig. 9). As a consequence, UV-spectra of DNCCs lack absorption maxima helpful for the (on-line) detection of these phyllobilins, which may therefore have escaped being described in the original analysis of some plant extracts, e.g. of barley20 and of A. thaliana.19 Mass spectrometry offers additional methods to detect phyllobilins routinely, and that allow the provisional identification of phyllobilins (‘on-line’). With the help of high-resolution mass spectrometry, the molecular formulas of phyllobilins may be delineated. In simple cases, the chemical constitution could also be derived from a detailed mass spectrometric analysis.27
The original classification of the (bilane-type) NCCs as ‘rusty pigments’1,4 reflected the ease, with which these colourless tetrapyrroles underwent apparently unspecific oxidation reactions to coloured materials. Indeed, NCCs (and YCCs) were shown to be excellent antioxidants.6,28 NCCs were found to undergo chemical oxidation reactions at the C15-(meso)-position, leading to yellow bilene-type YCCs (or 1-formyl-19-oxophyllobilenes-c), with double bond Z-configuration at C15. YCCs feature a chromophore, which is identical to the ‘western’ half of bilirubin.7,29 Moreover, YCCs are similarly effective as antioxidants, as bilirubin, and are easily oxidized to PiCCs (or 1-formyl-19-oxophyllobiladienes-b,c), with further extension of the conjugated chromophore system at C10.24
Phyllobilins show diagnostic UV/Vis-spectroscopic properties. With the exception of the colourless and ‘nonfluorescent’ phyllobilanes (NCCs and DNCCs), phyllobilins also display a correspondingly interesting spectrum of specific photochemical activities. UV-spectra of fluorescent Chl-catabolites (FCCs) exhibit absorption maxima near 317 and 360 nm, and FCCs correspondingly emit blue fluorescence with a maximum near 450 nm.18 Typical, natural FCCs isomerize readily to NCCs, making some photo-physical studies with them difficult.22 As FCC esters are persistent, a semi-synthetic FCC methyl ester was prepared and studied in detail. It was found not only to exhibit a relatively high fluorescence quantum yield (ϕf) of about 0.25 (at room temperature), but, surprisingly, also to sensitize the formation of singlet oxygen with a remarkable quantum yield of about 0.630 (for further discussion, see Section 2.7).
UV/Vis-spectra of the (15Z)-YCC (15Z)-32-hydroxy-1-formyl-19-oxo-16,19-dihydro-phyllobilene-c and of its isomer (15E)-YCC exhibit absorption maxima near 310 and 426 nm, or near 313 and 440 nm, respectively (see Fig. 10).23 In exploratory photochemical studies this (15Z)-YCC was seen to show weak luminescence and to undergo E/Z-photo-isomerization of its C15–C16 double bond.23 Absorption spectra of the PiCC (10E,15Z)-32-hydroxy-1-formyl-19-oxo-16,19-dihydro-phyllobiladiene-b,c displayed absorption maxima near 313 and 523 nm.24 Binding of Zn(II)-ions (or Cd(II)-ions) by this PiCC gave blue complexes, with absorption maxima near 578 and 620 nm (see Fig. 10). The PiCC was indicated to only exhibit weak luminescence at 620 nm,24 whereas its blue complexes with Zn(II)- and Cd(II)-ions gave rise to strong red luminescence at 650 nm, allowing the detection of these metal ions at nM concentrations.24 The photochemistry of phyllobilins is likely to be relevant for their possible physiological functions in plants. Indeed, for most phyllobilins, the detailed photochemical behaviour remains to be studied. Clearly, such knowledge promises to provide important insights into their eventual biological roles (see discussion in Section 2.7).
2.4. Naming of natural phyllobilins
In the earlier investigations, mostly concerned with the detection, isolation and identification of Chl-catabolites in senescent leaves, a phenomenological nomenclature was typically used. The newly found bilin-type catabolites were thus classified e.g. as red, fluorescent and nonfluorescent Chl-catabolites (RCC, FCC, NCC, resp.). Often they were specified further according to their plant source (e.g. Brassica napus) and their chromatographic polarity, i.e. as Bn-FCC-2, or for their position in the Chl-breakdown pathway (e.g. as ‘primary’: pFCC).17 When the structures of Chl-catabolites became available, semi-systematic names were used for these bilin-type tetrapyrroles, based on the phytoporphyrin core, and supplemented by atom numbering, as established for the Chls:6,31e.g. pFCC (Bn-FCC-2, see Fig. 4) was named a 31,32-didehydro-1,4,5,10,17,18,20-(22H)-octahydro-132-[methoxycarbonyl]-4,5-dioxo-4,5-seco-phytoporphyrinate,17 and Hv-NCC-1 a 31,32,82-trihydroxy-1,4,5,10,15,20-(22H,24H)-octahydro-132-[methoxycarbonyl]-4,5-dioxo-4,5-seco-phytoporphyrinate4 (see Fig. 2 and 3). Such long designations eventually became impractical in cases, where the full structural specification of the Chl-catabolites was relevant. Furthermore, with the recent advent of the discovery of the general relevance of 1,19-dioxo-phyllobilins, and their derivatives, the recommended nomenclature of bilins (see ref. 7 and references therein) was adapted for naming the Chl-derived phyllobilins, as described here systematically.The core structural unit for an NCC is thus represented by the phyllobilane (see Fig. 11), and Hv-NCC-1 from barley leaves is a 1-formyl-19-oxo-32,181,182-trihydroxy-16,19-dihydrophyllobilane. FCCs are 12,13-dihydro-phyllobilenes-b, i.e. pFCC is a (10Z)-1-formyl-19-oxo-12,13,16,19-tetrahydro-phyllobilene-b. Similarly, RCC is a (10Z,15Z)-1-formyl-19-oxo-12,13,19-(24H)-tetrahydro-phyllobiladiene-b,c. Likewise, Ap-DNCC from senescent leaves of Norway maple is named a 1,19-dioxo-32,181,182-trihydroxy-1,4,16,19-tetrahydro-phyllobilane (for complete atom numbering see ESI,‡ Fig. S1 and S2).
2.5. An unexpected manifestation of chlorophyll breakdown – blue luminescent bananas
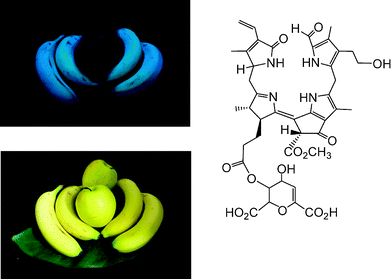
Typical FCCs are easily detectable by their fluorescence, yet only short lived metabolic precursors of the colourless and essentially photo-inactive NCCs. Strikingly, yellow bananas ‘glow’ bright blue, when excited by UV-light at wavelengths near 360 nm (‘black’ light), and when observed in the absence of white light (see Fig. 12).9,18The peels of other ripe fruit, such as of ‘Golden Delicious’ apples, which exhibit a similar yellow colour as bananas, show insignificant luminescence, when studied under the same conditions (see Fig. 12). Indeed, only NCCs were found in the peels of ripe apples, and no FCCs.28 The blue fluorescence of ripe bananas is largely caused by the accumulation of the special ‘hypermodified’ FCCs (hmFCCs), such as of Mc-FCC-56, in the banana peel (see Fig. 12, right).18,32 In fact, the ripeness of a banana (Musa acuminata) could be read from the intensity of its blue fluorescence, caused by temporal accumulation of hmFCCs. Similar hmFCCs, as in banana peels, were also found as major (or sole) tetrapyrrolic Chl-catabolites in some (blue luminescent) senescent leaves, e.g. of the banana plant (Musa acuminata)33,34 and of the Peace Lily (Spathiphyllum wallisii).35 The occurrence of blue luminescent, persistent FCCs in bananas (and, potentially, in other ripe fruit) may have a particular role in signaling ripeness of these fruit to frugivores, important in the context of seed distribution.6,18 Blue luminescence of senescent leaves is less well rationalized, at present. It could be relevant in helping to signal ripe fruit by ‘fruit flagging’.33,34
2.6. Relationships between bilins and phyllobilins
Phyllobilins, the natural, bilin-type breakdown products of chlorophyll (the green ‘pigment of leaves’), are ubiquitous linear tetrapyrroles. Their structures are remarkably similar to those of the bilins, linear tetrapyrroles named for their occurrence in bile, as catabolites of heme (the red ‘pigment of blood’).7 Phyllobilins are to be kept distinct from ‘phytobilins’ and ‘phycobilins’ of plants and other photosynthetic organisms,7,36 which are linear tetrapyrroles due to further biosynthetic transformations of the ‘heme-degradation product’ biliverdin.15 Note, these latter ‘plant bilins’, represent non-cyclic (tetrapyrrolic) chromophores that play important roles in light capture, when bound to phycobiliproteins, and light sensing in phytochromes.15,37,38Biologically relevant linear tetrapyrroles appear to occur along two main paths in the biosphere: either as early biosynthetic precursors of natural porphyrinoids, or as biosynthetic products, obtained from the porphyrinoids by cleavage of their macroring.7,37Via the latter paths, natural linear tetrapyrroles are generated by oxygen-dependent cleavages of the porphyrinoid macrocycles of Chl or of heme, at their respective ‘northern’ meso-positions. An iron center is crucial for oxygen activation and catalysis in both cases. In the course of Chl-breakdown, ring opening of metal-free Pheo a, is the key step that is achieved by an iron-dependent enzyme, the senescence regulated (Rieske-type) mono-oxygenase PaO. Oxygenolytic Pheo a cleavage occurs with retention of the meso-carbon atom and its conversion into a formyl group, and it generates RCC as the progenitor of the characteristic type-I phyllobilins.12 In contrast, the dioxo-bilin biliverdin is formed in the key step during heme-breakdown, in which the particular meso-carbon is lost as carbon monoxide. Thus, heme-oxygenases rely on the substrate (heme = iron-protoporphyrin) to present the iron center for activation by the protein (see Fig. 13).10 These catabolic enzymes are not specific for heme (iron-protoporphyrin IX) and can accept other iron-porphyrins: heme-oxygenase from rat even accepted an iron-complex of Pheo a as substrate and converted it into a metal-free dioxobilin-analogue, as a recent exploratory study suggested.6
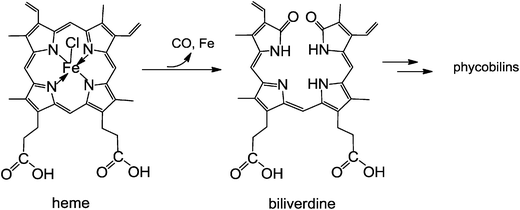 | ||
| Fig. 13 Oxidative opening of the porphyrin macrocycle of heme by heme-oxygenase liberates the iron-ion and CO, and furnishes biliverdin (BV). BV is the biosynthetic precursor of the (dioxo-)bilins, such as bilirubin and phycoviolobilin (see Fig. 14). | ||
Remarkably, in some plants, type-I phyllobilins (1-formyl-19-oxo-phyllobilins), which are generated via the ‘early’ steps of the ‘phyllobilin/PaO’ pathway, are subsequently deformylated oxidatively to type-II phyllobilins (or 1,19-dioxo-phyllobilins), rendering these Chl-catabolites more similar to the heme-derived (dioxo)-bilins.19 A puzzling hydroxymethylation at ring A of some type-II phyllobilins was observed recently, which is unprecedented among the known natural bilins,7 and which remains to be rationalized, both in the context of Chl-breakdown, as well as of other metabolic issues.25
Clearly, a distinct structural hallmark of the phyllobilins is the presence of an ‘additional’ cyclopentanone-unit (‘ring E’) annealed with the pyrrole ring B, a structural feature common to the Chls (where ‘ring E’ is annealed to ‘ring C’). When carrying the original methoxy-carbonyl group, or a free carboxylic acid function, the ‘extra’ ring E is likely to exert steric interactions with ring C, similar to those in the Chls. Interestingly, the colour-giving chromophore of YCCs is structurally related to the one of bilirubin,7 and the origin of the pink colour of PiCCs derives from a conjugated system similar to the chromophore of a protein-bound heme-catabolite, named phycoviolobilin (Cys-PVB, see Fig. 14).15,39 In the light-sensing phytochromes and cyanobacteriochromes the protein-bound bilin cofactors undergo a crucial photo-isomerization at their C15![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C16 double bond.38,39
C16 double bond.38,39
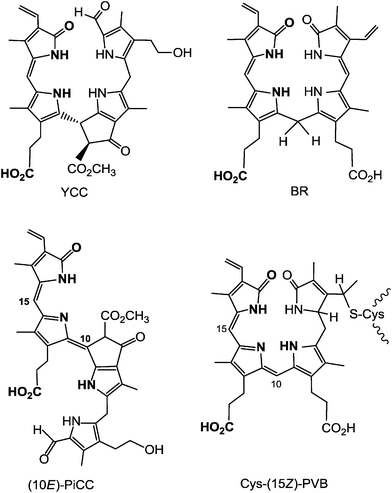 | ||
| Fig. 14 The main chromophores of YCCs and of bilirubin (BR) are strikingly similar, as are those of PiCCs and of (the protein-bound) phycoviolobilin (Cys-(15Z)-PVB). | ||
2.7. The question of physiological roles of phyllobilins
The biological significance of Chl-breakdown in higher plants has mainly been associated with the photo-toxicity of Chl.1,40 Indeed, the observed rapid degradation of the green plant pigments to colourless linear tetrapyrroles is consistent with this proposal.3 Remarkably, an entirely different strategy for Chl-detoxification has been observed in aquatic herbivores, such as unicellular and multicellular zooplankton, which feed on algae, and convert Chls from the ingested food, into its non-luminescent and practically photo-inactive pheophorbide a (Pheo a) derivative, named ‘132,173-cyclopheophorbide a enol’.6,41As a result of Chl-breakdown in higher plants, Chls are withdrawn from Chl-binding proteins (in light-harvesting complexes and in photoreaction centres) of the thylakoids. This, in turn, was found to labilize the protein matrix for controlled proteolytic decomposition, used for the purpose of recuperating protein-derived nitrogen.1,40 On the other hand, in contrast to earlier views, the four nitrogen atoms of the Chl-molecules appear not to be mobilized as nutrient, since they remain part of the known catabolites.6
Phyllobilins were observed as consequences of leaf senescence and of fruit ripening.8 Chl-breakdown implies reduction of the photosynthetic activity, with stringent consequences for the supply of the plant tissues with energy and crucial metabolites. The vitality of the transforming plant cells is particularly threatened in these developmental phases.1 Phyllobilins may, therefore, play a particular physiological role as antioxidants.28 Furthermore, phyllobilins are pigments likely to contribute to the apparent colour of leaves and fruit. Hence, further roles of these tetrapyrrolic Chl-catabolites are possible, either as ‘sun-screens’, or as (light-emitting) luminophores. To the latter, the exceptional hmFCCs may be counted, which are results of ‘biosynthetic Chl-breakdown steps’, and which may signal ripeness of bananas and other fruits to frugivores.6,18 Some phyllobilins, such as FCCs, are unexpectedly effective sensitizers of singlet oxygen, which may act as a stress signal42,43 or as a toxin for pathogens.44 In the latter context, the capacity of some phyllobilins to bind and activate transition metals may be relevant.24,45 On the other hand, this chemical property could also be important in plants for ‘heavy metal’ detoxification.46
The striking structural similarity of linear tetrapyrroles from Chl-breakdown with bilins from ‘heme-breakdown’ fuels speculations that phyllobilins may also play a variety of other physiological roles in plant cells. Phytobilins, such as phytochromobilin, and phycoviolobilin are chromophores of photoreceptor-proteins, named phytochromes15,47 or e.g. cyanobacterio-chromes.38,39 Phycobilins are important cofactors of phycobiliproteins in photo-system antenna complexes (phycobilisomes).15 Structurally similar (type-II) phyllobilins could bind well to photoreceptor and antenna proteins. Possibly, they could act as cofactor-mimics, as (competitive) inhibitors of these, or, in general, as ‘phycobilin dummies’. Phycobiliproteins have found application as coloured and luminescent additives to food and cosmetics.15 Furthermore, some phycocyanines, abundant in cyanobacteria, have been characterized as antioxidants, and were shown to have interesting anti-inflammatory effects, to function as phototoxic drugs against cancer, and to have a range of other beneficial health-effects, as deduced from experiments with mammalian cell lines and tissues.15,38 Likewise, the abundant chlorophyll-derived phyllobilins may also have a variety of physiological effects and find interesting applications, in which their bilin-like structures, as well as their photo-, redox-, and metal chelating chemistry would be important features. Indeed, similar to the situation with various heme-derived bilins, which are now known to arise by biosynthetic transformations of the heme-oxygenase product biliverdin (BV),38,39 some of the modifications observed in Chl-catabolites may be seen purposeful for their eventual biological function, and they may, likewise, be classified as ‘biosynthetic’.18,25
3. Research outlook and broader implications
A variety of bilin-type Chl-catabolites are now identified, their structures have been characterized, and their chemistry has been partially explored.6 This research has already given a solid basis to biochemical and plant-biological investigations of Chl-breakdown during leaf senescence and during fruit ripening.11,48 Colourless phyllobilanes typically accumulate in senescent leaves and ripe fruit, and mark an apparently ‘final’ stage of Chl-breakdown.40 Nowadays, most earlier questions concerning the basic biochemical processes along the ‘phyllobilin/PaO’ path appear to be clarified, up to the stage of the phyllobilanes.6,11,48The situation is far less advanced concerning the further whereabouts of Chl-breakdown products. In some freshly senescent leaves, the amounts of the phyllobilanes found came up roughly for the amounts of Chls present in the original green leaf. However, in other cases, and in the typical later phases of leaf senescence, phyllobilanes seem to ‘disappear’ and their further fate is still a puzzle. The observation of yellow phyllobilenes (YCCs) and pink phyllobiladienes (PiCCs) in a variety of de-greened, yellow leaves may suggest metabolic oxidative processes involving the phyllobilanes. Indeed, a still little characterized oxidative process that leads to highly stereo- and regio-selective hydroxylation of NCCs at the ‘western’ C15 position appears to be ubiquitous in leaves.6 The relevance of this process for the decrease of the amounts of phyllobilanes in senescent leaves, and their eventual disappearance, remains to be seen. Clearly, the formation of chemically rather labile, coloured bilin-type Chl-catabolites may point to new physiological roles of phyllobilins, and it may open a path to further breakdown of the Chls in senescent leaves, based on their subsequent transformations. In a related context, the further fate of the known phyllobilins in detached leaves (e.g. their further ‘breakdown’ by microorganisms in the soil) would be another interesting subject, into which, at present, well founded insights are not available.
Biochemical, molecular biological and various plant-biological studies11 have paved the way, over the last twenty years, to insights important for a deeper understanding of physiological processes and evolutionary factors that contribute to the development, growth and stress resistance of plants,49 as well as to the ripening of fruit.50 In this respect, knowledge and control of Chl-breakdown may help e.g. to increase the resistance of plants (such as grass) against drought and against lack of nutrients,49,51 in order to produce better crops and more nutritious crop products (such as barley and soy beans), and to increase the resistance of green vegetables (such as broccoli) against post-harvest deterioration.53 Along these lines, Chl-breakdown may also be a relevant subject, in a practical sense, in the context of pathogen-induced senescence of higher plants and a possible role in the plants’ defense program.6 Clearly, basic scientific insights into Chl-breakdown may prove to be economically and ecologically beneficial.
In view of these advances, phyllobilins are yet a strikingly little explored class of ubiquitous, plant-derived, amphiphilic heterocyclic natural products, which own a wide range of unusual structural and chemical properties. Remarkably, good evidence for relevant biological roles of phyllobilins in the plants is still lacking.6 The quest of finding any of the still elusive metabolic functions is an important (and presumably fruitful) challenge. Indeed, little is also known about the further fate of phyllobilins in the tissue of senescent leaves and of ripening fruit. Interestingly, the latter topic and the previous one may be strongly interrelated. Specific enzymatic modifications of the structures of the colourless phyllobilins may suggest further metabolic relevance of these Chl-catabolites and of their descendants, rather than simply be associated with metabolic ‘extravagances’.1 Likewise, as phyllobilins are part of the nutrition of plant-ingesting animals and humans, the effect of these natural products on animal and human metabolism and health is of interest.6,28 Indeed, the established health benefits of antioxidants in the peels of ripe apples and the overlooked accumulation of NCCs as antioxidants in these and in other fruit, have led to the proposal that NCCs should also be taken into consideration, in that respect (as the old proverb ‘An apple a day keeps the doctor away’ suggests).28
In contrast to the possibly beneficial effects of some phyllobilins, as part of the nutrition, Chls and pheophorbide a (Pheo a) should be considered to be phototoxic to mammals. In line with this, evidence for active cellular export of Pheo a (from plant derived food), as well as for endogenous breakdown to red RCC-like compounds in mice was provided in an animal study.53 The pathway for the inferred degradation of Pheo a is unknown. However, exploratory chemical evidence has been provided, which would suggest that heme-oxygenase could do the job of cutting up and eliminating (iron-complexes of) Chl-derived porphyrinoids in animals and humans.6
Chl-breakdown to bilin-type tetrapyrroles is not restricted to higher plants.6 It has been observed for several lower organisms, notably in the green alga Auxenochlorella protothecoides (which release red type-I phyllobilins, related to RCC),54 as well as for dinoflagellates, marine photo-organism, which generate bilin-type Chl-catabolites via opening of the Chl-macroring at the ‘western’ meso-position.55
In summary, our increasing insights into the occurrence and chemistry of phyllobilins, and of related Chl-derived bilins, have helped to develop a basic understanding of Chl-breakdown, with broad implications to plant biology and physiology.1,11,52 Hopefully, this will promote interest in this topic in food and agricultural chemistry, as well as in the nutritional sciences. Last but not least, such knowledge could have practical consequences in agriculture and horticulture, and it could also enlighten school education and botanical excursions.
Acknowledgements
I would like to thank Thomas Müller and the present ‘greenish’ part of my co-workers in Innsbruck (Chengjie Li, Xiujun Liu, Matthias Roiser, Markus Ruetz, Mathias Scherl, Gerhard Scherzer, Iris Süssenbacher and – not least – Clemens Vergeiner), as well as their previous colleagues, for their excellent research contributions to this topic, Stefan Hörtensteiner at the University of Zürich, as well as Michael Oberhuber (Laimburg, Italy), and their groups, for very fruitful collaborations. I am grateful to the Austrian National Science Foundation for generous financial support (recent FWF projects: P 19596, L-472 and I-563), as well as to an Austrian/Italian/EU interregional project (Interreg-IV ID 5345).References
- P. Matile, Exp. Gerontol., 2000, 35, 145 CrossRef CAS.
- G. A. F. Hendry, J. D. Houghton and S. B. Brown, New Phytol., 1987, 107, 255 CrossRef CAS PubMed.
- B. Kräutler and P. Matile, Acc. Chem. Res., 1999, 32, 35 CrossRef.
- B. Kräutler, B. Jaun, K. Bortlik, M. Schellenberg and P. Matile, Angew. Chem., Int. Ed. Engl., 1991, 30, 1315 CrossRef.
- C. Peisker, H. Thomas, F. Keller and P. Matile, J. Plant Physiol., 1990, 136, 544 CrossRef CAS.
- B. Kräutler and S. Hörtensteiner, in Handbook of Porphyrin Science, ed. G. C. Ferreira, K. M. Kadish, K. M. Smith and R. Guilard, World Scientific, Singapore, 2014, vol. 28, pp. 117–185 Search PubMed.
- H. Falk, Chemistry of Linear Oligopyrroles and Bile Pigments, Springer, 1989 Search PubMed.
- B. Kräutler, Photochem. Photobiol. Sci., 2008, 7, 1114 Search PubMed.
- S. Moser, T. Müller, M. Oberhuber and B. Kräutler, Eur. J. Org. Chem., 2009, 21 CAS.
- P. R. Ortiz de Montellano and K. Auclair, Heme Oxygenase Structure and Mechanism, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Amsterdam, 2003, vol. 12, pp. 183–210 Search PubMed.
- S. Hörtensteiner and B. Kräutler, Biochim. Biophys. Acta, Rev. Bioenerg., 2011, 1807, 977 CrossRef PubMed.
- S. Hörtensteiner, K. L. Wüthrich, P. Matile, K. H. Ongania and B. Kräutler, J. Biol. Chem., 1998, 273, 15335 CrossRef PubMed.
- A. Pružinska, I. Anders, S. Aubry, N. Schenk, E. Tapernoux-Lüthi, T. Müller, B. Kräutler and S. Hörtensteiner, Plant Cell, 2007, 19, 369 CrossRef PubMed.
- M. Sugishima, Y. Okamoto, M. Noguchi, T. Kohchi, H. Tamiaki and K. Fukuyama, J. Mol. Biol., 2010, 402, 879 CrossRef CAS PubMed.
- K. E. Overkamp and N. Frankenberg-Dinkel, in Handbook of Porphyrin Science', ed. G. C. Ferreira, K. M. Kadish, K. M. Smith and R. Guilard, World Scientific, Singapore, 2014, vol. 28, pp. 187–226 Search PubMed.
- M. Oberhuber, J. Berghold and B. Kräutler, Angew. Chem., Int. Ed., 2008, 47, 3057 CrossRef CAS PubMed.
- W. Mühlecker, K. H. Ongania, B. Kräutler, P. Matile and S. Hörtensteiner, Angew. Chem., Int. Ed. Engl., 1997, 36, 401 CrossRef.
- S. Moser, T. Müller, M.-O. Ebert, S. Jockusch, N. J. Turro and B. Kräutler, Angew. Chem., Int. Ed., 2008, 47, 8954 CrossRef CAS PubMed.
- B. Christ, I. Süssenbacher, S. Moser, N. Bichsel, A. Egert, T. Müller, B. Kräutler and S. Hörtensteiner, Plant Cell, 2013, 25, 1868 CrossRef CAS PubMed.
- F. G. Losey and N. Engel, J. Biol. Chem., 2001, 276, 8643 CrossRef CAS PubMed.
- T. Müller, M. Rafelsberger, C. Vergeiner and B. Kräutler, Angew. Chem., Int. Ed., 2011, 50, 10724 CrossRef PubMed.
- M. Oberhuber, J. Berghold, K. Breuker, S. Hörtensteiner and B. Kräutler, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 6910 CrossRef CAS PubMed.
- M. Ulrich, S. Moser, T. Müller and B. Kräutler, Chem. – Eur. J., 2011, 17, 2330 CrossRef CAS PubMed.
- C. Li, M. Ulrich, X. Liu, K. Wurst, T. Müller and B. Kräutler, Chem. Sci., 2014 10.1039/C4SC00348A.
- I. Süßenbacher, B. Christ, S. Hörtensteiner and B. Kräutler, Chem. – Eur. J., 2014, 20, 87 CrossRef PubMed.
- B. Kräutler, Chlorophyll Breakdown and Chlorophyll Catabolites, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Oxford, 2003, vol. 13, pp. 183–209 Search PubMed.
- T. Müller, S. Vergeiner and B. Kräutler, Int. J. Mass Spectrom., 2014, 48, 365–366 Search PubMed.
- T. Müller, M. Ulrich, K.-H. Ongania and B. Kräutler, Angew. Chem., Int. Ed., 2007, 46, 8699 CrossRef PubMed.
- D. Lightner and A. F. McDonagh, Acc. Chem. Res., 1984, 17, 417 CrossRef CAS.
- S. Jockusch, N. J. Turro, S. Banala and B. Kräutler, Photochem. Photobiol. Sci., 2014, 13, 407 CAS.
- H. Scheer, in Chlorophylls and Bacteriochlorophylls, ed. B. Grimm, R. J. Porra, W. Rüdiger and H. Scheer, Dordrecht, The Netherlands, 2006, pp. 1–26 Search PubMed.
- S. Moser, T. Müller, A. Holzinger, C. Lütz, S. Jockusch, N. J. Turro and B. Kräutler, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 15538 CrossRef CAS PubMed.
- S. Banala, S. Moser, T. Müller, C. Kreutz, A. Holzinger, C. Lütz and B. Kräutler, Angew. Chem., Int. Ed., 2010, 49, 5174 CrossRef CAS PubMed.
- C. Vergeiner, S. Banala and B. Kräutler, Chem. – Eur. J., 2013, 19, 12294 CrossRef CAS PubMed.
- B. Kräutler, S. Banala, S. Moser, C. Vergeiner, T. Müller, C. Lütz and A. Holzinger, FEBS Lett., 2010, 584, 4215 CrossRef PubMed.
- N. Frankenberg and J. C. Lagarias, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Oxford, UK, 2003, vol. 13, pp. 211–235 Search PubMed.
- M. J. Terry and A. C. McCormac, in Tetrapyrroles: Birth, Life and Death, ed. M. J. Warren and A. G. Smith, Landes Bioscience, Austin, Texas, 2008, pp. 221–234 Search PubMed.
- K.-H. Zhao, R. J. Porra and H. Scheer, in Handbook of Porphyrin Science, ed. G. C. Ferreira, K. M. Kadish, K. M. Smith and R. Guilard, World Scientific, Singapore, 2012, vol. 22, pp. 1–66 Search PubMed.
- N. C. Rockwell, S. S. Martin, A. G. Gulevich and J. C. Lagarias, Biochemistry, 2012, 51, 1449 CrossRef CAS PubMed.
- P. Matile, S. Hörtensteiner, H. Thomas and B. Kräutler, Plant Physiol., 1996, 112, 1403 CAS.
- Y. Kashiyama, A. Yokoyama, Y. Kinoshita, S. Shoji, H. Miyashiya, T. Shiratori, H. Suga, K. Ishikawa, A. Ishikawa, I. Inouye, K.-i. Ishida, D. Fujinuma, K. Aoki, M. Kobayashi, S. Nomoto, T. Mizoguchi and H. Tamiaki, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 17328 CrossRef CAS PubMed.
- K. Apel and H. Hirt, Annu. Rev. Plant Biol., 2004, 55, 373 CrossRef CAS PubMed.
- C. Triantaphylidès and M. Havaux, Plant, Cell Environ., 2009, 14, 219 Search PubMed.
- C. Flors and S. Nonell, Acc. Chem. Res., 2006, 39, 293 CrossRef CAS PubMed.
- A. Fürstner, Angew. Chem., Int. Ed., 2003, 42, 3582 CrossRef PubMed.
- T. Jaffré, Y. Pillon, S. Thomine and S. Merlot, Plant Sci., 2013, 4, 1 Search PubMed.
- N. Frankenberg and J. C. Lagarias, J. Biol. Chem., 2003, 278, 9219 CrossRef CAS PubMed.
- A. Tanaka and R. Tanaka, Curr. Opin. Plant Biol., 2006, 9, 248 CrossRef CAS PubMed.
- H. Thomas, L. Huang, M. Young and H. Ougham, BMC Evol. Biol., 2009, 9, 163 CrossRef PubMed.
- C. S. Barry, Plant Sci., 2009, 176, 325 CrossRef CAS PubMed.
- B. Christ, A. Egert, I. Süssenbacher, B. Kräutler, D. Bartels, S. Peters and S. Hörtensteiner, Plant, Cell Environ., 2014 DOI:10.1111/pce.12308.
- G. A. King and E. M. O'Donoghue, Exp. Gerontol., 1995, 6, 385 CAS.
- J. W. Jonker, M. Buitelaar, E. Wagenaar, M. A. van der Valk, G. L. Scheffer, R. J. Scheper, T. Plosch, F. Kuipers, R. P. J. O. Elferink, H. Rosing, J. H. Beijnen and A. H. Schinkel, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 15649 CrossRef CAS PubMed.
- N. Engel, T. A. Jenny, V. Moser and A. Gossauer, FEBS Lett., 1991, 293, 131 CrossRef CAS.
- H. Nakamura, Y. Kishi, O. Shimomura, D. Morse and J. W. Hastings, J. Am. Chem. Soc., 1989, 111, 7607 CrossRef CAS.
Footnotes |
| † Dedicated to Prof. Heinz Falk on the occasion of his 75th birthday. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cs00079j |
| This journal is © The Royal Society of Chemistry 2014 |