Efficient water oxidation with organometallic iridium complexes as precatalysts†
Abstract
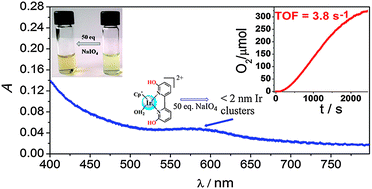
Catalytic water oxidation has been investigated using five iridium complexes as precatalysts and NaIO4 as an oxidant at various pH conditions. An increase in the activity of all complexes was observed with increasing pH. A detailed analysis of spectroscopic data together with O2-evolution experiments using Cp*Ir(6,6′-dihydroxy-2,2′-bipyridine)(OH2)2+ as a precatalyst indicate that the high catalytic activity is closely connected with transient species (A) that exhibits an absorption band at λmax 590 nm. The formation of this active form is strongly dependent on reaction conditions, and the species was distinctly observed using a small excess of periodate. However, another species absorbing at 600 nm (B), which seems to be a less active catalyst, was also observed and was more prominent at high oxidant concentration. Dynamic light scattering analysis and transmission electron microscopy have identified species B as 120 nm nanoparticles. The ultrafiltration method has revealed that species A can be attributed to particles with size in the range of 0.5–2 nm, possibly small IrOx clusters similar to those described previously by Harriman and co-workers (J. Phys. Chem., 1991, 95, 616–621).
- This article is part of the themed collection: Photosynthesis: From Natural to Artificial

 Please wait while we load your content...
Please wait while we load your content...