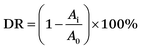Titanium dioxide macroporous materials doped with iron: synthesis and photo-catalytic properties†
Wei-Qiang
Fan
,
Hong-Ye
Bai
,
Ge-Hong
Zhang
,
Yong-Sheng
Yan
,
Chun-Bo
Liu
and
Wei-Dong
Shi
*
School of Chemistry & Chemical Engineering, Jiangsu University, Zhenjiang 212013, People's Republic of China. E-mail: shiwd999@163.com; Tel: +86 511 887901877
First published on 14th October 2013
Abstract
A series of iron doped three-dimensional (3D) ordered macroporous TiO2 hybrid materials (Fe–Ti–Oxide-M) have been successfully prepared by using polymethylmethacrylate nanospheres (PMMAs) as templates. The crystal structure of Fe–Ti–Oxide-M (50%) could be assigned to the Fe3Ti3O10 phase by XRD characterization. Synthetic samples were also studied by TEM and UV-vis absorption spectra in detail. All obtained samples possessed 3D ordered macroporous structures, and the absorption spectra of Fe–Ti–Oxide-M gradually extended to the visible light region with increased doping quantities of Fe. Furthermore, the photo-catalytic degradation of tetracycline (TC) was investigated, and the observed photo-catalytic performances of Fe–Ti–Oxide-M (1%, 2%, 5%, 10%, 20%, and 50%) were associated with their composition, which further indicates that the introduction of Fe has a remarkable influence on the photo-activity of 3D ordered macroporous TiO2 materials.
Introduction
Recently, much attention has been focused on strategies for the photo-catalytic purification of polluted air and wastewater.1–3 It is worth noting that attempts to tune the chemical composition and the morphology of the matrices could provide a flexible means to optimize the catalytic performance of catalysts.4,5 Therefore, it remains a fascinating challenge to design and synthesize novel photo-catalysts with suitable chemical modifications and microstructure for the photo-degradation of organic pollutants using solar energy.6–8Yablonovitch and John first proposed the concept of photonic crystals.9,10 In photonic crystals, the 3D ordered arrangement of two different refractive index materials generates a periodic potential field, which will lead to the emergence of the energy band gap. Therefore, the band-edge effect of photonic crystals plays a role in enhancing the light absorption of substances, namely the photonic crystal slow photon effect, which extends the interaction time of the light and gain materials.11 Recently, the use of opaline lattices as templates to assemble 3D ordered macroporous materials, as photonic crystals,12–14 has attracted intense attention, because of their applications in catalysis,15 magnetics,16,17 and photoluminescence.18–20
The photonic crystal structure with the regulation of light (especially the slow photon effect) will help to promote the activity of material, which endows 3D ordered macroporous materials with potential applications in the field of photo-catalysis.21 TiO2, due to its nature of chemical stability, environmental-friendliness, and high activity, has been widely used as a highly-efficient photo-catalyst. So, combining a 3D ordered macroporous structure and a TiO2 matrix into a single material has become a challenge to explore novel photo-catalysts. For example, Ozin’s group22 found that the photo-activity of TiO2 fashioned as inverse opals can be dramatically enhanced by utilizing the slow photon effect with energies close to the electronic band gap of the semiconductor. Moreover, Song et al.23 reported that the absorption of TiO2 in the ultraviolet region could be enhanced based on the photonic crystal slow photon effect by optimizing the photonic crystal band gap, which could increase the catalytic efficiency up to 2.3 times. Therefore, it can be considered that the catalytic properties of TiO2 can be further improved if it is endowed with 3D ordered macroporous materials.
However, as is well known, TiO2, due to its wide band gap (Eg = 3.2 eV), can only respond to ultraviolet light (less than 387 nm), which means a low utilization rate of solar energy.24 So, although the 3D ordered macroporous structure can further enhance the photo-activity of a TiO2 matrix, the wide band gap still limits its practical application. Despite this, it is still an interesting task to enhance the efficiency of TiO2 matrices through visible light sensitization.25 Recently, some studies have been conducted with a view to improving the visible light sensitivity of catalysts based on a TiO2 matrix.26,27 One of the major strategies for inducing a TiO2 matrix to possess a visible light response is chemical doping with transition metals containing partially filled d-orbitals, such as V, Ni, Cr, Mo, Fe, Sn, Mn, and so on.28–32 Moreover, it has been hypothesized that the addition of transition metals to a TiO2 matrix could also enhance the rate of photo-catalytic oxidation, due to electron scavenging by the metal ions at the semiconductor surface.33 Therefore, chemical doping of TiO2 matrices might be an alternative way to further design novel TiO2 catalysts with 3D ordered macroporous structures.
Inspired by the aforementioned points, in this work, we report on the preparation of a series of 3D ordered macroporous TiO2 hybrid materials with different doping quantities of Fe (Fe–Ti–Oxide-M; Fe/(Fe + Ti) = 1%, 2%, 5%, 10%, 20%, and 50%) by using polymethylmethacrylate nanospheres (PMMAs) as templates. The morphologies and absorption spectra of Fe–Ti–Oxide-M samples were systematically investigated. More importantly, the series of Fe–Ti–Oxide-M samples exhibited different photo-sensitized degradation of tetracycline (TC) dye under the illumination of visible light, and these properties were also found to be quantity of Fe dependent.
Experimental
Materials
All the reagents were used without further purification.Syntheses
Characterization
Powder X-ray diffraction (XRD) patterns were obtained on a D/MAX-2500 diffract meter (Rigaku, Japan) using a Cu Kα radiation source (λ = 1.54056 Å). Transmission electron microscopy (TEM) images were collected on an F20 S-TWIN electron microscope (Tecnai G2, FEI Co.), using a 200 kV accelerating voltage. UV-vis diffuse reflectance spectra of the samples were obtained from a UV-vis spectrophotometer (UV2550, Shimadzu, Japan). BaSO4 was used as a reflectance standard. Specific surface area was measured by using a NOVA2000e analytical system made by Quantachrome Corporation (USA), the BET surface areas were obtained from nitrogen adsorption at −196 °C, and the samples were previously outgassed overnight at 120 °C under vacuum conditions. Total organic carbon (TOC) analyses were conducted on a multi N/C 2100 (Analytik Jena AG, Germany) TOC analyzer.Photo-catalytic activity measurement
The photodegradative reaction for TC was carried out at room temperature under visible-light. The photochemical reactor contained 0.1 g sample and 100 mL TC solution (20 mg L−1). To determine the initial absorbency of samples, the reactor was kept in darkness for 30 min to reach absorption equilibrium. The photochemical reactor was irradiated with a 150 W Xenon lamp which was located with a distance of 8 cm at one side of the containing solution. UV lights with wavelengths less than 420 nm were removed by a UV-cutoff filter. The sampling analysis was conducted at 10 min intervals. The photocatalytic degradation ratio (DR) was calculated by the following formula:A0 is the initial absorbency of TC that reached absorption equilibrium, while Ai is the absorbency after the sampling analysis.
The TC concentration was measured by a UV-vis spectrophotometer with the maximum absorption wavelength at 357 nm.
Results and discussion
Structure and microscopy characterization of Fe–Ti–Oxide-M
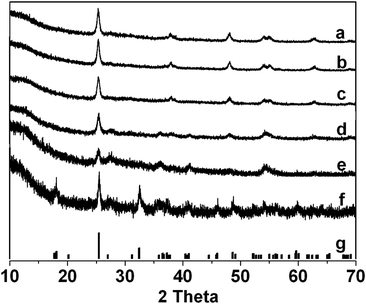
The synthesis steps of the macroporous materials are described in Scheme 1. The Fe–Ti–Oxide-M was prepared by using a Ti–Fe mixed solution as a precursor. In the mixed solutions, the quantities of acetic acid and ethanol took an important role, since the acetic acid as a chelating agent could effectively buffer the hydrolytic rate of isopropyl titanate, and the ethanol was used to adjust the viscosity of the solution. Then, the mixed solution can be filled into the interspace of assembled PMMAs through capillary infiltration, and solidified under mild conditions. The template PMMAs were finally removed by calcination, and the obtained products are Fe doped 3D macroporous materials based on a TiO2 matrix.In order to study the structures of the Fe–Ti–Oxide-M, X-ray diffraction patterns were characterized as shown in Fig. 1. The XRD pattern of Fe–Ti–Oxide-M (1%) in Fig. 1a was comparable with pure TiO2 (JCPDS ID: 21-1272). Moreover, no traces of impure reflections could be found when the quantity of Fe was increased even up to 5% (Fig. 1c), which indicated that the Fe might be doped into the TiO2 crystal lattice if the quantity of Fe is below 5%. Nevertheless, a new diffraction reflection at ca. 26.9°, which could be attributed to the Fe3Ti3O10 phase (JCPDS ID: 43-1011),34 appeared when increasing the quantity of Fe to 10% (Fig. 1d), while the relative intensity of reflections from TiO2 became weaker in contrast. This confirmed that the Fe3Ti3O10 phase began to form in the Fe–Ti–Oxide-M (10%) sample. Furthermore, when the quantity of Fe was equal to Ti (Fig. 1f), the final sample exhibited the pure Fe3Ti3O10 phase according to the simulated XRD pattern of Fe3Ti3O10 (Fig. 1g). Fe3Ti3O10 is a kind of iron titanium oxide, and it contains stoichiometric iron and titanium atoms. To the best of our knowledge, investigations on macroporous materials composed of pure Fe3Ti3O10 have been lacking. Therefore, the formation of the Fe3Ti3O10 phase in the product would offer alternative novel macroporous materials in the field of photo-catalysis. Moreover, the crystallite sizes play an important role in the stability of the macroporous skeleton, because crystallite sizes that are too large might make the skeleton prone to collapse. So, we further calculated the crystallite sizes using the Scherrer equation, and the crystallite sizes of all samples range from 16.0 to 24.0 nm. The small size could ensure the stability of the macroporous structure.
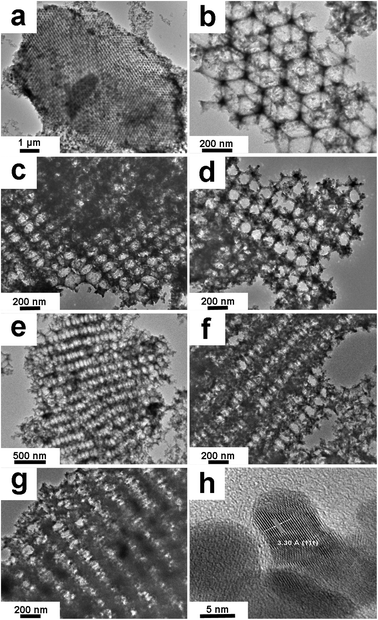
Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) provided insight into the morphology of Fe–Ti–Oxide-M. As depicted in Fig. 2a, the Fe–Ti–Oxide-M (1%) block is constructed with a uniform macroporous structure, and the block has a large size with a continuous macroporous structure. We can see from the magnified view of Fe–Ti–Oxide-M (1%) (Fig. 2b) that the sample consists of ca. 280 nm diameter spherical pores which are interconnected through ca. 85 nm wide apertures and surrounded by a continuous gel framework, indicating the presence of close-packed spherical pores inside the samples. Additionally, the TEM images of Fe–Ti–Oxide-M (2%, 5%, 10%, 20%, and 50%) all exhibit close-packed macroporous structures (Fig. 2c–g), indicating that the quantity of Fe inside the TiO2 matrix did not observably influence the macroporous structure of the samples. In Fig. 2h, the HRTEM image of Fe–Ti–Oxide-M (50%) reveals that Fe–Ti–Oxide-M is composed of the Fe3Ti3O10 phase with an interplanar spacing of about 3.30 Ǻ, which corresponds to the (111) crystalline plane.
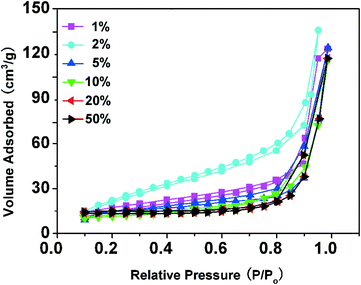
The macroporous structure of Fe–Ti–Oxide-M was also characterized by nitrogen adsorption–desorption isotherms. The N2 adsorption–desorption isotherms (Fig. 3) exhibit a hysteresis loop range of 0.8–1.0 P/P0. The BET surface areas of all samples are around 50 m2 g−1, due to their 3D ordered macroporous structure. This result is a little higher than the reported SiO235 and TiO236 materials with 3D ordered macroporous structures. Commonly, the measured BET surface areas of macroporous materials are related to the small particle sizes of the crystallites, so the BET surface area data also reveal that the crystallite sizes of Fe–Ti–Oxide-M are relatively small, in agreement with their XRD results calculated by the Scherrer equation. Moreover, the relative large surface areas of Fe–Ti–Oxide-M can facilitate the diffusion of solvent in the system of photo-degradation, which might make a key improvement on the catalytic efficiency.37
Generally, chemical doping with metal ions can alter the semiconductor properties of a TiO2 matrix. So, the UV-vis absorption spectra of Fe–Ti–Oxide-M were further characterized as shown in Fig. 4A. The Fe–Ti–Oxide-M (1%) exhibited a broad band from 400 to 200 nm in the UV region, and the maximal absorption was achieved at about 300 nm. In comparison, the Fe–Ti–Oxide-M (2–50%) demonstrated successive red shifts responding to the introduction of Fe, and the absorption edge of Fe–Ti–Oxide-M (50%) extended to about 700 nm. This phenomenon is in agreement with the previous reports.38–40 Furthermore, we calculated both the indirect and direct band gaps (Eg) of samples based on their UV-vis absorption spectra in order to fully investigate the photophysical properties of the materials. As shown in Fig. 4B and C, the Eg of the samples became gradually smaller with increasing quantities of Fe, which suggested that the ability to absorb visible light can be controlled by altering the amount of Fe inside the Fe–Ti–Oxide-M. Moreover, in regard to the XRD data, the absorption of Fe–Ti–Oxide-M (20–50%) should be attributed to the Fe3Ti3O10 phase. Therefore, we can speculate that two things may contribute to the improved red shift of the TiO2 matrix. One is that the Fe atoms were doped into the TiO2 crystal lattice, which embedded new energy levels into the band gap of TiO2, and a similar phenomenon has been reported by Miyauchi’s group recently.41 The additional reason is that the Fe3Ti3O10 phase with a narrow band gap had formed in the samples, which further induced the red shift of the absorption spectra of Fe–Ti–Oxide-M (20–50%).
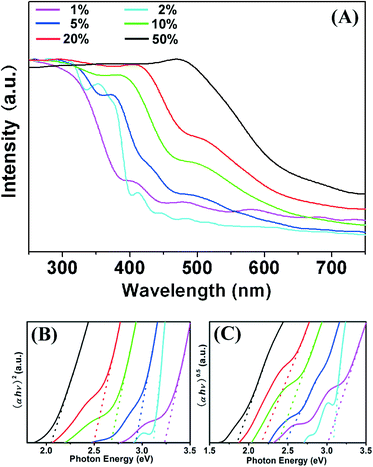 | ||
| Fig. 4 UV-vis absorption spectra (A) of Fe–Ti–Oxide-M (1–50%). The (B) and (C) spectra show the corresponding indirect and direct band gaps, respectively. | ||
Photocatalytic properties
Organic compounds are used extensively in industrial products in the printing, textile, medication, and photographic industries. Many organic compounds are nontoxic themselves, but they could form highly toxic complexes with some heavy metal ions in wastewater, and cause the pollution of water resources.42,43 TC, a popular broad-spectrum antibacterial agent, has been widely applied in bacterial infection treatment. However, there are serious threats to the ecosystem and human health if TC is released into the environment. So, the degradation of TC has been established to be one of the most promising technologies for environmental remediation.44 Recent suggestions for the degradation of TC have mainly focused on semiconductor-based photo-catalysts using solar energy,45 and the degradation efficiency directly determines the application of the photo-catalysts.Based on the interesting absorption spectra and crystal structure, the photo-catalytic activity of the Fe–Ti–Oxide-M was studied on degradation of TC in an aqueous solution (20 mg L−1). During the process of photo-degradation, the major absorbance (ca. 357 nm) from TC in the solution gradually decreased under visible light (λ ≥ 420 nm) for 1 hour (Fig. S1†), which suggested significant photo-degradation of TC by the Fe–Ti–Oxide-M.
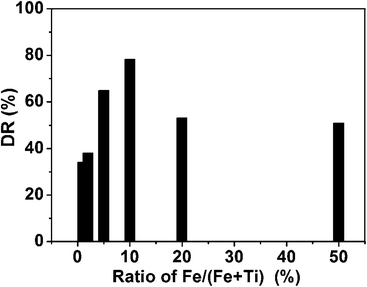
As shown in Fig. 5, the photocatalytic degradation ratios of Fe–Ti–Oxide-M (1%, 2%, 5%, 10%, 20%, and 50%) are 34.1%, 38.0%, 65.0%, 78.3%, 53.2%, and 51.0% after irradiation for 1 h, respectively. Furthermore, the blank experiments were also investigated under different conditions: first, the bare TC solution was exposed to visible light for 1 h; second, the Fe–Ti–Oxide-M and TC mixed solution was reacted for 1 h in the dark. The photocatalytic degradation ratios of both blank experiments are very low, which indicated that the TC could hardly be degraded without the samples under visible light irradiation, while the Fe–Ti–Oxide-M shows much higher photo-activity.
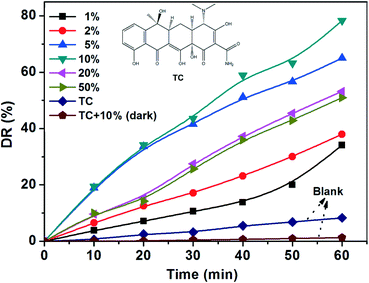 | ||
| Fig. 5 Photo-catalytic degradation ratios (detection wavelength is 357 nm) of Fe–Ti–Oxide-M (1–50%) under visible light. | ||
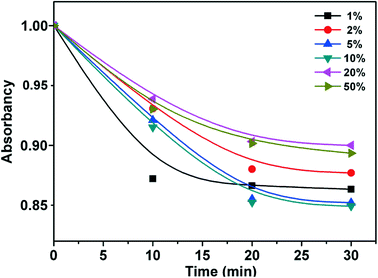
To ensure the accuracy of the photodegradation ratios in consideration of the influence of physical adsorption, control experiments of physical adsorption in the samples over 30 min were further carried out. As shown in Fig. 6, all samples reached adsorption equilibrium in 20 min, which revealed that the photo-activities of samples take the main role in the photodegradation of TC with little relation to physical adsorption. Moreover, the total organic carbon (TOC) under visible-light for the Fe–Ti–Oxide-M (10%) (Fig. S2†) has been further studied to determine the extent of mineralization during photodegradation. The TOC removal percentage was calculated, and 86% of the TOC was removed after 420 min under visible light irradiation, which indicated the obvious mineralization of TC.
In our work, the doping quantity of Fe could efficiently extend the absorption of the TiO2 macroporous materials to the visible region, and the Fe–Ti–Oxide-M (50%) possessed the largest absorption area in the visible region, so the Fe–Ti–Oxide-M (50%) should exhibit the best photo-catalytic performance in theory. However, Fe–Ti–Oxide-M (10%) demonstrated the highest photo-catalytic degradation ratio compared with other samples (Fig. 7). Some reasons are proposed to account for the high photocatalytic activity of the hierarchical Fe–Ti–Oxide-M (10%): as previously reported, metal ions can introduce new energy levels of the transition metal ions into the band gap of TiO2 without changing its valence band edge, which results in the absorption of visible light for metal doped TiO2.46 On the other hand, the solubility of Fe in the TiO2 matrix is commonly very low (about 1 at% for anatase), so the redundant Fe should be left as dopants on the surface of TiO2, which would depress the photo-activity of TiO2 by contrast.47 With the combination of the above two factors, there should be an optimal value of doping quantity of Fe in the TiO2 matrix, and deviation from the optimal value will work against the purpose of increasing the photo-activity of TiO2.
Therefore, the optimal value of doping quantity of Fe is 10% in the 3D macroporous Fe–Ti–Oxide-M system. Because the Fe–Ti–Oxide-M (50%) was composed of a Fe3Ti3O10 phase, the results further suggest that TiO2 doped with a lower concentration of Fe has a better photo-catalytic degradation ratio of TC than pure Fe3Ti3O10 under visible light.
Conclusion
Fe–Ti–Oxide-M (1%, 2%, 5%, 10%, 20%, and 50%) with a 3D macroporous structure has been successfully synthesized. Tuning the doping quantity of Fe can significantly affect the crystal structure of the resulting Fe–Ti–Oxide-M. The obtained samples exhibited the Fe3Ti3O10 phase when the quantity of Fe was equal to Ti. Moreover, the Fe–Ti–Oxide-M showed a significant red shift in response to the doping of Fe. The photo-catalytic properties of Fe–Ti–Oxide-M were studied under visible light in-depth. Taking into account the crystal structure and absorption spectra, the Fe–Ti–Oxide-M (10%) showed the optimal catalytic activity among the samples with different doping quantities of Fe in this system.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (21201085 and 21276116), Start-Up Foundation of Jiangsu University (11JDG105, and 11JDG153), and Natural Science Foundation of Jiangsu Province (BK2012294 and BK2011528).References
- Y. Q. Lei, S. Y. Song, W. Q. Fan, Y. Xing and H. J. Zhang, J. Phys. Chem. C, 2009, 113, 1280 CAS.
- A. Testino, I. R. Bellobono, V. Buscaglia, C. Canevali, M. D. Arienzo, S. Polizzi, R. Scotti and F. Morazzoni, J. Am. Chem. Soc., 2007, 129, 3564 CrossRef CAS PubMed.
- Y. B. Zhao, W. H. Ma, Y. Li, H. W. Ji, C. C. Chen, H. Y. Zhu and J. C. Zhao, Angew. Chem., Int. Ed., 2012, 51, 3188 CrossRef CAS PubMed.
- B. Mandlmeier, J. M. Szeifert, D. Fattakhova-Rohlfing, H. Amenitsch and T. Bein, J. Am. Chem. Soc., 2011, 133, 17274 CrossRef CAS PubMed.
- H. Tong, S. X. Ouyang, Y. P. Bi, N. Umezawa, M. Oshikiri and J. H. Ye, Adv. Mater., 2012, 24, 229 CrossRef CAS PubMed.
- Y. Q. Lei, G. H. Wang, S. Y. Song, W. Q. Fan, M. Pang, J. K. Tang and H. J. Zhang, Dalton Trans., 2010, 39, 3273 RSC.
- J. C. Zhao, C. C. Chen and W. H. Ma, Top. Catal., 2005, 35, 269 CrossRef CAS PubMed.
- Y. P. Huang, W. H. Ma, J. Li, M. M. Cheng, J. C. Zhao, L. J. Wan and J. C. Yu, J. Phys. Chem. B, 2003, 107, 9409 CrossRef CAS.
- E. Yablonovitch, Phys. Rev. Lett., 1987, 58, 2059 CrossRef CAS.
- S. John, Phys. Rev. Lett., 1987, 58, 2486 CrossRef CAS.
- J. I. L. Chen, G. Von Freymann, S. Y. Choi, V. Kitaev and G. A. Ozin, J. Mater. Chem., 2008, 18, 369 RSC.
- I. Soten, H. Miguez, S. M. Yang, S. Petrov, N. Coombs, N. Tetreault, N. Matsuura, H. E. Ruda and G. A. Ozin, Adv. Funct. Mater., 2002, 12, 71 CrossRef CAS.
- P. D. Yang, A. H. Rizvi, B. Messer, B. F. Chmelka, G. M. Whitesides and G. D. Stucky, Adv. Mater., 2001, 13, 427 CrossRef CAS.
- Y. A. Vlasov, N. Yao and D. J. Norris, Adv. Mater., 1999, 11, 165 CrossRef CAS.
- C. Wang, A. Geng, Y. Guo, S. Jiang and X. Qu, Mater. Lett., 2006, 60, 2711 CrossRef CAS PubMed.
- Y. N. Kim, S. J. Kim, E. K. Lee, E. O. Chi, N. H. Hur and C. S. Hong, J. Mater. Chem., 2004, 14, 1774 RSC.
- J. Gallery, M. Ginzburg, H. Miguez, S. M. Yang, N. Coombs, A. Safa-Sefat, J. E. Greedan, I. Manners and G. A. Ozin, Adv. Funct. Mater., 2002, 12, 382 CrossRef.
- Y. Liu and S. Wang, Colloids Surf., B, 2007, 58, 8 CrossRef CAS PubMed.
- R. Withnall, T. G. Ireland, M. I. Martinez-Rubio, G. R. Fern and J. Silver, J. Mod. Opt., 2002, 49, 965 CrossRef CAS.
- A. Ghadimi, L. Cademartiri, U. Kamp and G. A. Ozin, Nano Lett., 2007, 7, 3864 CrossRef CAS.
- B. L. Su, Q. J. Zhang, D. Bonifazi and J. L. Li, ChemSusChem, 2011, 4, 1327 CrossRef CAS PubMed.
- J. I. L. Chen, G. Von Freymann, S. Y. Choi, V. Kitaev and G. A. Ozin, Adv. Mater., 2006, 18, 1915 CrossRef CAS.
- J. Liu, G. L. Liu, M. Z. Li, W. Z. Shen, Z. Y. Liu, J. X. Wang, J. C. Zhao, L. Jiang and Y. L. Song, Energy Environ. Sci., 2010, 3, 1503 CAS.
- Q. Wang, C. C. Chen, W. H. Ma, H. Y. Zhu and J. C. Zhao, Chem.–Eur. J., 2009, 15, 4765 CrossRef CAS PubMed.
- A. J. Cowan, C. J. Barnett, S. R. Pendlebury, M. Barroso, K. Sivula, M. Grätzel, J. R. Durrant and D. R. Klug, J. Am. Chem. Soc., 2011, 133, 10134 CrossRef CAS PubMed.
- R. M. Navarro Yerga, M. C. Alvarez Galvan, F. del Valle, J. A. Villoria de la Mano and J. L. Fierro, ChemSusChem, 2009, 2, 471 CrossRef CAS PubMed.
- X. B. Chen, S. H. Shen, L. J. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503 CrossRef CAS PubMed.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891 CrossRef CAS PubMed.
- D. H. Kim, D. K. Choi, S. J. Kim and K. S. Lee, Catal. Commun., 2008, 9, 654 CrossRef CAS PubMed.
- D. W. Hwang, H. G. Kim, J. S. Lee, J. Kim, W. Li and S. H. Oh, J. Phys. Chem. B, 2005, 109, 2093 CrossRef CAS PubMed.
- E. Borgarello, J. Kiwi, M. Grätzel, E. Pelizzetti and M. Visca, J. Am. Chem. Soc., 1982, 104, 2996 CrossRef CAS.
- R. Konta, T. Ishii, H. Kato and A. Kudo, J. Phys. Chem. B, 2004, 108, 8992 CrossRef CAS.
- E. C. Butler and A. P. Davis, J. Photochem. Photobiol., A, 1993, 70, 273 CrossRef CAS.
- G. Chen, J. Chen, J. H. Peng and C. Srinivasakannan, J. Alloys Compd., 2011, 509, L244 CrossRef CAS PubMed.
- W. Q. Fan, J. Feng, S. Y. Song, Y. Q. Lei, S. Dang and H. J. Zhang, Opt. Mater., 2011, 33, 582 CrossRef CAS PubMed.
- B. T. Holland, C. F. Blanford and A. Stein, Science, 1998, 281, 538 CrossRef CAS.
- L. S. Zhong, J. S. Hu, L. J. Wan and W. G. Song, Chem. Commun., 2008, 1184 RSC.
- X. H. Wang, J.-G. Li, H. Kamiyama, M. Katada, N. Ohashi, Y. Moriyoshi and T. Ishigaki, J. Am. Chem. Soc., 2005, 127, 10982 CrossRef CAS PubMed.
- Y. Q. Wang, H. M. Cheng, Y. Z. Hao, J. M. Ma, W. H. Li and S. M. Cai, J. Mater. Sci., 1999, 34, 3721 CrossRef CAS.
- F. Gracia, J. P. Holgado, A. Caballero and A. R. Gonzalez-Elipe, J. Phys. Chem. B, 2004, 108, 17466 CrossRef CAS.
- M. Liu, X. Q. Qiu, M. Miyauchi and K. Hashimoto, J. Am. Chem. Soc., 2013, 135, 10064 CrossRef CAS PubMed.
- X. M. Zhou, H. C. Yang, C. X. Wang, X. B. Mao, Y. S. Wang, Y. L. Yang and G. Liu, J. Phys. Chem. C, 2010, 114, 17051 CAS.
- F. Cao, W. D. Shi, L. J. Zhao, S. Y. Song, J. H. Yang, Y. Q. Lei and H. J. Zhang, J. Phys. Chem. C, 2008, 112, 17095 CAS.
- X. Xiao, R. P. Hu, C. Liu, C. L. Xing, X. X. Zuo, J. M. Nan and L. Wang, Chem. Eng. J., 2013, 225, 790 CrossRef CAS PubMed.
- G. H. Zhang, X. Zhang, Y. F. Wu, W. D. Shi and W. S. Guan, Micro Nano Lett., 2013, 8, 177 CAS.
- K. Nagaveni, M. S. Hegde and G. Madras, J. Phys. Chem. B, 2004, 108, 20204 CrossRef CAS.
- W. Choi, A. Termin and M. R. Hoffmann, J. Phys. Chem., 1994, 98, 13669 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ce41627e |
| This journal is © The Royal Society of Chemistry 2014 |