Charge transport properties of spin crossover systems
Eliseo
Ruiz
Departament de Química Inorgànica and Centre de Recerca en Química Teòrica, Universitat de Barcelona, Diagonal 645, 08028 Barcelona, Spain. E-mail: eliseo.ruiz@qi.ub.es
First published on 25th October 2013
Abstract
The study of spin crossover compounds by means of theoretical or experimental approaches has provided interesting results in recent decades. The main feature of such compounds is the change in the spin state induced by many different external stimuli, i.e. temperature, light, pressure, solvent coordination and the electric field. Spin crossover systems are potentially more useful than other magnetic molecules because their switching behaviour can occur closer to room temperature, and they are thus candidates for use in spintronic devices. Here, I review the state of the art in quantum chemical approaches to the study of such systems and discuss experiments that have focused on transport properties in single-molecule, nano-objects or thin-film spin crossover systems.
1. Introduction
Spin crossover is an appealing magnetic property1–3 based on ground state spin-switching controlled by means of an external stimulus, i.e. temperature, light, pressure, solvent coordination or the electric field.4–8 This property is usually associated with a d4–d7 electron configuration of pseudo-octahedral mononuclear first-row transition metal complexes. Thus, for such coordination with a d4 configuration, the fourth electron can be placed in one of the singly-occupied t2g orbitals (low spin) or in one of the empty eg orbitals (high spin). Indeed, the stability of both states will depend on a subtle balance between inter-electronic repulsion energy in the t2g orbitals and the energy gap between the t2g and eg orbitals. An important feature of the spin crossover phenomenon is the change in size of the octahedral complexes, which is due to the different bonding nature of the t2g (non-bonding) and eg (antibonding) orbitals and results in a considerable increase in metal–ligand distances in the high-spin state in comparison with the low-spin one.9 Since the first spin crossover systems, FeIII dithiocarbamate complexes, were reported by Cambi et al. in 1931,10 hundreds of mononuclear and polynuclear iron complexes have been characterised.11–16 Although this property could appear with pseudo-octahedral coordination of the four electron configurations mentioned above, most of the systems studied have consisted of FeII d6 complexes with the metal coordinated to six nitrogen atoms. Spin crossover behaviour is extremely sensitive to ligand coordination, and subtle changes in the structure of the complex or the nature of the ligands can extinguish such behaviour.17,18 There are also examples of other elements, such as MnII, MnIII, CrII and CoIII cations.19–21 However, in practice, this property is basically restricted to first-row transition metals. Low-spin states become very stable for second- and third-row transition metals due to the large energy splitting of the orbitals and a weaker inter-electronic repulsion.22 Hence, only some low number coordination modes, or systems with a small number of electrons with occupation of nonbonding orbitals only,23i.e. MoIV d2 electron configuration,24 can be close for the spin crossover transition to occur. It is important to bear in mind that for non-octahedral coordination modes, spin crossover phenomena could appear for other electron configurations. The limit for this corresponds to the five non-degenerate d orbitals, in which case, such behaviour could be possible for the d2 to the d8 electron configuration.In spin crossover systems, the low-spin state is the ground state at low temperature. Although a low–high spin conversion can be promoted by diverse external stimuli, temperature change is usually employed to promote the transition.25 An increase in temperature favours the stability of the high-spin state in electronic and vibrational entropic terms.26 Indeed, the transition temperature corresponds to a situation in which such entropic contributions can compensate the enthalpic contribution favourable to a low-spin state. Another important aspect is the existence of cooperativity effects, which can be detected using magnetisation measurements.27 Molecules have a tendency to maintain the same state as their neighbouring molecules, resulting in a different magnetic curve when measurements are taken during cooling or heating of the sample, starting a hysteresis cycle. The transition can be abrupt, with all the molecules suddenly changing their spin state, in which case it is proof of high cooperativity, or more gradual.28,29 Strong cooperativity and communication between neighbouring metal centres, for instance that observed in the periodic Hofmann type-clathrate spin crossover systems synthesised by Real and coworkers, induce wider hysteresis cycles than systems with lower dimensionality,30,31 such as planes, chains or discrete molecules. In this latter case, the effect is only transmitted through weak intermolecular interactions.32 It is important to note that these materials can show fast switching effects and bistability at the molecular level near room temperature, in contrast to other magnetic molecular systems that present attractive physical properties but only at low temperatures. Consequently, these kinds of system are potential candidates in the design of switchable molecular spintronic devices.33–37
Light irradiation can promote a low temperature transition from the ground low-spin state to the excited high-spin state. Since the system in the high-spin state adopts some structural changes and de-excitation is a spin-forbidden process, the system remains trapped in a high-spin state at low temperatures. This mechanism is called LIESST (Light Induced Excited Spin State Trapping, see Fig. 1), and it causes the system to invert the relative stability of low- and high-spin states.7,38 Also worth mentioning is the mechanism usually known as LD-LISC (Ligand Driven-Light Induced Spin Changes),39 an effect that appears in complexes with one or more ligands, which can undergo a photo-induced structural modification resulting in metal spin transition. Pressure also has an influence on spin crossover transition. Due to the larger size of high-spin complexes, this state is not favoured when pressure is applied;40 hence, transitions occur at higher temperatures if pressure is applied. Such stability can also be modulated by chemical species such as solvent molecules. Thus, for some complexes, the coordination (or de-coordination) of solvent molecules can trigger a spin transition in the metal centre.25,41–43
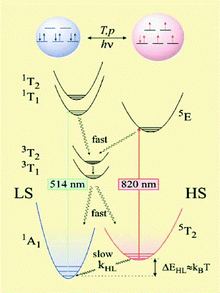 | ||
| Fig. 1 Electronic structure of a spin-crossover FeII complex indicating some of the external stimuli that can provoke the transition and the mechanism of LIESST and reverse-LIESST effects. (From ref. 25.) | ||
2. Quantum chemical studies of spin crossover systems
From the theoretical point of view, the quantum chemical study of spin crossover entails some important difficulties, as has been pointed out in recent extensive reviews (and references therein).44–46 In principle, the comparison of theoretical results with experimental data is not an easy task because the key parameter of a spin crossover system is normally its transition temperature, typically quantified by T1/2, which corresponds to the transition of 50% of the molecules, and typically determined in a solid sample rather than in discrete isolated molecules. Hence, to calculate the transition temperature it is also necessary to calculate the system's vibrational spectra, where low frequencies play a fundamental role in the transition.47–49 It is well known that the calculation of energy differences between electronic configurations with different total spin is a problematic task when using DFT methods;50,51 however, despite these obstacles, many quantum chemical studies have been conducted for these systems. In the first studies to use DFT methods,50,52,53 the goal was to reproduce “experimental” energy difference values between both states for the [Fe(phen)2(NCS)2] complex, and the results obtained indicated that the choice of the exchange–correlation functional exerted a strong influence.50,51,54 Thus, GGA and LDA functionals would overestimate low-spin state stability, resulting in an excessive energy difference, while the popular hybrid B3LYP functional gives the opposite effect. In order to circumvent such problems, a B3LYP* functional was proposed which included only 15% exact-type exchange instead of the 20% in the original B3LYP functional.55,56 Furthermore, a scaling factor which corrected the deficiencies of the functionals was employed to estimate the thermodynamic magnitudes57 involved in spin crossover phenomena.58 In recent years, most of the studies using DFT methods have focused on reproducing the correct ground state and obtaining reasonable electronic energy differences between the two spin states.50,51,59–63 The calculation of thermodynamic magnitudes, such as the enthalpy or the free energy, which allows a direct comparison with the experimental data, has recently been devised using hybrid meta-GGA functionals for a family of FeII systems showing LD-LISC behaviour. The TPSSh functional correctly reproduces the correct ground state, but an average error of more than 50 K is obtained in the computed T1/2 values.64 In addition to mononuclear spin crossover systems, trinuclear65 and tetranuclear66,67 complexes have also been theoretically studied. Cooperativity effects can be mimicked through the inclusion of periodic boundary conditions, although this complicates the calculation of thermodynamic contributions. Periodic DFT + U methods have been employed to overcome the functional problem48,68 and dispersion corrections have also been added, showing that the transition enthalpy for the prototypical [Fe(phen)2(NCS)2] system was larger for the crystalline phase than for the isolated molecule. This finding indicates that intermolecular contacts stabilise the low-spin state in comparison to the high-spin state for this type of system. Tarafder et al. employed the same theoretical approach, periodic DFT + U calculations, to perform molecular dynamics simulations of a spin-crossover FeIINbIV metal–organic framework in order to analyse the effects of temperature and pressure and the influence of the spin state on exchange interactions between the paramagnetic centres.69Among the post Hartree–Fock methods, the multiconfigurational CASPT2 calculation70 can provide more accurate energy values for the spin states than DFT methods, but its application is limited to the study of the thermodynamic properties of such systems due to the lack of analytical frequencies in the computer codes and heavy computational requirements. However, such approaches have been employed to determine the electronic structure of spin crossover and particularly to focus on the nature and energy excitations of the excited states.71–76 The nature of the excited states can be analysed using this methodology due to the interaction of the complex with light, and consequently, to study the states involved in the LIESST effect.74,75 An unusual theoretical approach in Chemistry, but with similar expected accuracy as that provided by the multiconfigurational methods, is the diffusion Monte Carlo method. Droghetti et al. recently conducted a comparative study using this approach with DFT functionals, and reported the strong influence of ground state energies depending on whether the molecule was in gas or solid phase.77
3. Charge transport properties of spin-crossover systems
Due to their switching activity, spin-crossover systems have been proposed as prime candidates in Molecular Electronics and Spintronics for use in molecular-based devices.78 They have been considered both in theoretical studies based on DFT methods combined with the NEGF approach to calculate I–V characteristics79,80 and from the experimental point of view with STM experiments. From the theoretical point of view, the results for spin-crossover FeII d6 complexes are very attractive because they present a large spin polarisation of high-spin S = 2 t2g4eg2 state (five alpha and one beta electrons) showing relatively high conductivity. Charge transport is mainly due to beta electrons (minority spin electrons) induced by the energy splitting of beta orbitals for such electron configuration (as the energy level in comparison with the Fermi level of the electrodes).79 Hence, high spin electron configuration leads to such large conductivity (considering that there is only one beta electron) because the orbital bearing this electron, as well as the first unoccupied beta orbital are close in energy to the Fermi level of the gold electrodes, providing effective channels for molecular conductivity (see Fig. 2). However, in the low spin S = 0 t2g6eg0 electron configuration, both sets of orbitals are relatively distant from the Fermi level, resulting in lower conductivity, at least for the ligands involved in the studied FeII complexes.79,80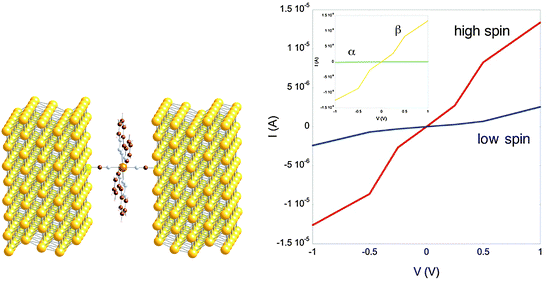 | ||
| Fig. 2 Model structure of the gold electrodes and the trans bis(3-(2-pyridyl)(1,2,3)triazolo(1–5)pyridine)bis(isothiocyanato)iron(II) complex (left). I–V characteristics calculated at the DFT-NEGF level for the high- (red) and low-spin (blue) states. The inset contains the alpha (green) and beta (orange) contributions of the current in the high-spin system. (From ref. 79. Reproduced with the permission of the American Chemical Society.) | ||
From an experimental point of view, one of the first attempts to study the spin crossover system deposited on a surface using the STM technique was performed in 2010 by Alam et al., who studied spin-switchable iron(II) complexes of bis(pyrazolyl)pyridine ligands on graphite surfaces81 and employed current-imaging tunnelling spectroscopy to detect the iron centres. These authors reported that low-spin states presented a higher local tunnelling conductance than high spin-states for this system. Although MnII centres are relatively uncommon in the spin crossover field, a single-molecule three-terminal device was constructed using the [Mn(terpy-O-(CH2)6-Sac)2]2+ complex placed in the gap between two gold electrodes generated by electromigration.82 By changing the gate voltage, it was possible to reduce the complex. The additional electron in the terpyridine ligand destabilises the antibonding eg orbitals resulting in a stabilization of the low-spin state in the MnII centres. A similar combined theoretical and experimental study of the transport properties of a spin crossover [FeII(bpp)2]2+ [bpp: 2,6-bis(pyrazol-1-yl)pyridine] complex was carried out by Meded et al.83 using DFT and CASSCF calculations to characterise the electronic structure of this complex and to compute the relative energy of the states involved. Transport properties were determined using a nano-device, placing single molecules in a gap between two gold electrodes generated by electromigration. These measurements were performed at low temperatures and the Kondo effect was detected; however, the authors have claimed that the spin state of the molecule can be controlled in the device with the electric field.
Control of the spin state with the electric field was previously reported in 2009 by Baadji et al.84 in a theoretical study of di-cobaltocene-based molecules. For these systems, they show that the destabilization energies due to the electric field, Stark energy, are different for the singlet and the triplet state. Thus, theoretical estimations indicate that the singlet ground state could be switched for a single-molecule if an electric field of the order of 1 V nm−1 was applied in a two-terminal device with a triplet ground state. In the same year, experimental evidence was provided by Mahfoud et al. for cyanometalate Prussian blue analogues, Rb0.8Mn[Fe(CN)6]0.93·1.62 H2O and Co3[W(CN)8]2(pyrimidine)4·6H2O, following the effect of the electric field by switching the ground state through the changes in the Raman spectra.85,86 The first compound exhibited a charge-transfer phase transition between the high-temperature FeIII(S = 1/2)-CN-MnII(S = 5/2) and the low-temperature FeII(S = 0)-CN-MnIII(S = 2) states, while in the second compound, the high-temperature state was a CoHSII(S = 3/2)-NC-WV(S = 1/2) complex and the low-temperature system presented a CoLSIII(S = 0)-NC-WIV(S = 0) electronic configuration. Application of an electric field above a threshold value and within the thermal hysteresis region leads to a transition from the high- to low-temperature phase in these compounds. The importance of control of the spin of the magnetic molecules by an external electric field was outlined by Sessoli.87 More recently, a similar concept has been proposed in theoretical studies by changing the exchange coupling constants with the electric field in Cu3 complexes88 and for valence tautomeric interconversion in cobalt dioxolene complexes.89
Coming back to the topic of the charge transport properties of spin crossover on surfaces, following the two experiments mentioned above, other authors have performed similar experiments.90 A crucial aspect of these is the thin film growth and patterning of spin crossover compounds on surfaces, a subject which has been extensively discussed by Cavallini in recent reviews.91,92 Thus, key elements include the relative low-spin and high-spin stabilities of the molecules deposited on surfaces in comparison with bulk behaviour, and how to control the state with the electric field (or current). An excellent example using STM at low temperature (5 K) was provided by Gopakumar et al.93 showing that [Fe(H2Bpz2)2(bipy)] molecules (phen = 1,10-phenanthroline) deposited on an Au(111) surface (see Fig. 3) can be switched from the low-spin to high-spin state by the STM tip (I = 0.5 nA and V = 1.8 V). On a timescale of seconds, the low-spin state was once again recovered. A high-spin state can be confirmed by the existence of Kondo resonance due to the presence of unpaired electrons (see Fig. 3). High-spin states show higher conductance than low-spin ones. High-spin to low-spin transitions of a second layer of molecules can also be controlled with elevated currents and a voltage of 1.5 V. The absence of molecule switching in the first layer is due to the shorter lifetime of the electronically high-spin excited state in direct contact with the metal surface.
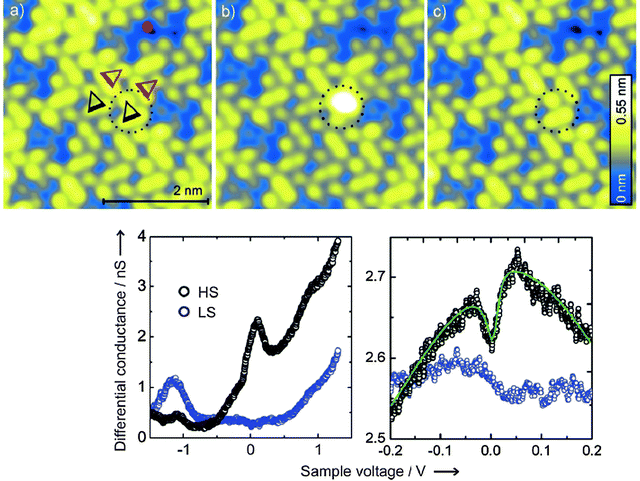 | ||
| Fig. 3 STM topographs of a double layer of [Fe(H2Bpz2)2(bipy)] (a) triangles indicated four selected molecules. One of them switched from the LS to HS state (b) and back to the LS state (c). Differential conductance spectra showing the larger conductance of the HS state and the presence of the Kondo effect at V = 0. (From ref. 92. Reproduced with the permission of Wiley – Angewandte Chemie International Edition.) | ||
Miyamachi et al. have performed outstanding low-temperature STM experiments with a prototypical spin crossover system [Fe(phen)2(NCS)2] deposited on Cu(001) surfaces94 (also introducing an interfacial CuN layer, previously the mobility of a thin layer of the same molecule was also explored).95 The results obtained using X-ray absorption spectroscopy showed the coexistence of low- and high-spin states even though the experiment was performed at 50 K (bulk Tc 175 K), consistent with the presence of two different types of molecule (low- and high-spin) in the STM images. Furthermore, Kondo resonance was found for the high-spin state and it presented a higher conductance than the low-spin molecule on CuN/Cu(100) surfaces. On bare Cu(100) surfaces, this is not possible, but on CuN/Cu(100) surfaces, the FeII complex can be electrically switched between high- and low-spin states at low temperatures (see Fig. 4). Thus, cyclic I(V) curves are obtained showing a switch with memristive effects (electric control of conductance) from high- to low-spin (smaller size and abrupt current decrease) at 1.2 V, while the opposite switching event appears at −0.8 V. An analysis of the two switching rates R reveals the existence of two different mechanisms, depending on the current (R ∝ IN): a low- to high-spin state is a high order process (N ≈ 8) involving heating by the tunnelling electrons, whereas the transition from high-spin to low-spin states only requires one electron (N ≈ 1) and involves an intermediate and short-lived excited state.
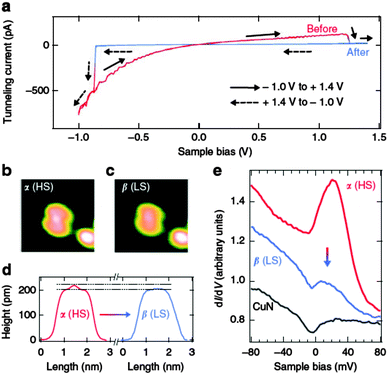 | ||
| Fig. 4 (a) I–V characteristics of the [Fe(phen)2(NCS)2] deposited CuN/Cu(100) surface showing the hysteretic behavior. (b) and (c) STM images of the molecule in the two states and the height profile showing the smaller size of the LS molecules. (d) Height profiles along the molecular long axis before and after the transition. (e) Differential conductance spectra determined before and after the switching process showing the transition from the high-conductance HS state (b) to the low-conductance LS state (c) and also corresponding to the bare CuN surface. (From ref. 94. Reproduced with the permission of Macmillan Publishers Ltd. – Nature Communications.) | ||
At the theoretical level, the state stability of the [Fe(phen)2(NCS)2] complex deposited on a Cu(001) surface has been studied using DFT methods, including the GGA functional +U and dispersion corrections.96 The results showed an increased stability of the low-spin state when the molecule was deposited on the Cu(001) surface compared to the isolated molecule. This trend between the energy of the two states is equivalent to that found between isolated molecules and the bulk phase mentioned previously.48,68 However, the presence of the CuN interfacial layer has been observed experimentally to reduce this energy gap, facilitating the spin switch, due to a weaker molecule–substrate interaction. The use of graphitic substrates preserves spin-crossover behaviour even for molecules in direct contact with the surface, as has been shown by Bernien et al. using X-ray absorption spectroscopy.97
The role of the surface was also studied by Zhang et al.,98 who used spectroscopic techniques to perform a comparative study of a thin film of the [Fe(H2Bpz2)2(bipy)] complex (H2Bpz2 = bis(hydrido)bis(1H-pyrazol-1-yl)borate on gold and organic ferroelectric copolymer poly(vinylidene fluoride)) with trifluoroethylene substrates. The molecular films showed a significant energy shift of the unoccupied density of states with temperature, detected by inverse photoemission spectroscopy, indicating that some thermal spin crossover occurred on the organic ferroelectric substrate. Experimentally, Pronschinske et al. employed STM and local conductance mapping to also show spin-state coexistence in bilayer films of the same [Fe(H2Bpz2)2(bipy)] on Au(111) which, in contrast to bulk behaviour, is independent of temperature between 131 and 300 K.99 Thus, many molecules on the surface (STM imaging and local conductance measurements) remain in a low-spin state at room temperature. Analysis of the calculated DFT density of states in this system also confirms that the high-spin molecules have closer states to the Fermi level than the low-spin ones, leading to greater transport properties. The LIESST effect has also been detected in a film of [Fe(H2Bpz2)2(bipy)] molecules on gold;100 however, only a fraction of the molecules (around 20%) had a reversible transition such as in the bulk phase, while most of the molecules remained trapped in a definite spin state, further corroboration of the important influence of the surface on such properties.
Other research groups have also conducted studies on the charge transport of spin-crossover nano-objects.101–103 Coronado et al. obtained 15 nm diameter nanoparticles showing spin crossover behaviour from [Fe(R-trz)3]X2 compounds (trz = 1,2,4-triazole) using the reverse micelle technique. Prins et al. performed an innovative study of the charge transport of such nanoparticles, ([Fe(trz)3](BF4)2), using a device built from a single nanoparticle (see Fig. 5).104 They observed I–V characteristics at 10 K for a 100 nm wide electrode equidistant Coulomb blockade step, indicating that the transport mechanism in the device was due to sequential single-electron tunnelling through an asymmetric double-barrier junction (nanoparticle core-surfactant layer). At higher temperatures, a hysteresis loop was observed in the I–V characteristics: at constant temperature, a transition between the low-spin and high-spin state occurred when changing the bias voltage.
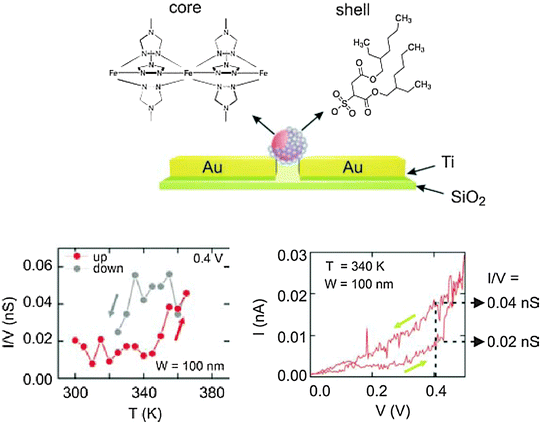 | ||
| Fig. 5 (above) Scheme of the device with ([Fe(trz)3](BF4)2) nanoparticles and (below) I–V dependence with the temperature and I–V characteristics at 340 K showing the hysteretic behavior and the transition from the LS to HS state at V = 0.4 V. (From ref. 104. Reproduced with the permission of Wiley – Advanced Materials.) | ||
Recently, nanorods of the same FeII compound, as that employed by Prins et al. for nanoparticles,104 were studied by Rotaru et al., but the transport properties presented the opposite behaviour to that of the equivalent nanoparticles (conductivity of the low-spin state higher than the high-spin state).105,106 This finding could be attributed to the different mechanism responsible for transport, expected for the nanorods due to their larger size, whereas tunnelling should be dominant in the smaller nanoparticles. Spin-crossover nanoparticles of [Fe(hptrz)3](OTs)2 (hptrz = 4-heptyl-1,2,4-triazole, OTs = p-toluenesulfonyl) were employed as sensors for a high sensitivity magnetometer due to the change in the voltage due to the spin transition.107 The device shows a large detection capability because of a limit of detection of 2 × 10−6 mm3 for the size of spin-crossover compound and the goal should be the detection of the properties of a single nanoparticle.
Mahfoud et al. reported a non-volatile memory effect of a [Fe(HBpz3)2] spin crossover complex deposited on thin films using Raman microspectroscopy, and confirmed that the films maintained the structure and properties of the bulk material.108 The compound underwent an irreversible thermal phase change at 380 K, resulting in crystallographic transformation. The properties of the thin film were compared with microcrystalline powder by performing AC conductivity measurements whilst varying frequency and temperature. These measurements indicated that the thin film exhibited a greater dependence on frequency than the microcrystalline powder, probably due to a higher degree of disorder in the thin film. I–V characteristics of the thin film were dramatically modified after heating to above the transition temperature, causing a large reduction in conductivity. This effect was employed in a device working at 370 K (just below the transition temperature) and increasing the electric bias in increments of 1 V per minute. At 2 V (due to the heat caused by the Joule effect), a phase transition occurred and consequently, an important reduction in the current (from 42 nA to 0.4 nA). The authors have suggested that this effect could be used to advantage in a ROM memory device, where the writing process could be performed by heating the cell while the read out would performed by measuring the resistivity of the device at room temperature.
Finally, it is noteworthy to mention that there is extensive research in the field of merging electrical and spin-crossover properties in bulk materials.109 Two strategies have been mainly employed to obtain such multifunctional materials: double salts by combining spin-crossover cations/anions and also charged molecular conductors, i.e. TCNQ or [Ni(dmit)2]− or the inclusion of ligands that can be conductors. The possibility to merge the LIESST effect with conductivity is particularly interesting to promote by irradiation the conductivity (or superconductivity). Also, the combination of liquid crystal behaviour and spin-crossover has been explored basically using long alkyl chains on the ligands.110,111
As conclusion and perspectives, spin-crossover systems have a tremendous potential in molecular electronic devices due to the switching ability at high (room) temperature and the possibility to use the electric field as external stimulus for switching. Relatively strong electric fields can be easily localized in small regions of molecular devices. But still many aspects should be considered in more detail: (i) the role of ligands in order to tune the charge transport properties; (ii) a deeper analysis of the influence of the molecule–electrode contact; (iii) full knowledge of the charge transport mechanism from the single-molecule to larger nanostructured systems; (iv) accurate control of the spin state using electric fields and currents and (v) use of magnetically-active electrodes in the devices with spin crossover systems.
Acknowledgements
This research was funded by the Ministerio de Ciencia e Innovación and Generalitat de Catalunya through grants CTQ2011-23862-C02-01 and 2009SGR-1459.References
- P. Gütlich, Eur. J. Inorg. Chem., 2013, 581 CrossRef.
- P. Gütlich, A. B. Gaspar and Y. Garcia, Beilstein J. Org. Chem., 2013, 9, 342 CrossRef PubMed.
- Spin-Crossover Materials: Properties and Applications, ed. M. A. Halcrow, Wiley, Chichester, 2013 Search PubMed.
- P. Gütlich and H. A. Goodwin, in Spin Crossover in Transition Metal Compounds I, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 233, p. 1 Search PubMed.
- P. Gütlich, Y. Garcia and T. Woike, Coord. Chem. Rev., 2001, 219, 839 CrossRef.
- P. Gütlich, Y. Garcia and H. A. Goodwin, Chem. Soc. Rev., 2000, 29, 419 Search PubMed.
- P. Gütlich, A. Hauser and H. Spiering, Angew. Chem., Int. Ed. Engl., 1994, 33, 2024 CrossRef.
- P. Gütlich, Struct. Bonding, 1981, 44, 83 CrossRef.
- P. Guionneau, M. Marchivie, G. Bravic, J. F. Létard and D. Chasseau, in Spin Crossover in Transition Metal Compounds II, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 234, p. 97 Search PubMed.
- L. Cambi and L. Szego, Ber. Dtsch. Chem. Ges., 1931, 64, 2591 CrossRef.
- P. J. van Koningsbruggen, in Spin Crossover in Transition Metal Compounds I, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 233, p. 123 Search PubMed.
- H. Toftlund and J. J. McGarvey, in Spin Crossover in Transition Metal Compounds I, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 233, p. 151 Search PubMed.
- J. A. Real, A. B. Gaspar, M. C. Muñoz, P. Gütlich, V. Ksenofontov and H. Spiering, in Spin Crossover in Transition Metal Compounds I, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 233, p. 167 Search PubMed.
- H. A. Goodwin, in Spin Crossover in Transition Metal Compounds I, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 233, p. 59 Search PubMed.
- P. J. van Koningsbruggen, Y. Maeda and H. Oshio, in Spin Crossover in Transition Metal Compounds I, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 233, p. 259 Search PubMed.
- I. A. Gass, S. R. Batten, C. M. Forsyth, B. Moubaraki, C. J. Schneider and K. S. Murray, Coord. Chem. Rev., 2011, 255, 2058 CrossRef CAS PubMed.
- S. Alvarez, J. Am. Chem. Soc., 2003, 125, 6795 CrossRef CAS PubMed.
- M. A. Halcrow, Chem. Soc. Rev., 2011, 40, 4119 RSC.
- H. A. Goodwin, in Spin Crossover in Transition Metal Compounds II, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 234, p. 23 Search PubMed.
- Y. Garcia and P. Gütlich, in Spin Crossover in Transition Metal Compounds II, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 234, p. 49 Search PubMed.
- S. Hayami, Y. Komatsu, T. Shimizu, H. Kamihata and Y. N. Lee, Coord. Chem. Rev., 2011, 255, 1981 CrossRef CAS PubMed.
- B. N. Figgis and M. A. Hitchman, Ligand Field Theory and Its Applications, Wiley-VCH, New York, 2000 Search PubMed.
- J. Cirera, E. Ruiz and S. Alvarez, Inorg. Chem., 2008, 47, 2871 CrossRef CAS PubMed.
- M. A. Arrio, J. Long, C. C. D. Moulin, A. Bachschmidt, V. Marvaud, A. Rogalev, C. Mathoniere, F. Wilhelm and P. Sainctavit, J. Phys. Chem. C, 2010, 114, 593 CAS.
- J. A. Real, A. B. Gaspar and M. C. Muñoz, Dalton Trans., 2005, 2062 RSC.
- J. P. Tuchagues, A. Bousseksou, G. Molnar, J. J. McGarvey and F. Varret, in Spin Crossover in Transition Metal Compounds III, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 235, p. 85 Search PubMed.
- A. Bousseksou, F. Varret, M. Goiran, K. Boukheddaden and J. P. Tuchagues, in Spin Crossover in Transition Metal Compounds III, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 235, p. 65 Search PubMed.
- J. A. Real, A. B. Gaspar, V. Niel and M. C. Muñoz, Coord. Chem. Rev., 2003, 236, 121 CrossRef CAS.
- K. S. Murray and C. J. Kepert, in Spin Crossover in Transition Metal Compounds I, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 233, p. 195 Search PubMed.
- M. Carmen Muñoz and J. A. Real, Coord. Chem. Rev., 2011, 255, 2068 CrossRef PubMed.
- Y. Garcia, V. Niel, M. C. Muñoz and J. A. Real, in Spin Crossover in Transition Metal Compounds I, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 233, p. 229 Search PubMed.
- J. Tao, R.-J. Wei, R.-B. Huang and L.-S. Zheng, Chem. Soc. Rev., 2012, 41, 703 CAS.
- S. Sanvito, Chem. Soc. Rev., 2011, 40, 3336 RSC.
- P. Gamez, J. Sánchez Costa, M. Quesada and G. Aromí, Dalton Trans., 2009, 7845 RSC.
- J. F. Létard, P. Guionneau and L. Goux-Capes, in Spin Crossover in Transition Metal Compounds III, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 235, p. 221 Search PubMed.
- O. Kahn and C. J. Martinez, Science, 1998, 279, 44 CrossRef CAS.
- O. Kahn, J. Krober and C. Jay, Adv. Mater., 1992, 4, 718 CrossRef CAS.
- A. Hauser, in Spin Crossover in Transition Metal Compounds II, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 234, p. 155 Search PubMed.
- M. L. Boillot, J. Zarembowitch and A. Sour, in Spin Crossover in Transition Metal Compounds II, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 234, p. 261 Search PubMed.
- V. Ksenofontov, A. B. Gaspar and P. Gütlich, in Spin Crossover in Transition Metal Compounds III, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 235, p. 23 Search PubMed.
- P. N. Martinho, Y. Ortin, B. Gildea, C. Gandolfi, G. McKerr, B. O'Hagan, M. Albrecht and G. G. Morgan, Dalton Trans., 2012, 41, 7461 RSC.
- J. P. Lomont, S. C. Nguyen, J. P. Schlegel, M. C. Zoerb, A. D. Hill and C. B. Harris, J. Am. Chem. Soc., 2012, 134, 3120 CrossRef CAS PubMed.
- R.-J. Wei, J. Tao, R.-B. Huang and L.-S. Zheng, Inorg. Chem., 2011, 50, 8553 CrossRef CAS PubMed.
- H. Paulsen, V. Schuenemann and J. A. Wolny, Eur. J. Inorg. Chem., 2013, 628 CrossRef CAS.
- K. P. Kepp, Coord. Chem. Rev., 2013, 257, 196 CrossRef CAS PubMed.
- R. J. Deeth, C. M. Handley and B. J. Houghton, in Spin-Crossover Materials, John Wiley & Sons Ltd, 2013, p. 443 Search PubMed.
- G. Brehm, M. Reiher and S. Schneider, J. Phys. Chem. A, 2002, 106, 12024 CrossRef CAS.
- T. Bucko, J. Hafner, S. Lebegue and J. G. Angyan, Phys. Chem. Chem. Phys., 2012, 14, 5389 RSC.
- Y. Qu, Int. J. Quantum Chem., 2013, 113, 943 CrossRef CAS.
- A. Fouqueau, S. Mer, M. E. Casida, L. M. L. Daku, A. Hauser, T. Mineva and F. Neese, J. Chem. Phys., 2004, 120, 9473 CrossRef CAS PubMed.
- L. M. L. Daku, A. Vargas, A. Hauser, A. Fouqueau and M. E. Casida, ChemPhysChem, 2005, 6, 1393 CrossRef CAS PubMed.
- H. Paulsen and A. X. Trautwein, in Spin Crossover in Transition Metal Compounds III, ed. P. Gütlich and H. A. Goodwin, Topics in Current Chemistry, 2004, vol. 235, p. 197 Search PubMed.
- J. A. Wolny, H. Paulsen, A. X. Trautwein and V. Schuenemann, Coord. Chem. Rev., 2009, 253, 2423 CrossRef CAS PubMed.
- M. Swart, Int. J. Quantum Chem., 2013, 113, 2 CrossRef CAS.
- M. Reiher, O. Salomon and B. A. Hess, Theor. Chem. Acc., 2001, 107, 48 CrossRef CAS PubMed.
- M. Reiher, Inorg. Chem., 2002, 41, 6928 CrossRef CAS PubMed.
- H. Paulsen, L. Duelund, H. Winkler, H. Toftlund and A. X. Trautwein, Inorg. Chem., 2001, 40, 2201 CrossRef CAS.
- G. Baranovic, Chem. Phys. Lett., 2003, 369, 668 CrossRef CAS.
- R. J. Deeth and N. Fey, J. Comput. Chem., 2004, 25, 1840 CrossRef CAS PubMed.
- G. Ganzenmuller, N. Berkaine, A. Fouqueau, M. E. Casida and M. Reiher, J. Chem. Phys., 2005, 122, 234321 CrossRef PubMed.
- M. Swart, J. Chem. Theory Comput., 2008, 4, 2057 CrossRef CAS.
- M. Swart, M. Guell and M. Sola, Phys. Chem. Chem. Phys., 2011, 13, 10449 RSC.
- K. P. Jensen and J. Cirera, J. Phys. Chem. A, 2009, 113, 10033 CrossRef CAS PubMed.
- J. Cirera and F. Paesani, Inorg. Chem., 2012, 51, 8194 CrossRef CAS PubMed.
- J. A. Wolny, S. Rackwitz, K. Achterhold, Y. Garcia, K. Muffler, A. D. Naik and V. Schuenemann, Phys. Chem. Chem. Phys., 2010, 12, 14782 RSC.
- E. M. Zueva, E. R. Ryabikh, A. M. Kuznetsov and S. A. Borshch, Inorg. Chem., 2011, 50, 1905 CrossRef CAS PubMed.
- E. M. Zueva, E. R. Ryabikh and S. A. Borshch, Inorg. Chem., 2011, 50, 11143 CrossRef CAS PubMed.
- S. Lebegue, S. Pillet and J. G. Angyan, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 024433 CrossRef.
- K. Tarafder, S. Kanungo, P. M. Oppeneer and T. Saha-Dasgupta, Phys. Rev. Lett., 2012, 109, 077203 CrossRef CAS.
- D. Roca-Sanjuan, F. Aquilante and R. Lindh, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 585 CrossRef CAS.
- C. de Graaf and C. Sousa, Chem.–Eur. J., 2010, 16, 4550 CrossRef CAS PubMed.
- A. Domingo, M. Angels Carvajal and C. De Graaf, Int. J. Quantum Chem., 2010, 110, 331 CrossRef CAS.
- M. Kepenekian, V. Robert, B. Le Guennic and C. De Graaf, J. Comput. Chem., 2009, 30, 2327 CAS.
- M. Papai, G. Vanko, C. de Graaf and T. Rozgonyi, J. Chem. Theory Comput., 2013, 9, 509 CrossRef CAS.
- C. Boilleau, N. Suaud and N. Guihery, J. Chem. Phys., 2012, 137, 224304 CrossRef PubMed.
- L. M. L. Daku, F. Aquilante, T. W. Robinson and A. Hauser, J. Chem. Theory Comput., 2012, 8, 4216 CrossRef.
- A. Droghetti, D. Alfe and S. Sanvito, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 205114 CrossRef.
- S. V. Aradhya and L. Venkataraman, Nat. Nanotechnol., 2013, 8, 399 CrossRef CAS PubMed.
- D. Aravena and E. Ruiz, J. Am. Chem. Soc., 2012, 134, 777 CrossRef CAS PubMed.
- N. Baadji and S. Sanvito, Phys. Rev. Lett., 2012, 108, 217201 CrossRef CAS.
- M. S. Alam, M. Stocker, K. Gieb, P. Mueller, M. Haryono, K. Student and A. Grohmann, Angew. Chem., Int. Ed., 2010, 49, 1159 CrossRef CAS PubMed.
- E. A. Osorio, K. Moth-Poulsen, H. S. J. van der Zant, J. Paaske, P. Hedegard, K. Flensberg, J. Bendix and T. Bjornholm, Nano Lett., 2010, 10, 105 CrossRef CAS PubMed.
- V. Meded, A. Bagrets, K. Fink, R. Chandrasekar, M. Ruben, F. Evers, A. Bernand-Mantel, J. S. Seldenthuis, A. Beukman and H. S. J. van der Zant, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 245415 CrossRef.
- N. Baadji, M. Piacenza, T. Tugsuz, F. Della Sala, G. Maruccio and S. Sanvito, Nat. Mater., 2009, 8, 813 CrossRef CAS PubMed.
- T. Mahfoud, G. Molnar, S. Bonhommeau, S. Cobo, L. Salmon, P. Demont, H. Tokoro, S.-I. Ohkoshi, K. Boukheddaden and A. Bousseksou, J. Am. Chem. Soc., 2009, 131, 15049 CrossRef CAS PubMed.
- O. Sato, Nat. Chem., 2010, 2, 10 CrossRef CAS PubMed.
- R. Sessoli, Nat. Chem., 2010, 2, 346 CrossRef CAS.
- M. F. Islam, J. F. Nossa, C. M. Canali and M. Pederson, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 155446 CrossRef.
- A. Droghetti and S. Sanvito, Phys. Rev. Lett., 2011, 107, 047201 CrossRef CAS.
- A. Grohmann, M. Haryono, K. Student, P. Mueller and M. Stocker, Eur. J. Inorg. Chem., 2013, 662 CrossRef CAS.
- M. Cavallini, Phys. Chem. Chem. Phys., 2012, 14, 11867 RSC.
- D. Gentili and M. Cavallini, Coord. Chem. Rev., 2013, 257, 2456 CrossRef CAS PubMed.
- T. G. Gopakumar, F. Matino, H. Naggert, A. Bannwarth, F. Tuczek and R. Berndt, Angew. Chem., Int. Ed., 2012, 51, 6262 CrossRef CAS PubMed.
- T. Miyamachi, M. Gruber, V. Davesne, M. Bowen, S. Boukari, L. Joly, F. Scheurer, G. Rogez, T. K. Yamada, P. Ohresser, E. Beaurepaire and W. Wulfhekel, Nat. Commun., 2012, 3, 938 CrossRef PubMed.
- S. Shi, G. Schmerber, J. Arabski, J.-B. Beaufrand, D. J. Kim, S. Boukari, M. Bowen, N. T. Kemp, N. Viart, G. Rogez, E. Beaurepaire, H. Aubriet, J. Petersen, C. Becker and D. Ruch, Appl. Phys. Lett., 2009, 95, 043303 CrossRef.
- S. Gueddida and M. Alouani, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 144413 CrossRef.
- M. Bernien, D. Wiedemann, C. F. Hermanns, A. Kruger, D. Rolf, W. Kroener, P. Muller, A. Grohmann and W. Kuch, J. Phys. Chem. Lett., 2012, 3, 3431 CrossRef CAS.
- X. Zhang, T. Palamarciuc, P. Rosa, J.-F. Létard, B. Doudin, Z. Zhang, J. Wang and P. A. Dowben, J. Phys. Chem. C, 2012, 116, 23291 CAS.
- A. Pronschinske, Y. F. Chen, G. F. Lewis, D. A. Shultz, A. Calzolari, M. B. Nardelli and D. B. Dougherty, Nano Lett., 2013, 13, 1429 CAS.
- B. Warner, J. C. Oberg, T. G. Gill, F. El Hallak, C. F. Hirjibehedin, M. Serri, S. Heutz, M. A. Arrio, P. Sainctavit, M. Mannini, G. Poneti, R. Sessoli and P. Rosa, J. Phys. Chem. Lett., 2013, 4, 1546 CrossRef CAS.
- H. J. Shepherd, G. Molnar, W. Nicolazzi, L. Salmon and A. Bousseksou, Eur. J. Inorg. Chem., 2013, 653 CrossRef CAS.
- P. N. Martinho, C. Rajnak and M. Ruben, Spin-Crossover Materials, John Wiley & Sons Ltd, 2013, p. 375 Search PubMed.
- A. Bousseksou, G. Molnar, L. Salmon and W. Nicolazzi, Chem. Soc. Rev., 2011, 40, 3313 RSC.
- F. Prins, M. Monrabal-Capilla, E. A. Osorio, E. Coronado and H. S. J. van der Zant, Adv. Mater., 2011, 23, 1545 CrossRef CAS PubMed.
- A. Rotaru, I. y. A. Gural'skiy, G. Molnar, L. Salmon, P. Demont and A. Bousseksou, Chem. Commun., 2012, 48, 4163 CAS.
- A. Rotaru, J. Dugay, R. P. Tan, I. A. Gural'skiy, L. Salmon, P. Demont, J. Carrey, G. Molnar, M. Respaud and A. Bousseksou, Adv. Mater., 2013, 25, 1745 CrossRef CAS PubMed.
- T. Q. Hung, F. Terki, S. Kamara, M. Dehbaoui, S. Charar, B. Sinha, C. Kim, P. Gandit, I. A. Gural'skiy, G. Molnar, L. Salmon, H. J. Shepherd and A. Bousseksou, Angew. Chem., Int. Ed., 2013, 52, 1185 CrossRef CAS PubMed.
- T. Mahfoud, G. Molnar, S. Cobo, L. Salmon, C. Thibault, C. Vieu, P. Demont and A. Bousseksou, Appl. Phys. Lett., 2011, 99, 053307 CrossRef.
- O. Sato, Z.-Y. Li, Z.-S. Yao, S. Kang and S. Kanegawa, Spin-Crossover Materials, John Wiley & Sons Ltd, 2013, p. 303 Search PubMed.
- A. B. Gaspar, M. Seredyuk and R. Gütlich, Coord. Chem. Rev., 2009, 253, 2399 CrossRef CAS PubMed.
- S. Hayami, Spin-Crossover Materials, John Wiley & Sons Ltd, 2013, p. 321 Search PubMed.
| This journal is © the Owner Societies 2014 |
