Free energy landscape of G-protein coupled receptors, explored by accelerated molecular dynamics†
Abstract
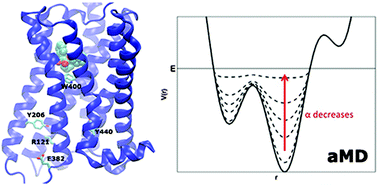
G-protein coupled receptors (GPCRs) mediate cellular responses to various hormones and neurotransmitters and are important targets for treating a wide spectrum of diseases. They are known to adopt multiple conformational states (e.g., inactive, intermediate and active) during their modulation of various cell signaling pathways. Here, the free energy landscape of GPCRs is explored using accelerated molecular dynamics (aMD) simulations as demonstrated on the M2 muscarinic receptor, a key GPCR that regulates human heart rate and contractile forces of cardiomyocytes. Free energy profiles of important structural motifs that undergo conformational transitions upon GPCR activation and allosteric signaling are analyzed in detail, including the Arg3.50–Glu6.30 ionic lock, the Trp6.48 toggle switch and the hydrogen interactions between Tyr5.58–Tyr7.53.
- This article is part of the themed collection: The free energy landscape: from folding to cellular function

 Please wait while we load your content...
Please wait while we load your content...