The energy landscape of a protein switch
Abstract
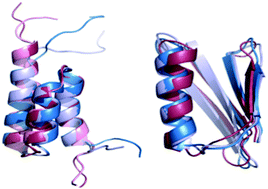
Protein switches are made of highly similar sequences that fold to dramatically different structures. A structural switching system with 31 sequence variants for α and α+β folds has been illustrated experimentally by He et al., Structure, 2012, 20, 283 and is investigated computationally in the present study. Methods to assign a sequence to one of the two folds are reported and analyzed. A fast and accurate protocol to identify the correct fold of the 31 sequences is based on enriching modeled structures using short molecular dynamics (MD) trajectories and scoring these structures with coarse-grained energy functions. We examine five coarse-grained energy functions and illustrate that the Hinds–Levitt potential works the best for this task. We show that enrichment by MD significantly enhances prediction accuracy. Finally, we find that melting temperature correlates well with the energy difference between the two folds (correlation coefficient ∼−0.7). The correlation reduces dramatically (∼0.4) if the absolute energy of the correct fold is considered. Moreover, prediction of melting temperature is sensitive to the structural templates. We emphasize in our analyses the use of native structures as templates since these folds are more readily available from structural biology experiments.
- This article is part of the themed collection: The free energy landscape: from folding to cellular function

 Please wait while we load your content...
Please wait while we load your content...