Open Access Article
Open Access ArticleDrug–drug salt forms of ciprofloxacin with diflunisal and indoprofen†
Partha Pratim
Bag
,
Soumyajit
Ghosh
,
Hamza
Khan
,
Ramesh
Devarapalli
and
C.
Malla Reddy
*
Indian Institute of Science Education and Research (IISER) Kolkata, Mohanpur Campus, Mohanpur 741252, India. E-mail: cmallareddy@gmail.com; cmreddy@iiserkol.ac.in; Fax: +91 33 25873020; Tel: +91 33 25873118, Ext: 238
First published on 13th June 2014
Abstract
Two salt forms of a fluoroquinolone antibacterial drug, ciprofloxacin (CIP), with non-steroidal anti-inflammatory drugs, diflunisal (CIP/DIF) and indoprofen (CIP/INDP/H2O), were synthesized and characterized by PXRD, FTIR, DSC, TGA and HSM. Crystal structure determination allowed us to study the drug–drug interactions and the piperazine-based synthon (protonated piperazinecarboxylate) in the two forms, which is potentially useful for the crystal engineering of new salt forms of many piperazine-based drugs.
Multicomponent pharmaceutical forms consisting of an active pharmaceutical ingredient (API) and an inactive co-former, which is ideally a generally recognized as safe (GRAS) substance, have been well explored in recent times.1–3 Formation of co-crystals and salt forms can improve an API's physicochemical properties, such as solubility and bioavailability, and the mechanical properties of individual drugs without changing any covalent bonds in either of the species.4–7 Due to the simple and convenient preparation methods, there is an increased interest in the discovery of multi-API forms as evident from the recent rise in the number of publications and patent applications.8,9 Examples of these multi-API forms include the crystalline forms of theophylline with phenobarbital,10 ethenzamide with gentisic acid,11 meloxicam with aspirin,12 acetylsalicylic acid with (L)-theanine,13 acetaminophen with theophylline,14 lamivudine with zidovudine,15 sulfamethazine with theophylline,16 isoniazid with 4-aminosalicylic acid17 and pyrazinamide with isoniazid.18 Salt formation is the most widely practiced method to greatly improve the solubility and stability of drugs.19
Crystal engineering approach has been effectively utilized in recent times in the synthesis of new forms particularly by exploiting supramolecular synthons. Hence the identification of synthons that can be transferred across different systems is important. For example, synthon transferability in cytosine and lamivudine salts was recently demonstrated by Desiraju and co-workers by IR spectroscopy studies.20a Aakeröy and co-workers successfully established the role of synthon transferability (intermolecular amide⋯amide synthons) in the assembly and organization of bidentate acetylacetonate (acac) and acetate “paddlewheel” complexes of a variety of metal(II) ions.20b Recently Das et al. have reported the gelation behaviour in various diprimary ammonium monocarboxylate salts by exploiting the synthon transferability.20c
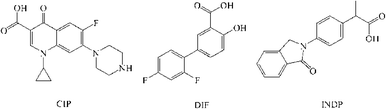
Here we report the two drug–drug salt forms of ciprofloxacin (CIP) with diflunisal and indoprofen. Ciprofloxacin is a widely used drug, belonging to the fluoroquinolone antibacterial family (Scheme 1).21 It is a broad-spectrum antibiotic drug and is active against both Gram-positive and Gram-negative bacteria; however, it is known to have poor solubility and absorption, particularly in basic media. CIP is available in several brand names, such as Cifran OD and Ciprolet in India and Cipro and Cipro XR in the USA. Diflunisal and indoprofen are non-steroidal anti-inflammatory drugs (NSAID). A search of the Cambridge Structural Database (CSD version 5.35) revealed 82 structure reports containing ciprofloxacin. Among these, 17 structures belong to the multi-component forms of CIP, which include hydrates,22 solvates,23 co-crystals and salts,24 while the remaining structures belong to metal complexes. In our study we attempted to prepare co-crystals/salts of CIP with a few APIs as co-formers (Table S2†). We succeeded in obtaining only two salt forms of CIP with diflunisal (DIF) and indoprofen (INDP). The salt formation of these drugs is consistent with the ΔpKa rule (see the ESI,† Table S2). These two new solid forms, CIP/DIF (salt) and CIP/INDP/H2O (salt hydrate), were then investigated by various characterization techniques.
The solid form screening was conducted by neat grinding (NG),25a solvent drop grinding (SDG)25b in the presence of methanol, and fast evaporation (FE)26 using rotary evaporation technique (Table S2†). The resulting solid powders were characterized by PXRD and FT-IR (Fig. S1 and S2†). We observed that the NG method did not produce the new salt forms, but the LAG and FE methods were successful. This manifests the efficiency of the latter two methods for screening purposes. In some recent systematic studies, we demonstrated the effectiveness of the FE method (which is widely used but poorly documented) in the screening of polymorphs, solvates and co-crystals.26
For single-crystal preparation, 50 mg (0.1509 mmol) of CIP and the same equivalent of co-former DIF or INDP were dissolved in 10 mL of acetonitrile–methanol mixture in a flask and heated until a clear solution was obtained. Good-quality single crystals suitable for X-ray diffraction studies were obtained after 3 to 4 days using the slow evaporation method under ambient conditions. The crystallographic data for the two salt forms, CIP/DIF and CIP/INDP/H2O, are listed in Table S3 (ESI†). A hydrogen bond table (Table S1) and the ORTEP diagrams (Fig. S3–S4†) are also given in the ESI.†
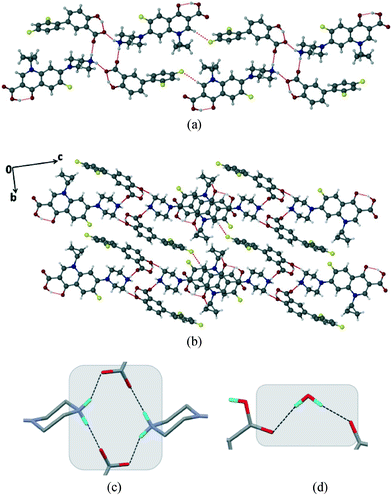
The salt CIP/DIF crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with one molecule of each ionised drug in the asymmetric unit (Fig. 1). Transfer of proton from the acid group of DIF to the secondary N-atom on the piperazine ring of CIP results in the ionization of molecules. In the structure, piperazine ring of CIP adopts a chair conformation. The two phenyl rings of DIF are tilted with respect to each other by ~39.63° while the hydroxyl group of DIF forms an intramolecular hydrogen bond with the carboxylate group via O–H⋯O interactions (O(6)–H(6)⋯O(5)
space group with one molecule of each ionised drug in the asymmetric unit (Fig. 1). Transfer of proton from the acid group of DIF to the secondary N-atom on the piperazine ring of CIP results in the ionization of molecules. In the structure, piperazine ring of CIP adopts a chair conformation. The two phenyl rings of DIF are tilted with respect to each other by ~39.63° while the hydroxyl group of DIF forms an intramolecular hydrogen bond with the carboxylate group via O–H⋯O interactions (O(6)–H(6)⋯O(5)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(18); d/Å, θ/°: 1.62(4) Å, 151(4)°). The carboxylic acid group of CIP also forms an intramolecular hydrogen bond with the adjacent carbonyl group (O(2)–H(2)⋯O(3); 1.62(4) Å, 156(4)°). The carboxylate group of DIF interacts with one axial and one equatorial H–N(piperazine) group of two adjacent CIP molecules via synthon 1 (N(3)–H(3B)⋯O(4): 1.83 Å, 180° and O(5)⋯H(3A)–N(3): 1.81 Å, 159°). The hydrogen bonding between CIP and DIF generates a R44(12) ring motif (Fig. 1). These two successive centrosymmetric tetrameric motifs are connected by weak C–H⋯F interactions (C(10)–H(10)⋯F(3): 2.44 Å, 160°) (Fig. 1a).27
C(18); d/Å, θ/°: 1.62(4) Å, 151(4)°). The carboxylic acid group of CIP also forms an intramolecular hydrogen bond with the adjacent carbonyl group (O(2)–H(2)⋯O(3); 1.62(4) Å, 156(4)°). The carboxylate group of DIF interacts with one axial and one equatorial H–N(piperazine) group of two adjacent CIP molecules via synthon 1 (N(3)–H(3B)⋯O(4): 1.83 Å, 180° and O(5)⋯H(3A)–N(3): 1.81 Å, 159°). The hydrogen bonding between CIP and DIF generates a R44(12) ring motif (Fig. 1). These two successive centrosymmetric tetrameric motifs are connected by weak C–H⋯F interactions (C(10)–H(10)⋯F(3): 2.44 Å, 160°) (Fig. 1a).27
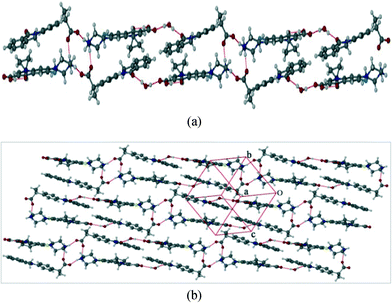
The salt hydrate CIP/INDP/H2O crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with one molecule of each component in the asymmetric unit (Fig. 2). The piperazine ring of CIP exists in a chair conformation and N(3) is protonated. The carboxylic acid group of CIP forms an intramolecular hydrogen bond with the carbonyl group (O(2)–H(2)⋯O(3): 1.74 Å, 155°) by accepting a proton from the carboxyl group of INDP. The carboxylate group of INDP interacts with both axial and equatorial H–N(piperazine) groups of two adjacent CIP molecules via synthon 1 (N(3)–H(3B)⋯O(4): 1.91 Å, 148° and N(3)–H(3A)⋯O(5): 1.79 Å, 170°), forming a centrosymmetric tetrameric ring motif (R44(12)). Notably, synthon 1, which involves the protonated piperazine rings, is very similar to that seen in CIP/DIF, demonstrating its transferability (Fig. 2a).20c In this structure, a water molecule acts as a bridge between CIP and INDP via O–H⋯O interactions by synthon 2 (O(7)–H(35)⋯O(6): 1.96(4) Å, 172(5)° and O(7)–H(35A)⋯O(1): 1.98(5) Å, 171(4)°). Interestingly, the water molecule here does the job of C–H⋯F interactions in CIP/DIF by linking the tetramers. This leads to the formation of ribbons running parallel to each other along the [111] axis (Fig. 2b).
space group with one molecule of each component in the asymmetric unit (Fig. 2). The piperazine ring of CIP exists in a chair conformation and N(3) is protonated. The carboxylic acid group of CIP forms an intramolecular hydrogen bond with the carbonyl group (O(2)–H(2)⋯O(3): 1.74 Å, 155°) by accepting a proton from the carboxyl group of INDP. The carboxylate group of INDP interacts with both axial and equatorial H–N(piperazine) groups of two adjacent CIP molecules via synthon 1 (N(3)–H(3B)⋯O(4): 1.91 Å, 148° and N(3)–H(3A)⋯O(5): 1.79 Å, 170°), forming a centrosymmetric tetrameric ring motif (R44(12)). Notably, synthon 1, which involves the protonated piperazine rings, is very similar to that seen in CIP/DIF, demonstrating its transferability (Fig. 2a).20c In this structure, a water molecule acts as a bridge between CIP and INDP via O–H⋯O interactions by synthon 2 (O(7)–H(35)⋯O(6): 1.96(4) Å, 172(5)° and O(7)–H(35A)⋯O(1): 1.98(5) Å, 171(4)°). Interestingly, the water molecule here does the job of C–H⋯F interactions in CIP/DIF by linking the tetramers. This leads to the formation of ribbons running parallel to each other along the [111] axis (Fig. 2b).
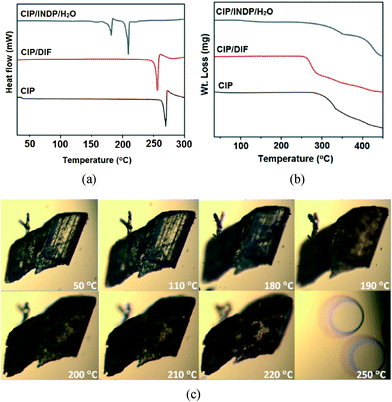
The new forms were also characterized by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) (Fig. 3a and b). In the DSC experiment, the endothermic peak for melting of CIP/DIF was found at 255.9 °C. In the case of CIP/INDP/H2O the first endothermic peak in the DSC curve was found at 182 °C, followed by a second large endotherm at 209 °C. TGA experiments on CIP/INDP/H2O showed the first weight loss (2.92%) from about 100 °C, which matches well with the expected weight loss (2.85%) corresponding to the loss of one water molecule in the lattice, and the second weight loss from 230 °C most likely corresponding to the sublimation of INDP (mp = 208–210 °C) from the melt. Further evaluation of this salt hydrate by hot-stage microscopy (HSM) revealed that the integrity of the crystal was not lost upon solvent evaporation from about 100 °C, but a phase transition at 182 °C (see the morphology changes at 180–190 °C in Fig. 3c) is apparent, after which melting occurs upon further heating. This suggests that after the loss of water, the crystal probably forms an anhydrate form (salt/co-crystal), which upon further heating undergoes a phase transition before melting. We plan to investigate this further in the future by variable temperature PXRD.
A search of the CSD (version 5.35) for structures containing both a piperazine ring and a carboxyl group resulted in 238 hits, the majority (233) of which were salts with a protonated piperazine ring (by an acid group), while only 5 structures were neutral. This is not surprising as the piperazine group is highly basic (the pKa of an unsubstituted piperazine is 9.8) and protonation by acid groups is expected (due to the large ΔpKa). The cyclic synthon 1 was found in 19 of the salts. This finding demonstrates that synthon 1 is not rare and shows some degree of transferability, especially when at least one secondary N(piperazine) group is available.20c Hence salt formation using the general ΔpKa rule can be exploited for the crystal engineering of a large library of piperazine-based drugs (such as trimetazidine, amoxapine, 6-nitroquipazine, etc.). For example, by carefully choosing acid co-formers with a ΔpKa > 3, the formation of salts may be promoted in piperazine drugs, where synthon 1 may play a role.
In conclusion, two new drug–drug salt forms, CIP/DIF and CIP/INDP/H2O, were characterized by single-crystal X-ray diffraction, DSC, TGA and HSM. The DSC and hot-stage microscopy experiments on the salt hydrate indicate a phase transition before melting. Notably, in the screening process, the liquid-assisted grinding and fast evaporation methods have successfully identified the two new forms, whereas the neat grinding method failed. This study demonstrates the effectiveness of the FE method in screening salt forms. The ability of piperazine-based drugs to form salts as well as the transferability of synthon 1, which is important in the context of crystal engineering to generate new salt forms of a large group of piperazine-based drugs, was established by analyzing the present and CSD structures. As the drug–drug forms of CIP are important in the context of drug development, we further plan to study their properties, such as solubility and stability, under different relative humidity conditions in the future.
Notes and references
- Ö. Almarsson and M. J. Zaworotko, Chem. Commun., 2004, 1889 RSC.
- S. Aitipamula, et al. , Cryst. Growth Des., 2012, 12, 2147 CAS.
- (a) N. R. Hornedo, S. J. Nehm and A. Jayasankar, Cocrystals: design, properties and formation mechanisms, in Encyclopedia of Pharmaceutical Technology, Taylor & Francis, London, 3rd edn, 2007, p. 615 Search PubMed; (b) U.S. Food and Drug Administration – Database of Select Committee on GRAS Substances (SCOGS) Reviews. http://www.accessdata.fda.gov/scripts/fcn/fcnNavigation.cfm?rpt=scogsListing (accessed 15/02/2011).
- (a) C. C. Sun and H. Hou, Cryst. Growth Des., 2008, 8, 1575 CrossRef CAS; (b) M. S. R. N. Kiran, S. Varughese, C. M. Reddy, U. Ramamurty and G. R. Desiraju, Cryst. Growth Des., 2010, 10, 4650 CrossRef CAS; (c) C. M. Reddy, G. R. Krishna and S. Ghosh, CrystEngComm, 2010, 12, 2296 RSC; (d) S. Ghosh and C. M. Reddy, Angew. Chem., Int. Ed., 2012, 51, 10319 CrossRef CAS PubMed; (e) N. Schultheiss and A. Newman, Cryst. Growth Des., 2009, 9, 2950 CrossRef CAS PubMed; (f) N. A. Meanwell, Annu. Rep. Med. Chem., 2008, 43, 373 CAS; (g) N. Shan and M. J. Zaworotko, Drug Discovery Today, 2008, 13, 440 CrossRef CAS PubMed.
- U.S. Food and Drug Administration, Office of Combination Products, http://www.fda.gov/oc/combination/, 21 CFR Part 3.2(e).
- (a) S. G. Chrysant, Clin. Drug Invest., 2008, 28, 713–734 CrossRef CAS; (b) A. I. Wertheimer and A. Morrison, Pharmacol. Ther., 2002, 27, 44 Search PubMed; (c) K. K. Bucci and C. J. Possidente, Am. J. Health-Syst. Pharm., 2006, 63, 1654 CrossRef PubMed; (d) C. S. Gautam and L. Saha, Br. J. Clin. Pharmacol., 2007, 65, 795 CrossRef PubMed.
- M. D. Eddleston, B. Patel, G. M. Day and W. Jones, Cryst. Growth Des., 2013, 13, 4599 CAS.
- (a) I. Nugrahani, S. Asyarie, S. N. Soewandhi and S. Ibrahim, Int. J. Pharmacol., 2007, 3, 475 CrossRef CAS; (b) S. P. Velaga, S. Basavoju and D. Boström, J. Mol. Struct., 2008, 889, 150 CrossRef CAS PubMed; (c) A. T. M. Serajuddin, Adv. Drug Deliv. Rev., 2007, 59, 603 CrossRef CAS PubMed; (d) J. F. Remenar, S. L. Morissette, M. L. Peterson, B. Moulton, J. M. MacPhee, H. R. Guzman and Ö. Almarsson, J. Am. Chem. Soc., 2003, 125, 8456 CrossRef CAS PubMed; (e) A. V. Trask, W. D. S. Motherwell and W. Jones, Int. J. Pharm., 2006, 320, 114 CrossRef CAS PubMed.
- (a) G. R. Desiraju, Angew. Chem., Int. Ed., 2011, 50, 52 CrossRef CAS PubMed; (b) K. Biradha, C.-Y. Su and J. J. Vittal, Cryst. Growth Des., 2011, 11, 875 CrossRef CAS; (c) G. R. Desiraju, Crystal engineering: The design of organic solids, Elsevier, Amsterdam, 1989 Search PubMed; (d) P. Vishweshwar, J. A. McMahon and M. J. Zaworotko, in Frontiers in Crystal Engineering, ed. E. R. T. Tiekink and J. J. Vittal, Wiley, Chichester, 2006, pp. 25–49 Search PubMed.
- M. L. Peterson, E. A. Collier, M. B. Hickey, H. Guzman and O. Almarsson, in Multi-Component Pharmaceutical Crystalline Phases: Engineering for performance, Organic crystal engineering: Frontiers in Crystal Engineering, ed. E. R. T. Tiekink, J. Vittal and M. Zaworotko, John Wiley Publishers, New York, 2010 Search PubMed.
- S. Aitipamula, P. S. Chow and R. B. H. Tan, CrystEngComm, 2009, 11, 1823 RSC.
- M. L. Cheney, D. R. Weyna, N. Shan, M. Hanna, L. Wojtas and M. Zaworotko, J. Pharm. Sci., 2011, 100, 2172 CrossRef CAS PubMed.
- H. G. Brittain and P. V. Felice, Intravenous formulation with water-soluble co-crystals of acetylsalicylic acid and theanine, US Patent Application, US/2010-0286099, 2010 Search PubMed.
- H. G. Lee, G. G. Zhang and D. R. Flanagan, J. Pharm. Sci., 2011, 100, 1736 CrossRef CAS PubMed.
- P. M. Bhatt, Y. Azim, T. S. Thakur and G. R. Desiraju, Cryst. Growth Des., 2009, 9, 951 CAS.
- J. Lu and S. Rohani, J. Pharm. Sci., 2010, 99, 4042 CAS.
- P. Grobelny, A. Mukherjee and G. R. Desiraju, CrystEngComm, 2011, 13, 4358 RSC.
- S. Cherukuvada and A. Nangia, CrystEngComm, 2012, 14, 2579 RSC.
- M. Pudipeddi, A. T. M. Serajuddin, D. J. W. Grant and P. H. Stahl, Solubility and dissolution of weak acids, bases, and salts, in Handbook of pharmaceutical salts, properties, selection and use, ed. P. H. Stahl and C. G. Wermuth, Weinheim: Wiley-VCH, 1st edn, 2002, pp. 19–40 Search PubMed.
- (a) S. Chakraborty, S. Ganguly and G. R. Desiraju, CrystEngComm, 2014, 16, 4732 RSC; (b) C. B. Aakeröy, B. M. T. Scott, M. M. Smith, J. F. Urbina and J. Desper, Inorg. Chem., 2009, 48, 4052 CrossRef PubMed; (c) U. K. Das, V. G. Puranik and P. Dastidar, Cryst. Growth Des., 2012, 12, 5864 CrossRef CAS.
- C. M. Oliphant and G. M. Green, Am. Fam. Physician, 2002, 65(3), 455 Search PubMed.
- (a) I. Turel, P. Bukovec and M. Quiros, Int. J. Pharm., 1997, 152, 59 CrossRef CAS; (b) F. P. A. Fabbiani and B. Dittrich, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, 64, o2354 CAS; (c) F. P. A. Fabbiani, B. Dittrich, A. J. Florence, T. Gelbrich, M. B. Hursthouse, W. F. Kuhs, N. Shankland and H. Sowa, CrystEngComm, 2009, 11, 1396 RSC.
- (a) F. P. A. Fabbiani, J.-B. Arlin, G. Buth, B. Dittrich, A. J. Florence, R. Herbst-Irmer and H. Sowa, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2011, 67, o120 CAS; (b) A. D. Vasiliev, N. N. Golovnev and I. A. Baidina, J. Struct. Chem., 2009, 50, 165 CrossRef PubMed; (c) X. Li, Y. Hu, Y. Gao, G. G. Z. Zhang and R. F. Henry, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, o5803 CAS; (d) I. Turel and A. Golobic, Anal. Sci., 2003, 19, 329 CrossRef CAS.
- (a) M. D. Prasanna and T. N. Guru Row, J. Mol. Struct., 2001, 559, 255 CrossRef CAS; (b) B. Lou, D. Boström and S. Velaga, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2007, 63, o731 CAS; (c) J. S. Reddy, S. V. Ganesh, R. Nagalapalli, R. Dandela, K. A. Solomon, K. A. Kumar, N. R. Goud and A. Nangia, J. Pharm. Sci., 2011, 100, 3160 CrossRef CAS PubMed; (d) S. Basavoju, D. Boström and S. P. Velaga, Mol. Cryst. Liq. Cryst., 2012, 562, 254 CrossRef CAS; (e) C. B. Romanuk, Y. G. Linck, A. K. Chattah, G. A. Monti, S. L. Cuffini, M. T. Garland, R. Baggio, R. H. Manzo and M. E. Olivera, Int. J. Pharm., 2010, 391, 197 CrossRef CAS PubMed.
- (a) S. Karki, T. Friščič, W. Jones and W. D. S. Motherwell, Mol. Pharmaceutics, 2007, 4, 347 CrossRef CAS PubMed; (b) A. V. Trask, N. Shan, W. D. S. Motherwell, W. Jones, S. Feng, R. B. H. Tan and K. J. Carpenter, Chem. Commun., 2005, 880 RSC.
- (a) P. P. Bag, M. Patni and C. M. Reddy, CrystEngComm, 2011, 13, 5650 RSC; (b) P. P. Bag and C. M. Reddy, Cryst. Growth Des., 2012, 12, 2740 CrossRef CAS; (c) P. P. Bag, R. R. Kothur and C. M. Reddy, CrystEngComm, 2014, 16, 4706 RSC; (d) S. F. Chow, M. Chen, L. Shi, A. H. L. Chow and C. C. Sun, Pharm. Res., 2012, 89, 1854 CrossRef PubMed; (e) D. A. Haynes, W. Jones and W. D. S. Motherwell, CrystEngComm, 2006, 8, 830 RSC.
- V. R. Thalladi, H.-C. Weiss, D. Bläser, R. Boese, A. Nangia and G. R. Desiraju, J. Am. Chem. Soc., 1998, 120, 8702 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental method, geometrical parameters of molecules from crystal structures, powder X-ray diffraction patterns, infrared spectra, and ORTEP diagrams. CCDC 986495–986496. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ce00631c |
| This journal is © The Royal Society of Chemistry 2014 |