Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Proton tunnelling and promoting vibrations during the oxidation of ascorbate by ferricyanide?
Shaun M.
Kandathil
,
Max D.
Driscoll
,
Rachel V.
Dunn
,
Nigel S.
Scrutton
* and
Sam
Hay
*
Manchester Institute of Biotechnology (MIB) and Faculty of Life Sciences, University of Manchester, 131 Princess St., Manchester, UK M1 7DN. E-mail: nigel.scrutton@manchester.ac.uk; sam.hay@manchester.ac.uk; Fax: +44 (0)161 3065201; Tel: +44 (0)161 3065141
First published on 6th January 2014
Abstract
A combination of the temperature- and pressure-dependencies of the kinetic isotope effect on the proton coupled electron transfer during ascorbate oxidation by ferricyanide suggests that this reference reaction may exploit vibrationally assisted quantum tunnelling of the transferred proton.
The catalytic effect of enzymes – the rate enhancement relative to the uncatalysed rate (kcat/kuncat) – is often unparalleled, yet the precise origin(s) of this catalytic effect remain unresolved. A major problem1 remains the identification of good reference reactions that can be used to study the uncatalysed reaction.2–4 One class of potential reference reactions involve the oxidation of L-ascorbate (HAsc−; vitamin C)5–12 and are similar to the reactions catalysed by ascorbate peroxidase enzymes13 and cytochrome b561 proteins.14 In aqueous solution, ascorbate can be oxidised in two sequential 1-electron transfer reactions (Scheme 1; DHA is dehydroascorbic acid) by oxidants including ferricyanide ([Fe(CN)6]3−).5,11,12
During the first step of the reaction (k1), the electron transfer from ascorbate to ferricyanide appears to be kinetically-coupled to the proton transfer from ascorbate to solvent (i.e. a proton-coupled electron transfer; PCET), as modest solvent kinetic isotope effects (KIEs) have been observed on ferricyanide reduction.7,11,12 Under certain solvent conditions, the magnitude of the observed KIE at room temperature is larger than the semi-classical limit of ∼7, suggesting that nuclear quantum mechanical tunnelling (NQMT) of the transferred proton occurs during the PCET reaction.15 In addition, the temperature dependence of the KIE (ΔEa or ΔΔH‡) varies with solvent composition and, in some cases is quite large (ΔEa ∼ 10 kJ mol−1).9,12 Strongly temperature-dependent KIEs have been used as evidence to support the promoting vibrations hypothesis, where H transfer is coupled to molecular vibration(s) between the donor and acceptor atoms that modulate the rate of H-transfer by transiently reducing the apparent classical barrier height and/or width.1,16–18
Recent studies of PCET during ascorbate oxidation reactions have suggested that the degree of NQMT during this reaction is variable and dependent on solvent composition and/or the oxidant.9–12 In these cases, NQMT was inferred from either the magnitude of the KIE (KIE ≫ 7) or the Arrhenius prefactor ratio (AH/AD ≪ 1).8,10 A model PCET system where the degree of NQMT and the temperature dependence of the KIE varies would allow more rigorous testing of the promoting vibration hypothesis and the possibility that NQMT is catalytic.1 However, further evidence that the solvent modulates the degree of NQMT during these reactions is desirable.
In the absence of NQMT, KIEs on H-transfers arise due to differences in vibrational zero-point energy and thus the stretching frequency of the transferred H, D or T.15 Generally, C–H stretches are insensitive to several kbar changes in pressure (the typical experimental range; 1 bar = 100 kPa),19,20 so pressure dependent KIEs have been used as evidence for the involvement of NQMT during H transfer.21,22 In this communication, we use both variable temperature and hydrostatic pressure23 to further characterise the ascorbate–ferricyanide reaction and to determine whether it is possible to alter the degree of NQMT by altering the solvent composition; in this case, by the addition of tetraethylammonium chloride (TEA), a salt that has been shown to significantly increase both the magnitude and temperature dependence of the KIE on this reaction.12
While both ascorbic acid (H2Asc) and ascorbate (HAsc−) reduce ferricyanide, they do so with significantly different rate constants and associated thermodynamic parameters.5 As we will focus on the more physiologically-relevant ascorbate reaction, we chose to work in buffered solution at pH 6.0,† where ascorbate is relatively stable24 and the concentration of ascorbic acid negligible; the pKa of the H2Asc deprotonation to HAsc− is 4.1.5 Further, as the corresponding reaction volume is reported to be −9.6 cm3 mol−1,5 the pKa of ascorbic acid decreases with increasing pressure (Δln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = −ΔVa/RT), so the concentration of ascorbic acid will be further diminished at elevated pressures.
Ka = −ΔVa/RT), so the concentration of ascorbic acid will be further diminished at elevated pressures.
The temperature- (Fig. 1) and pressure- (Fig. 2) dependencies of ferricyanide reduction by ascorbate were determined using stopped-flow spectrometry by monitoring the loss of ferricyanide absorbance at 420 nm. The PCET rate constant, k1 (Scheme 1) was determined by: k1 = kobs/2[HAsc−]5,11,12 and these data were fit to eqn (1) or (2), with the resulting thermodynamic parameters given in Table 1.
| k(T) = (kBT/h) exp(ΔS‡/R) exp(−ΔH‡/RT) | (1) |
| k(p,T) = k0(T) exp(−ΔV‡(T)/RpT) | (2) |
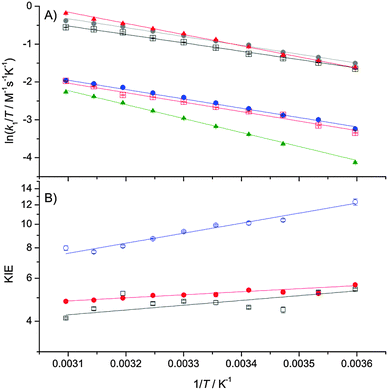 | ||
| Fig. 1 Eyring plots of k1 (A) and the temperature dependence of the KIE on k1 (B) fit to eqn (1). Key: (A) black squares, 5 mM HAsc−, H2O; red squares, 5 mM HAsc−, D2O; grey circles, 10 mM HAsc−, H2O; blue circles, 10 mM HAsc−, D2O; red triangles, 10 mM HAsc−, 0.85 M TEA, H2O; green triangles, 10 mM HAsc−, 0.85 M TEA, D2O. (B) Black squares, 5 mM HAsc; red circles, 10 mM HAsc; blue circles, 10 mM HAsc, 0.85 M TEA. | ||
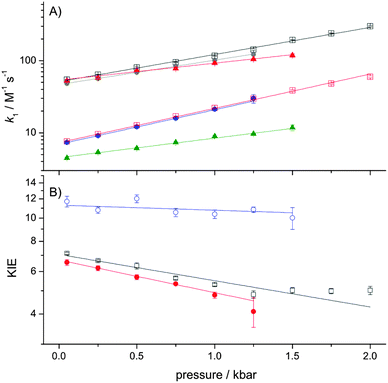 | ||
| Fig. 2 The pressure dependence of k1 (A) and the KIE on k1 (B) at 278 K. The data are fit to eqn (2) and the experimental conditions and symbol/colour scheme is the same as used in Fig. 1. Experiments were performed at 278 K in order to prevent significant ascorbate degradation during the relatively long lifetime of the experiment, which was typically 1–2 hours. | ||
| The parameters are derived by fitting the data in Fig. 1A and 2A to eqn (1) and (2), respectively. The quoted errors are standard errors determined during the data fitting.a The tetraethylammonium chloride (TEA) concentration of 0.85 M was chosen to be >0.5 M (where the effect is saturated12) and still in solution under all experimental conditions used in our study.b k1 was measured at 298 K.c k1,0, ΔV‡H and ΔΔV‡ were determined at 278 K. ΔΔH‡ = ΔH‡D − ΔH‡H; ΔΔS‡ = ΔS‡D − ΔS‡H; ΔΔV‡ = ΔV‡H − ΔV‡D. | |||
|---|---|---|---|
| [HAsc−] (mM) | 5 | 10 | 10 |
| [TEA]a (M) | 0 | 0 | 0.85 |
| k H1 (M−1 s−1)b | 99 ± 2 | 119 ± 2 | 123 ± 1 |
| k H1/kD1 | 4.8 ± 0.1 | 5.1 ± 0.1 | 9.9 ± 0.1 |
| ΔH‡H (kJ mol−1) | 18.4 ± 0.5 | 19.4 ± 0.4 | 24.5 ± 0.4 |
| ΔΔH‡ (kJ mol−1) | 2.4 ± 1.6 | 1.1 ± 0.9 | 6.1 ± 0.9 |
| ΔS‡H (J mol−1 K−1) | −145 ± 2 | −140 ± 1 | −123 ± 1 |
| ΔΔS‡ (J mol−1 K−1) | −5 ± 5 | −10 ± 3 | 2 ± 3 |
| k H1,0 (M−1 s−1)c | 51.2 ± 0.8 | 46.7 ± 0.3 | 53.7 ± 1.8 |
| k H1,0/kD1,0 | 7.0 ± 0.2 | 6.7 ± 0.1 | 11.8 ± 0.6 |
| ΔV‡H (cm3 mol−1) | −20.0 ± 0.4 | −18.0 ± 0.2 | −12.5 ± 0.9 |
| ΔΔV‡ (cm3 mol−1) | +5.3 ± 0.7 | +7.5 ± 0.4 | +1.8 ± 1.4 |
The reactions were repeated in buffered D2O solutions in order to determine the solvent KIE on the PCET during the reaction (Fig. 1 and 2; Table 1). The pH of the D2O solutions (pH* 6.0, pD 6.4) was chosen to offset the change in ascorbate pKa caused by deuteration.25 The reactions were performed with both 5 mM and 10 mM ascorbate (both in vast excess of ferricyanide) to confirm that the reaction is both strictly second-order, and that the KIE is independent of ascorbate concentration.
Like previous reports,9,12 in the absence of TEA the observed KIE on k1 is within the semi-classical limit (<7) at 25 °C and is only marginally temperature dependent. However, while the pressure dependence of kH1 (ΔV‡H) is not remarkable, and similar to previous reports,5 we found the KIE to be significantly pressure-dependent (|ΔΔV‡| ≫ 0; Table 1). As the pressure dependence of a KIE is a diagnostic of NQMT,21,22 these data provide compelling evidence for proton tunnelling from HAsc− to solvent during the PCET reaction.
Like previous reports,12 in the presence of high concentrations of TEA, the magnitude of the KIE becomes larger than the semiclassical limit, suggesting that NQMT plays a significant role during the PCET.15 As the difference in activation enthalpy is significantly increased, while the pressure dependencies of both k1 and the KIE are significantly reduced in the presence of TEA (Table 1), it would appear that TEA may influence the (vibrational) coupling of the H-transfer to the environment – i.e. the apparent promoting vibration(s) that give rise to the temperature-dependence of the KIE.23,26
We recently determined the pressure- and temperature-dependence of the large KIE on proton transfer catalysed by aromatic amine dehydrogenase (AADH). In this case, the pressure dependence of the KIE was found to vary with temperature27 and we concluded that while the AADH reaction clearly employs significant NQMT,28 the pressure-dependence of this KIE is not a good diagnostic of NQMT in this reaction. In the presence of TEA, the ascorbate–ferricyanide reaction behaves in a similar manner: both the magnitude of the KIE and ΔΔH‡ suggest that significant NQMT is involved, yet ΔΔV‡ is negligible (Table 1). More generally, the data in the present study provide further evidence that while strongly pressure-dependent KIEs are likely to arise as a result of NQMT, the absence of a pressure dependence of a KIE on H transfer is not evidence for a lack of NQMT during the reaction.
Taken together, the temperature and pressure dependencies of the KIE on the ascorbate–ferricyanide reaction suggest that NQMT plays a role during the reaction both in the presence (exalted KIE and increased ΔΔH‡) and absence of TEA (exalted ΔΔV‡). Further, the temperature dependence of the reaction in the presence of TEA is supportive evidence for the role of ‘promoting vibrations’.1,16
There are few examples of small molecule systems that manifest strongly temperature-dependent KIEs. It has been suggested that the unusual temperature dependencies of the KIE on quinol oxidation29,30 may arise due to the involvement of donor–acceptor vibrational modes (i.e. promoting vibration(s)) as well as excited vibronic states in the Marcus inverted region.31 In the case of the ascorbate–ferricyanide reaction, the PCET is likely to occur in a (transient) collisional complex formed between ascorbate and ferricyanide, as the reaction is second-order. Also, as the proton acceptor is likely to be solvent water, the nature of any persistent donor–acceptor vibrational modes will be very different to those promoting vibrations proposed to play a role during enzyme-catalysed reactions.1,16–18 It is noteworthy that both the magnitude and temperature dependence of the KIE on the ascorbate–ferricyanide reaction is modulated by changes in solvent composition (Table 1 and e.g. ref. 9–12). The reorganisation energy is sensitive to the solvent composition, and if the PCET reaction involves significant contribution from donor and/or acceptor excited vibronic states, then the KIE may be significantly temperature dependent (as is the case when TEA is added; Fig. 1) and sensitive to the solvent reorganisation.31 The ascorbate–ferricyanide reaction should be tractable for further theoretical and/or computational analysis to determine the precise origins of the magnitude and temperature dependence of the KIE on its PCET reaction.
In summary, the combination of the temperature- and pressure-dependencies of the KIE on the ascorbate–ferricyanide reaction have demonstrated that NQMT appears to play a role in the PCET during this reaction, both in the presence and absence of TEA, and is likely to be a general feature of the PCET during ascorbate oxidation. Significantly, the combined use of pressure and temperature allows one, in this case, to uncover NQMT contributions, which are not apparent when only looking at the magnitude or temperature-dependence of the KIE. Also, as the temperature dependence of the KIE is readily modulated by solvent composition,9–12 this is an ideal reference reaction for related studies of tunnelling and the potential involvement of promoting vibrations in related enzyme systems.
S.H. is a Biotechnology and Biological Sciences Research Council (BBSRC) David Phillips Fellow. N.S.S. is a Royal Society Wolfson Research Merit Award holder and an Engineering and Physical Sciences Research Council Established Career Fellow in Catalysis.
Notes and references
- S. Hay and N. S. Scrutton, Nat. Chem., 2012, 4, 161–168 CrossRef CAS PubMed.
- A. Radzicka and R. Wolfenden, Science, 1995, 267, 90–93 CAS.
- K. M. Doll, B. R. Bender and R. G. Finke, J. Am. Chem. Soc., 2003, 125, 10877–10884 CrossRef CAS PubMed.
- D. T. Major, A. Heroux, A. M. Orville, M. P. Valley, P. F. Fitzpatrick and J. L. Gao, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20734–20739 CrossRef CAS PubMed.
- N. Kagayama, M. Sekiguchi, Y. Inada, H. D. Takagi and S. Fanahashi, Inorg. Chem., 1994, 33, 1881–1885 CrossRef CAS.
- D. Vuina, V. Pilepic, D. Ljubas, K. Sankovic, I. Sajenko and S. Ursic, Tetrahedron Lett., 2007, 48, 3633–3637 CrossRef CAS PubMed.
- I. Sajenko, V. Pilepic, C. J. Brala and S. Ursic, J. Phys. Chem. A, 2010, 114, 3423–3430 CrossRef CAS PubMed.
- C. J. Brala, V. Pilepic, I. Sajenko, A. Karkovic and S. Ursic, Helv. Chim. Acta, 2011, 94, 1718–1731 CrossRef CAS.
- A. Karkovic, C. J. Brala, V. Pilepic and S. Ursic, Tetrahedron Lett., 2011, 52, 1757–1761 CrossRef CAS PubMed.
- I. Sajenko, V. Pilepic and S. Ursic, Z. Phys. Chem., 2011, 225, 805–820 CrossRef CAS.
- C. J. Brala, A. Karkovic, K. Klepac, A. M. Vucinovic, V. Pilepic and S. Ursic, Z. Phys. Chem., 2011, 225, 821–841 CrossRef CAS.
- C. J. Brala, A. Karkovic, I. Sajenko, V. Pilepic and S. Ursic, Z. Phys. Chem., 2012, 226, 29–46 CrossRef CAS.
- E. L. Raven, L. Lad, K. H. Sharp, M. Mewies and P. C. Moody, Biochem. Soc. Symp., 2004, 27–38 CAS.
- D. Njus, M. Wigle, P. M. Kelley, B. H. Kipp and H. B. Schlegel, Biochemistry, 2001, 40, 11905–11911 CrossRef CAS PubMed.
- R. Bell, The Tunnel Effect in Chemistry, Chapman and Hall, 1980 Search PubMed.
- Z. D. Nagel and J. P. Klinman, Chem. Rev., 2010, 110, PR41–PR67 CrossRef PubMed.
- S. D. Schwartz, Top. Curr. Chem., 2013, 337, 189–208 CrossRef CAS.
- S. Hammes-Schiffer, Biochemistry, 2013, 52, 2012–2020 CrossRef CAS PubMed.
- N. S. Isaacs, in Isotope Effects in Organic Chemistry, ed. E. Buncel and C. C. Lee, Elsevier, London, 1984, pp. 67–105 Search PubMed.
- D. B. Northrop, Philos. Trans. R. Soc., B, 2006, 361, 1341–1349 CrossRef CAS PubMed.
- N. S. Isaacs, K. Javaid and E. Rannala, Nature, 1977, 268, 372 CrossRef CAS.
- D. B. Northrop, J. Am. Chem. Soc., 1999, 121, 3521–3524 CrossRef CAS.
- S. Hay, M. J. Sutcliffe and N. S. Scrutton, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 507–512 CrossRef CAS PubMed.
- R. M. C. Dawson, D. C. Elliot, W. H. Elliot and K. M. Jones, Data for biochemical research, Oxford University Press, Oxford, 3rd edn, 1986 Search PubMed.
- A. Krezel and W. Bal, J. Inorg. Biochem., 2004, 98, 161–166 CrossRef CAS PubMed.
- S. Hay and N. S. Scrutton, Biochemistry, 2008, 47, 9880–9887 CrossRef CAS PubMed.
- S. Hay, L. O. Johannissen, P. Hothi, M. J. Sutcliffe and N. S. Scrutton, J. Am. Chem. Soc., 2012, 134, 9749–9754 CrossRef CAS PubMed.
- L. Masgrau, A. Roujeinikova, L. O. Johannissen, P. Hothi, J. Basran, K. E. Ranaghan, A. J. Mulholland, M. J. Sutcliffe, N. S. Scrutton and D. Leys, Science, 2006, 312, 237–241 CrossRef CAS PubMed.
- S. Nagaoka, M. Inoue, C. Nishioka, Y. Nishioku, S. Tsunoda, C. Ohguchi, K. Ohara, K. Mukai and U. Nagashima, J. Phys. Chem. B, 2000, 104, 856–862 CrossRef CAS.
- J. L. Cape, M. K. Bowman and D. M. Kramer, J. Am. Chem. Soc., 2005, 127, 4208–4215 CrossRef CAS PubMed.
- M. K. Ludlow, A. V. Soudackov and S. Hammes-Schiffer, J. Am. Chem. Soc., 2009, 131, 7094–7102 CrossRef CAS PubMed.
- N. E. Good, G. D. Winget, W. Winter, T. N. Connolly, S. Izawa and R. M. M. Singh, Biochemistry, 1966, 5, 467–477 CrossRef CAS.
- Y. Kitamura and T. Itoh, J. Solution Chem., 1987, 16, 715–725 CrossRef CAS.
Footnote |
| † All materials were purchased from Sigma-Aldrich except D2O, which was purchased from Goss Scientific Equipment Ltd. L-H2Asc solutions were prepared on the day of use and their concentration was determined spectrophotometrically in ∼1 mM HCl by ε = 7.5 mM−1 cm−1 at 245 nm.24 All measurements were performed with 0.1 mM K3[Fe(CN)6], 0.5 mM Na2EDTA and 50 mM MES (2-(N-morpholino)ethanesulfonic acid; pKa = 6.15) buffer, pH or pH* 6.0. MES was chosen due to its relatively low temperature (ΔpKa/T = −0.011 K−1)32 and pressure (ΔV0 = +3.9 cm3 mol−1)33 coefficients. Temperature-dependent stopped-flow experiments were performed with an Applied Photophysics (Leatherhead, UK) SX.18MV-R stopped-flow spectrophotometer. High-pressure experiments were performed using a Hi-Tech Scientific HPSF- 56 high-pressure stopped-flow spectrophotometer (TgK Scientific, Bradford on Avon, UK). In both cases, the reaction was monitored at 420 nm and fit to a single or double (to account for a minor slow-phase)-exponential function to determine kobs. |
| This journal is © the Owner Societies 2014 |