Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polymorphism and tautomerism in [dienH2][Co(dien)2][Ge2S6] leading to different hydrogen bonded networks†
Jessica
Lichte
,
Christian
Näther
and
Wolfgang
Bensch
*
Christian-Albrechts Universität, Institut für Anorganische Chemie, D-24118 Kiel, Germany. E-mail: wbensch@ac.uni-kiel.de; Fax: +49 431 880 1520; Tel: +49 431 880 2091
First published on 2nd May 2014
Abstract
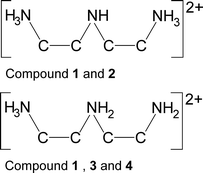
Four compounds of composition [dienH2][Co(dien)2][Ge2S6] (dien = diethylenetriamine) were obtained under solvothermal conditions changing the reaction time and educt ratio in the reaction slurries. In three compounds the [Co(dien)2]2+ complexes adopt the s-fac and in one the u-fac configuration. The main differences between the samples are found in the number and type of protonated dien molecules. In one distinct cation of compound 1 as well as in compound 2 the terminal N atoms are protonated, whereas in the second cation of 1 as well as in 3 and 4, one terminal and the central N atom are protonated. Therefore, the compounds with different protonated cations are tautomers, whereas compounds 3 and 4 represent polymorphic modifications. The occurrence of tautomerism and polymorphism for compounds with identical chemical composition is unprecedented in the chemistry of thiometallates and was never reported until now. In the crystal structure of all compounds the (dienH2)2+ cations and the [Ge2S6]4− anions are linked by intermolecular N–H⋯S hydrogen bonding into different supramolecular networks.
Introduction
The thiometallate chemistry of main group elements Ge, Sn, In, As or Sb was substantially developed during the last two decades.1–6 The main synthetic method applied for the generation of thiometallates is the solvothermal approach allowing the usage of different solvents and starting materials as well as structure directing molecules. Upon analyzing the crystal structures and the primary building blocks in the Ge, Sn, In, As or Sb based thiometallate compounds several differences are obvious. In thiogermanates and -indates the central cations are in a tetrahedral environment of four S anions,7 while in thiostannates the coordination number varies depending on the oxidation state of Sn.8 The [GeS4]4− anion can act as a ligand to metal cation centres,9–11 but the tetrahedron is more often found in condensation products like the [Ge2S6]4− anion,12–15 in the adamantine-like anion [Ge4S10]4− (ref. 16–20) or as [Ge8S19]6− (ref. 21).The situation is different for As and Sb, especially if these atoms are in the oxidation state +3 forming [AsS3]3− resp. [SbS3]3− trigonal pyramids as primary building units which often undergo condensation reactions to form more complex anions.22,23 Combination of the tetrahedral [GeS4]4− group with the [SbS3]3− pyramid should generate new compounds exhibiting unique structures. Indeed there are some examples for such combinations reported in literature like [Co(dien)2]2[GeSb4S10] (dien = diethylenetriamine) or [Mn(en)3][GeSb2S6] (en = ethylenediamine). In the former compound semi-cube like [GeSb2S7] clusters and chain-like Sb4S10 tetramers are observed being interconnected into a double layer with composition [GeSb4S10]. In the latter compound a GeS4 tetrahedron, one SbS3 pyramid and a SbS4 moiety are joined to form a [GeSb2S8] group and each of these groups is interconnected to three adjacent units via their four terminal S atoms to generate a 2-D anionic network with composition [GeSb2S6].24 A semi-cube like moiety [GeSb2S7] is also the main structural motif in [Ni(dien)2]3[Ge3Sb8S21]·0.5H2O. Alternating [GeSb2S7] units and SbS3 pyramids sharing a common S atom lead to the formation of a layered anion [Ge3Sb8S21] containing large pores with dimensions of about 13·13 Å.25 Remarkable ion exchange properties were reported for the three-dimensional network compound [(Me)2NH2]2[Sb2GeS6] ([(Me)2NH2]2 = dimethylammonium). Two different left-handed helices generated by corner-sharing of SbS4 and GeS4 units are joined to form channels hosting the cations.26 In the compound [(Me)2NH2]6[Ge2Sb2S7][Ge4S10] the adamantane-like [Ge4S10]4− anion coexists with the [GeSb2S7]2− anion. In the latter anion a Sb–Sb bond is observed which is very rare in thioantimonates. The structure of [dabcoH]2[Ge2Sb3S10] (dabco = 1,4-diazabicyclo[2.2.2]octane) features a [Ge2Sb3S10]n3n− ribbon constructed by two [GeSbS5] chains interconnected by a SbS4 moiety.27 A one-dimensional anionic ribbon [GeSb2S6]n2n− containing the unique {GeSb3S11} building unit was observed in the structure of [AEPH2][GeSb2S6]·CH3OH (AEP = N-(2-aminoethyl)piperazine). This compound exhibits photocatalytic activity in the degradation of rhodamine B.28
In the past few years we demonstrated that transition metal complexes are suitable structure directing molecules forcing the formation of new thiometallate compounds exhibiting unusual and unique crystal structures.29–33 During further explorative syntheses to combine thioantimonates with thiogermanates and using an in situ generated Co2+ transition metal complex to prepare new compounds exhibiting new structural motifs, we obtained three compounds with composition [dienH2][Co(dien)2][Ge2S6] by increasing the amount of Sb in the reaction mixture. Another compound with identical composition was isolated using slightly different educt ratios and increasing the reaction time. The appearance of tautomeric and polymorphic forms of a thiometalate is unique and was never reported before. Here we report the solvothermal syntheses and the crystal structures of these new compounds exhibiting dienH2 molecules being protonated at different N atoms.
Single-crystal structure analysis
The data collections were performed using an imaging plate diffraction system (IPDS-2) with Mo-Kα radiation from STOE & CIE and for all compounds a numerical absorption correction was performed using X-Red and X-Shape from STOE & CIE. The structure solutions were done with direct methods using SHELXS-97 and structure refinements were performed against F2 using SHELXL-97.34 All non-hydrogen atoms were refined with anisotropic displacement parameters except one disordered N atom of lower occupancy in compound 1. The C–H and some of the N–H H atoms were located in difference map but were positioned with idealized geometry (ammonium H atoms allowed to rotate but not to tip) and were refined isotropic using a riding model. Those H atoms which cannot be positioned were located in difference map, their bond lengths set to ideal values and afterwards they were refined isotropic using riding model. In compound 1 one N atom and in compound 2 and 4 one C atom is disordered and was refined using a split model. In compound 2 the non-coordinating amine ligand is disordered around a center of inversion, which is also the case if the structure is refined in space group P1. The absolute structure of compound 4 was determined and is in agreement with the selected setting (Flack-x-parameter: 0.002(2)). Details of the structure determinations are given in Table 1.| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Formula | C12H41CoGe2N9S6 | C12H41CoGe2N9S6 | C12H41CoGe2N9S6 | C12H41CoGe2N9S6 |
| MW/g mol−1 | 708.01 | 708.01 | 708.01 | 708.01 |
| Crystal system | Triclinic | Triclinic | Orthorhombic | Orthorhombic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Pbca | Pca21 |
| a/Å | 11.3224(3) | 7.2034(5) | 15.2110(3) | 14.7043(3) |
| b/Å | 14.6492(4) | 9.2773(6) | 16.7025(4) | 9.0099(2) |
| c/Å | 18.3710(5) | 11.4365(8) | 21.8821(4) | 21.4540(5) |
| α/° | 71.000(2) | 74.107(5) | 90 | 90 |
| β/° | 78.352(2) | 73.402(5) | 90 | 90 |
| γ/° | 73.441(2) | 71.293(5) | 90 | 90 |
| V/Å3 | 2741.5(2) | 679.62(8) | 5559.4(2) | 2842.3(2) |
| T/K | 293 | 293 | 293 | 293 |
| Z | 4 | 1 | 8 | 4 |
| D calc/g cm−3 | 1.715 | 1.730 | 1.692 | 1.655 |
| μ/mm−1 | 3.254 | 3.282 | 3.209 | 3.139 |
| θ max/deg | 28.0 | 28.0 | 28.0 | 26.0 |
| Measured refl. | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 362 362 |
7633 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 958 958 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098 098 |
| R int | 0.0462 | 0.0198 | 0.0704 | 0.0404 |
| T min/max | 0.5285/0.6177 | 0.5423/0.6735 | 0.5149/0.7110 | 0.5386/0.7204 |
| Unique refl. | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 102 102 |
3205 | 6648 | 5573 |
| Refl. [F0 > 4σ(F0)] | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 537 537 |
2933 | 5672 | 5486 |
| Parameters | 552 | 148 | 273 | 283 |
| R 1 [F0 > 4σ(F0)] | 0.0388 | 0.0295 | 0.0474 | 0.0199 |
| wR2 [all data] | 0.0908 | 0.0744 | 0.0832 | 0.0482 |
| GOF | 1.043 | 1.110 | 1.142 | 1.042 |
| Δρmax/min/e Å−3 | 0.851/−0.606 | 0.818/−0.701 | 0.503/−0.537 | 0.284/−0.249 |
CCDC – 985614 (1), CCDC – 985615 (2), CCDC – 985616 (3) and CCDC – 985617 (4) contain the supplementary crystallographic data for this paper.
Syntheses
The chemicals GeO2, Co, Sb, S and dien were used as purchased. For all syntheses glass tubes with a volume of 7 mL were used which were sealed with an aluminum cap. The amine (2 mL) was diluted with 200 μL water. The mixtures were heated in an oven at 140 °C between 1 and 26 days. Educt ratios (in mmol) for compounds 1 to 4: GeO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb
Sb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S: (1) 1
S: (1) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; (2) 1
3; (2) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.34
1.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; (3) 1
3; (3) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; (4) 0.7
3; (4) 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The reaction times for 1–3 were 1–4 days, and for crystallization of 4 26 days were necessary. All compounds were obtained as yellow needles with a yield of about 10% based on GeO2. The reaction products were washed with diethylether. Stibnite (Sb2S3) could be identified with X-ray powder diffractometry as main product. EDX analyses of selected crystals yield Co
3. The reaction times for 1–3 were 1–4 days, and for crystallization of 4 26 days were necessary. All compounds were obtained as yellow needles with a yield of about 10% based on GeO2. The reaction products were washed with diethylether. Stibnite (Sb2S3) could be identified with X-ray powder diffractometry as main product. EDX analyses of selected crystals yield Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ge
Ge![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratios close to that determined with single crystal structure refinements.
S ratios close to that determined with single crystal structure refinements.
Results and discussion
The four new compounds were obtained during a systematic investigation to prepare new thiogermanate–thioantimonates. Attempts to synthesize the samples by applying only GeO2, Co, S and the amine yielded no crystalline products. Performing experiments with GeO2, Sb, S, and Co salts like Co(NO3)2·6H2O, CoSO4·7H2O, CoCl2·6H2O or Co2O3 did not lead to formation of the compounds. For Co sources containing crystal water, the water concentration of the reaction mixtures was adjusted to give the desired ratios for dien![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. All syntheses done with Ge instead of GeO2 were also not successful. The syntheses were only successful and the compounds could only be isolated in the presence of elemental antimony in the reaction slurry. While compounds 1–3 could be obtained by increasing the amount of Sb in the reaction mixture, compound 4 was obtained by reducing the GeO2 content. The reaction time plays also a crucial role. Compounds 1–3 could only be obtained exclusively besides Sb2S3 (see above) if the reaction time did not exceed 4 days, while compound 4 was obtained after 26 days and also at longer reaction times up to 40 days. Extending the reaction times for 1–3 afforded crystallization of a second compound mainly as very thin needles which were too tiny for single crystal structure determination. Experiments were also undertaken to elucidate whether the compound can be transformed into each other to determine which is more stable. Combinations of handpicked single crystals of 1–4 were stirred at room temperature in glass tubes containing ether, methanol, ethanol, acetone or n-hexane and after 72 h the residue was filtered off, dried and then examined with X-ray powder diffractometry. In all cases the powder patterns are identical to that of the compounds applied at the beginning of the experiment, which can be traced back to the fact that their solubility is not large enough to allow interconversion.
H2O. All syntheses done with Ge instead of GeO2 were also not successful. The syntheses were only successful and the compounds could only be isolated in the presence of elemental antimony in the reaction slurry. While compounds 1–3 could be obtained by increasing the amount of Sb in the reaction mixture, compound 4 was obtained by reducing the GeO2 content. The reaction time plays also a crucial role. Compounds 1–3 could only be obtained exclusively besides Sb2S3 (see above) if the reaction time did not exceed 4 days, while compound 4 was obtained after 26 days and also at longer reaction times up to 40 days. Extending the reaction times for 1–3 afforded crystallization of a second compound mainly as very thin needles which were too tiny for single crystal structure determination. Experiments were also undertaken to elucidate whether the compound can be transformed into each other to determine which is more stable. Combinations of handpicked single crystals of 1–4 were stirred at room temperature in glass tubes containing ether, methanol, ethanol, acetone or n-hexane and after 72 h the residue was filtered off, dried and then examined with X-ray powder diffractometry. In all cases the powder patterns are identical to that of the compounds applied at the beginning of the experiment, which can be traced back to the fact that their solubility is not large enough to allow interconversion.
The role of antimony for the formation of the samples is not understood. But there are examples reported in literature that an element or a compound which were not in the final product was a necessary ingredient for the formation of the material.35,36
All four compounds contain the well known [Ge2S6]4− anion composed of two edge-sharing GeS4 tetrahedra (Fig. 1), [Co(dien)2]2+ complexes and diprotonated dien molecules (see Scheme 1). In all [Ge2S6]4− anions of the title compounds the Ge–S bonds exhibit the typical pattern of two long Ge–Sbr–Ge bonds (range in the four compounds: 2.2658(9)–2.2973(7) Å) and two shorter Ge–Sterm bonds (range in the four compounds: 2.1416(8)–2.1714(10) Å) (see tables in ESI†).
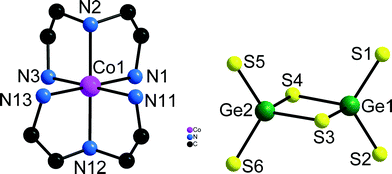 | ||
| Fig. 1 The Co1 centred [Co(dien)2]2+ complex in 1 with numbering of selected atoms. Note that the other two [Co(dien)2]2+ complexes have the same configuration. H atoms are not displayed. | ||
Surprisingly, the dien molecules in these compounds are differently protonated. In compound 2 only the terminal N atoms are protonated, whereas in 3 and 4 only cations are observed, in which one terminal and the central N atom is protonated, leading to two different polymorphic modifications. In compound 1 both tautomeric dien cations are found.
Compounds 1 and 2 crystallize in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Table 1) but with different number of molecules in the unit cell. In the structure of 1 three crystallographically independent Co2+ ions are present of which two occupy special positions. The four unique Ge atoms and all other atoms in 1 are located on general positions. The three independent Co2+ ions are each surrounded by two dien molecules yielding distorted CoN6 octahedra in s-fac configuration (Fig. 1).
(Table 1) but with different number of molecules in the unit cell. In the structure of 1 three crystallographically independent Co2+ ions are present of which two occupy special positions. The four unique Ge atoms and all other atoms in 1 are located on general positions. The three independent Co2+ ions are each surrounded by two dien molecules yielding distorted CoN6 octahedra in s-fac configuration (Fig. 1).
The Co–N bond lengths in the three complexes (Co1: 2.159(3)–2.188(3) Å; Co2: 2.140(3)–2.184(3) Å; Co3: 2.141(3)–2.197(3) Å, Table S1†) are typical for [Co(dien)2]2+ with the s-fac configuration.
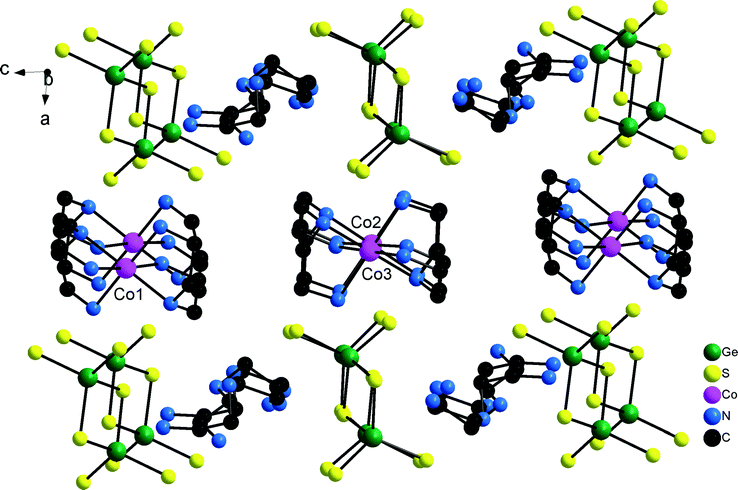
A remarkable structural detail of 1 is the occurrence of both tautomeric dien cations (see Scheme 1). In the structure of 1 anions and Co2+ centred cations alternate along [100] while along [001] the sequence is ⋯[Ge2S6]4−–[dienH2]2+–[Ge2S6]4−⋯ (Fig. 2). Along [010] the different constituents form rods. Alternatively, the arrangement of the constituents may be described as alternating layers composed of [dienH2]2+–[Ge2S6]4− and of Co2+ centred complexes. The H atoms of the [dienH2]2+ bound to the N atoms are involved in S⋯H–N bonding interactions and a layer-like arrangement of cations and anions is realized within the (010) plane (Fig. 3). The two unique [dienH2]2+ cations each join three [Ge2S6]4− anions via their terminal S atoms generating different types of ring-like motifs (Fig. 3). All three H atoms of the terminal NH3 group and both H atoms of the central NH2 moiety of molecule 1 are involved in S⋯H interactions while only one H atom of the terminal NH2 unit has such an interaction. In the second protonated dien molecule containing two terminal NH3 groups five H atoms are engaged in S⋯H bonding and also the H atom of the central NH unit. Hence, in both molecules six out of seven H atoms bonded to N have S⋯H bonds (Table S2†). Taking into account the hydrogen bonding interactions between S atoms of the anions and the H atoms of the Co2+ centred complexes a very complex N–H⋯S bonding pattern is generated yielding a 3D supramolecular assembly.
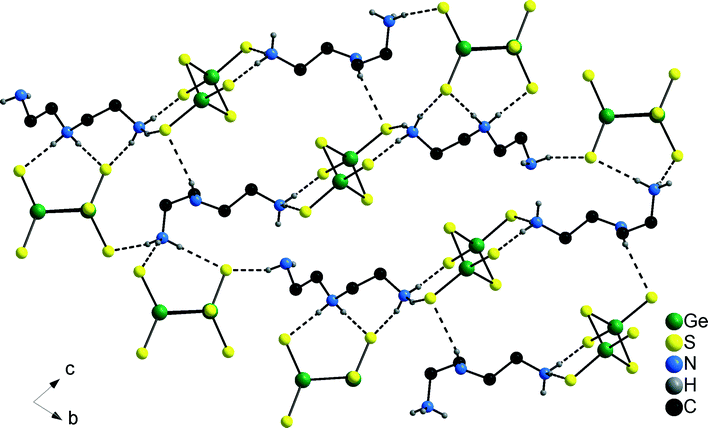 | ||
| Fig. 3 Layers in the structure of 1 generated by intermolecular S⋯H–N bonding interactions. Only H atoms bonded to N atoms are displayed. | ||
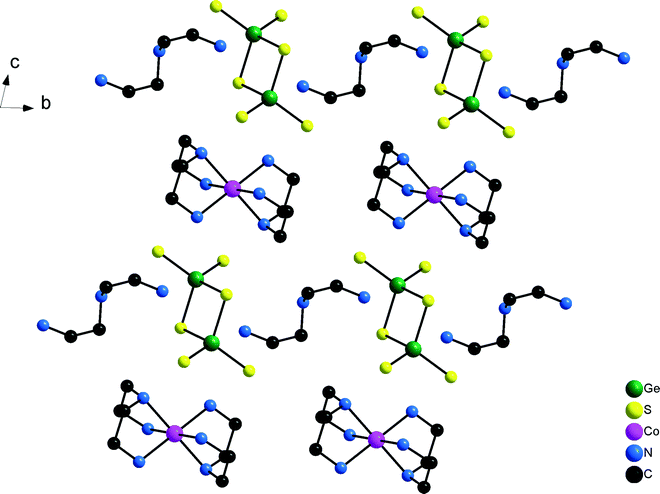
In the structure of compound 2 with one formula unit in the cell the unique Co2+ ion is located on a special position while all remaining independent atoms are located on general positions. Like in compound 2 the [Co(dien)2]2+ complex adopts the s-fac configuration with comparable Co–N bond lengths as observed in 1 (Co–N: 2.153(2)–2.188(2) Å, Table S3†). In contrast to 1 only one type of protonated dien molecule is observed (Scheme 1) with two terminal NH3 groups. In the structure of 2 a similar arrangement of the anions and cations like in 1 is found with [Ge2S6]4− anions and [Co(dien)2]2+ complexes alternating along [001] and the protonated amine molecules and the anions along [010] (Fig. 4).
The two NH3 groups of the dien molecule and the terminal S atoms of the anion exhibit N–H⋯S bonding interactions leading to formation of a two dimensional supramolecular layer-like arrangement within the (001) plane being characterized by rings composed of two dienH2 cations and two [Ge2S6]4− anions (Fig. 5). We note that all H atoms of the NH3 groups are engaged in S⋯H–N bonding to two different anions (Table S4†) while the H atom of the central NH group does not show S⋯H interactions. Along [100] adjacent [Ge2S6]4− anions are interconnected into rods by the [dienH2]2+ ions.
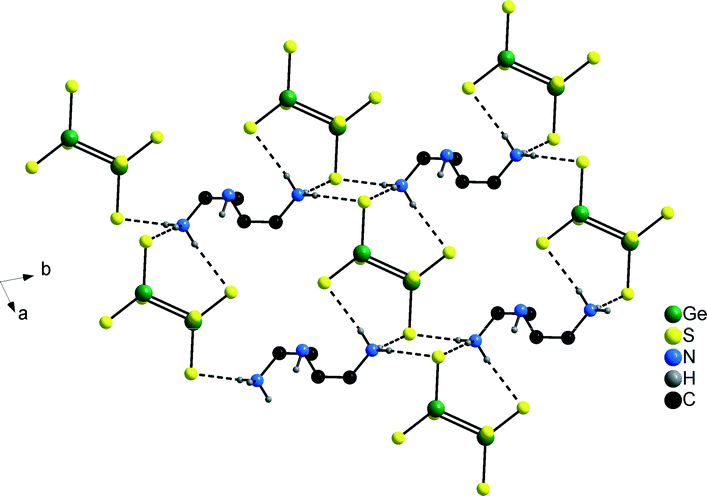 | ||
| Fig. 5 N–H⋯S hydrogen bonding network in the structure of compound 2 involving [Ge2S6]4− anions and [dienH2]2+ cations. Note that not all H atoms are displayed. | ||
The [Co(dien)2]2+ complexes are located between the layers composed of [dienH2]2+ cations and [Ge2S6]4− and including the S⋯H–N interactions between the anions and the [Co(dien)2]2+ complex a three-dimensional array of the cations and anions is generated.
Compound 3 crystallizing in the orthorhombic space group Pbca (Table 1) contains eight formula units and all unique atoms sit on general positions. The unique Co2+ ion is surrounded by two dien ligands (Co–N bond lengths: 2.139(3)–2.210(3) Å, Table S5†) and adopts the u-fac configuration (Fig. 6). In this compound charge neutrality is achieved by the presence of one dien molecule with one protonated terminal N atom and the protonated central N atom (Scheme 1).
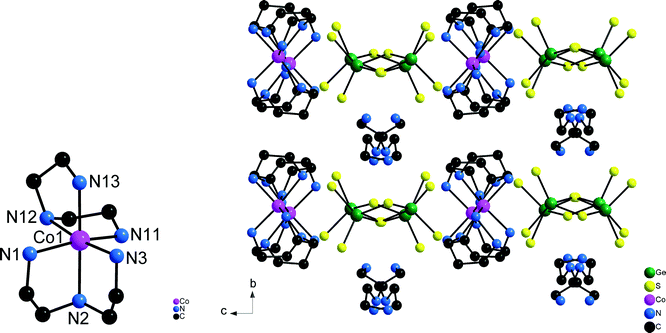 | ||
| Fig. 6 The [Co(dien)2]2+ cation with u-fac configuration (left) and the arrangement of the cations and anions in the structure of compound 3 (right). | ||
The arrangement of the cations and anions follows the same pattern as observed for 1 and 2 where cations and anions alternate along two directions (here: [010] and [001]) and individual ions are arranged to form rods along the third direction.
The N–H⋯S bonding interactions (Table S6†) generate a layer-like arrangement within the (001) plane containing rings composed of three cations and anions (Fig. 7). Two H atoms of the terminal NH3 group and both H atoms of the central and terminal NH2 units exhibit such interactions (Table S6†). In contrast to compounds 1 and 2, one bridging S atom of the [Ge2S6]4− anion has a short S⋯H–N contact besides the four terminal S atoms. The N–H atoms of the [Co(dien)2]2+ complex exhibit also hydrogen bonds to S atoms to form a three-dimensional supramolecular network.
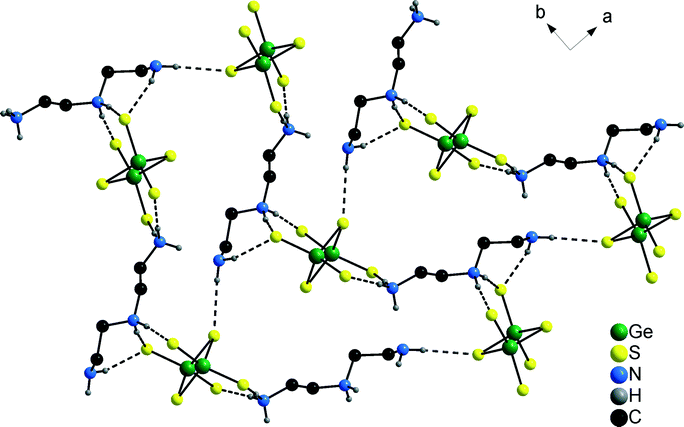 | ||
| Fig. 7 The S⋯H–N bonding network in the structure of compound 3. The C–H H atoms are omitted for clarity. | ||
Compound 4 crystallizes in the non-centrosymmetric space group Pca21 (Table 1) with four formula units and all atoms being located on general positions. The unique [Co(dien)2]2+ complex adopts the s-fac configuration with Co–N bond length very similar to those observed for the other three compounds (Co–N: 2.139(3)–2.210(3) Å, Table S7†). The charge compensating protonated dien molecule contains a terminal NH3 group and a central NH2 unit (Scheme 1). In the structure rows composed of [Co(dien)2]2+ complexes and [Ge2S6]4− ions are arranged along [001] and along [010] columns consisting of either only [Co(dien)2]2+ complexes or [Ge2S6]4−/[dienH2]2+ ions alternate (Fig. 8).
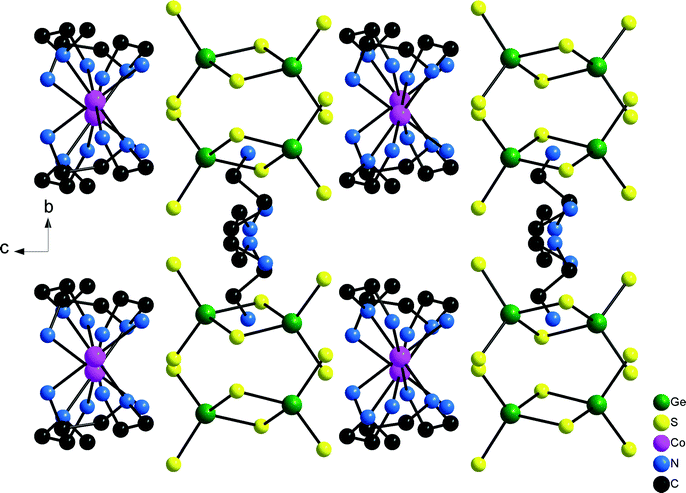 | ||
| Fig. 8 Arrangement of the constituents in the structure of compound 4. Note that H atoms are not drawn for clarity. | ||
The N–H⋯S bonding pattern between [dienH2]2+ cations and [Ge2S6]4− anions leads to the formation of chains being directed along [010] (Fig. 9). In contrast to compounds 1–3 only five of the seven H atoms bound to N atoms are involved in the hydrogen bonding interactions (Table S8†).
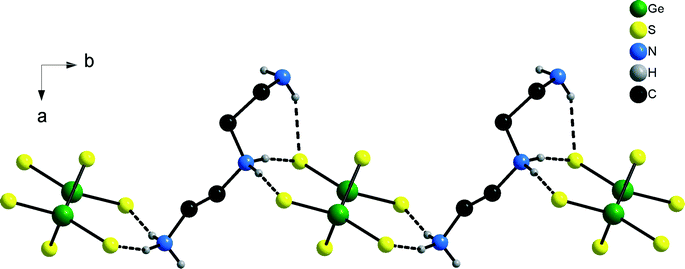 | ||
| Fig. 9 The N–H⋯S bonding pattern between [dienH2]2+ ions and [Ge2S6]4− anions. Only the H atoms bonded to the N atoms are shown. | ||
Like for the other three compounds the S atoms have also hydrogen bonding interactions to H atoms of the [Co(dien)2]2+ complex.
As mentioned above, in all compounds a completely different topology of the hydrogen-bonded network between [Ge2S6]4− anions and dienH2 cations is observed. The topology not only depends on the position where the nitrogen atoms of the dien ligands – central or terminal – are protonated. It also depends on the number of hydrogen bonds formed by the different amine and ammonium groups. This is schematically shown in Fig. 10, displaying the numbers of hydrogen bonds formed by the different H atoms. From this representation it is obvious that e.g. despite the fact that both terminal N atoms of the dien molecules in compounds 1 and 2 are protonated not all H atoms are equally involved in hydrogen bonding interactions even if the overall number of the H-bonds is identical. Consequently a different H-bonding pattern must result in the structures.
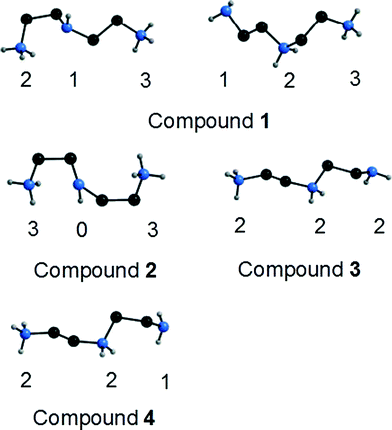 | ||
| Fig. 10 The [dienH2]2+ cations in the four compounds. The numbers of N–H⋯S interactions to the [Ge2S6]4− anions is indicated. Only N–H H atoms are shown. | ||
Conclusions
In the present contribution four different compounds of same composition were obtained under different reaction conditions. In all compounds discrete [Co(dien)2]2+ cations and [Ge2S6]4− anions are observed and charge compensation is achieved by diprotonated dien molecules. Interestingly, the dien cations are differently protonated leading to tautomerism, which is a rare phenomenon. However, in two of these compounds the same tautomeric cations are observed, and therefore, they also represent polymorphic modifications. In all of these compounds the anions and the dien cations are linked by intermolecular N–H⋯S hydrogen bonding, which, because of the different arrangement of the building blocks and the occurrence of different tautomers in the crystal lead to completely different hydrogen bonded networks. These results clearly show the advantage of solvothermal synthesis for the preparation of different stable and metastable compounds. Moreover, we also demonstrate here that even for relatively simple chemical compounds one can observe highly unusual phenomena if the product formation is investigated in more detail.Acknowledgements
Financial support by the State of Schleswig-Holstein and Deutsche Forschungsgemeinschaft (DFG) is gratefully acknowledged.References
- P. Feng, X. Bu and N. Zheng, Acc. Chem. Res., 2005, 38, 293 CrossRef CAS PubMed.
- X. Bu, N. Zheng and P. Feng, Chem. – Eur. J., 2004, 10, 3356 CrossRef CAS PubMed.
- S. Dehnen and M. Melullis, Coord. Chem. Rev., 2007, 251, 1259 CrossRef CAS PubMed.
- J. Zhou, J. Dai, G.-Q. Bian and C.-Y. Li, Coord. Chem. Rev., 2009, 253, 1221 CrossRef CAS PubMed.
- A. Kromm, T. van Almsick and W. S. Sheldrick, Z. Naturforsch., B: J. Chem. Sci., 2010, 65, 918 CAS.
- B. Seidlhofer, N. Pienack and W. Bensch, Z. Naturforsch., B: J. Chem. Sci., 2010, 65, 937 CAS.
- B. Krebs, Angew. Chem., Int. Ed. Engl., 1983, 22, 113 CrossRef.
- N. Pienack, C. Näther and W. Bensch, Eur. J. Inorg. Chem., 2009, 937 CrossRef CAS.
- J. Wang, C. Näther, J. Djamil and W. Bensch, Z. Anorg. Allg. Chem., 2012, 638, 1452 CrossRef CAS.
- M. Melullis, M. K. Brandmayer and S. Dehnen, Z. Anorg. Allg. Chem., 2006, 632, 64 CrossRef CAS.
- A. Choudhury and P. K. Dorhout, Z. Anorg. Allg. Chem., 2008, 634, 649 CrossRef CAS.
- G.-N. Liu, G.-C. Guo, M.-S. Wang, L.-Z. Cai and J.-S. Huang, J. Mol. Struct., 2010, 983, 104 CrossRef CAS PubMed.
- J.-F. Chen, Q.-Y. Jin, Y.-L. Pan, Y. Zhang and D.-X. Jia, Z. Anorg. Allg. Chem., 2010, 636, 230 CrossRef CAS.
- X. Liu, J. Zhou, J. He and Z.-W. Huang, Z. Naturforsch., B: J. Chem. Sci., 2011, 66, 659 CrossRef CAS PubMed.
- X. Liu, F. Hu, J. Zhou, L. An, D. Liang and J. Lin, CrystEngComm, 2012, 14, 3464 RSC.
- J. Y. Pivan, O. Achak, M. Louer and D. Louer, Chem. Mater., 1994, 6, 827 CrossRef CAS.
- B. Krebs and S. Pohl, Z. Naturforsch., B: Anorg. Chem., Org. Chem., Biochem., Biophys., Biol., 1971, 26, 853 CAS.
- S. Pohl and B. Krebs, Z. Anorg. Allg. Chem., 1976, 424, 265 CrossRef CAS.
- G.-N. Liu, J.-D. Lin, Z.-N. Xu, Z.-F. Liu, G.-C. Guo and J.-S. Huang, Cryst. Growth Des., 2011, 11, 3318 CAS.
- W.-Q. Mu, Q.-Y. Zhu, L.-S. You, X. Zhang, W. Luo, G.-Q. Bian and J. Dai, Inorg. Chem., 2012, 51, 1330 CrossRef CAS PubMed.
- B. Krebs, H.-U. Hurter, D. Voelker and H.-J. Wallstab, Z. Kristallogr., 1980, 154, 63 Search PubMed.
- R. Stähler and W. Bensch, Z. Anorg. Allg. Chem., 2002, 628, 1657 CrossRef.
- V. Vater and W. S. Sheldrick, Z. Naturforsch., B: J. Chem. Sci., 1997, 52, 1119 CAS.
- J. Zhou, L. An, X. Liu, L. Huang and X. Huang, Dalton Trans., 2011, 40, 11419 RSC.
- J. Zhou, X. Liu, G. Liang, W. Liang, F. Hu and L. Zhu, Inorg. Chem. Commun., 2013, 27, 92 CrossRef CAS PubMed.
- M.-L. Feng, D.-N. Kong, Z.-L. Xie and X.-Y. Huang, Angew. Chem., Int. Ed., 2008, 47, 8623 CrossRef CAS PubMed.
- M.-L. Feng, W.-W. Xiong, D. Ye, J.-R. Li and X.-Y. Huang, Chem. – Asian J., 2010, 5, 1817 CrossRef CAS PubMed.
- M.-L. Feng, C.-L. Hu, K.-Y. Wang, C.-F. Du and X.-Y. Huang, CrystEngComm, 2013, 15, 5007 RSC.
- Z. Rejai, C. Näther, R. K. Kremer and W. Bensch, Inorg. Chem., 2010, 49, 1651 CrossRef CAS PubMed.
- N. Herzberg, C. Näther and W. Bensch, Z. Kristallogr. - Cryst. Mater., 2012, 227, 552 CrossRef CAS.
- B. Seidlhofer, J. Djamil, C. Näther and W. Bensch, Cryst. Growth Des., 2011, 11, 5554 CAS.
- B. Seidlhofer, C. Näther and W. Bensch, CrystEngComm, 2012, 14, 5441 RSC.
- A. Puls, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2006, 632, 1239 CrossRef CAS.
- G. M. Sheldrick, Programs for the solution refinement of crystal structures, University of Göttingen, 1997 Search PubMed.
- B. Seidlhofer, V. Spetzler, E. Quiroga-Gonzalez, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2011, 637, 1295 CrossRef CAS.
- W. S. Sheldrick and B. Schaaf, Z. Naturforsch., B: J. Chem. Sci., 1994, 49, 993 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Tables with interatomic distances and angles. CCDC 985614–985617. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ce00312h |
| This journal is © The Royal Society of Chemistry 2014 |