Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Germane vs. digermane formation†‡
P.
Steiniger
,
G.
Bendt
,
D.
Bläser
,
C.
Wölper
and
S.
Schulz
*
University of Duisburg-Essen, Universitätsstr. 5-7, S07 S03 C30, 45117 Essen, Germany. E-mail: stephan.schulz@uni-due.de; Fax: +49 201 1833830; Tel: +49 201 1834635
First published on 17th October 2014
Abstract
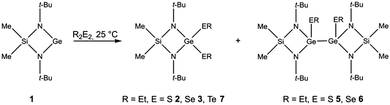
Oxidative addition reactions of dialkylchalcogenanes R2E2 and [Me2Si(Nt-Bu)2]Ge 1 yielded bis(alkylchalcogeno)germanes Me2Si(Nt-Bu)2Ge(ER)2 (R = Et, E = S 2, Se 3; R = Me, E = Se 4) and digermanes [Me2Si(Nt-Bu)2Ge(EEt)]2 (E = S 5, Se 6). The reaction of 1 with Et2Te2 proceeds with formation of Me2Si(Nt-Bu)2Ge(TeEt)27, which slowly converts into the Te-bridged complex [Me2Si(Nt-Bu)2GeTe]28. 1–6 and 8 were characterized by single crystal X-ray diffraction.
Germylenes R2Ge, which have singlet ground states with a low-lying s lone-pair orbital and a higher-lying p orbital,1 have evolved from exotic reaction intermediates to important reagents in organic chemistry.2 They were shown to activate a large variety of bonds including P–Cl,3 O–H,4 Ge–C5 and C–H bonds6 and to react in [2+1]- and [4+1]-cycloaddition reactions with alkenes and alkynes.2,7 Germylenes tend to dimerize to digermenes Ge2R4 or oligomerize into polygermanes, but monomeric R2Ge were kinetically stabilized by bulky organic ligands.8 Lappert's [(Me3Si)2CH]2Ge is monomeric in the gas phase and in solution9,10 and dimeric in the solid state,11 while [(Me3Si)3C]2Ge12 and [(Me3Si)2N]2Ge13 are monomeric in solution and in the solid state. [Me2Si(Nt-Bu)2]Ge 1 is monomeric in solution, while its solid state structure was not reported.14
Germylenes react with elemental chalcogenes with the formation of complexes containing chalcogen-bridges15 or terminal Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E bonds16 as well as polychalcogenides.17 In addition, insertion reactions in E–C bonds18 and E–E bonds (E = S, Se)19 as well as reactions with R3P
E bonds16 as well as polychalcogenides.17 In addition, insertion reactions in E–C bonds18 and E–E bonds (E = S, Se)19 as well as reactions with R3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E20 were reported. Our general interest in the reactivity of complexes with low-valent main group elements21 prompted us to investigate reactions of [Me2Si(Nt-Bu)2]Ge 1 with dialkyldichalcogenanes R2E2, which were recently shown to react with monovalent tin (RSnSnR), antimony (RSb), bismuth (RBi) and zinc complexes (R2Zn2) in oxidative addition reactions.22,23 In addition, we report on the solid state structure of [Me2Si(Nt-Bu)2]Ge 1 (Fig. 1).
E20 were reported. Our general interest in the reactivity of complexes with low-valent main group elements21 prompted us to investigate reactions of [Me2Si(Nt-Bu)2]Ge 1 with dialkyldichalcogenanes R2E2, which were recently shown to react with monovalent tin (RSnSnR), antimony (RSb), bismuth (RBi) and zinc complexes (R2Zn2) in oxidative addition reactions.22,23 In addition, we report on the solid state structure of [Me2Si(Nt-Bu)2]Ge 1 (Fig. 1).
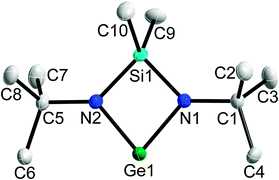 | ||
| Fig. 1 Solid state structure of 1 as determined at 100(1) K. Non-H-atoms shown as thermal ellipsoids at 50% probability levels, H atoms omitted for clarity. | ||
Crystals of 1 were grown in closed quartz glass capillaries under an Ar atmosphere at 100 K (1lt) and 230 K (1ht) using an IR-laser-assisted technique.301 crystallizes in the monoclinic space group P21/n with four molecules in the unit cell. The shortest Ge–Ge distance is 4.158 Å, so 1 is monomeric in the solid state.
Reactions of equimolar amounts of [Me2Si(Nt-Bu)2]Ge 1 and Et2E2 (E = S, Se) at 25 °C yielded two products in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (E = S) and 4
1 (E = S) and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (E = Se) molar ratios as was shown using 1H and 77Se NMR spectroscopy (Scheme 1). The relative intensities of the signals due to the Me2Si(Nt-Bu)2 and the Et groups within each set of resonances in the 1H NMR spectra were 1
1 (E = Se) molar ratios as was shown using 1H and 77Se NMR spectroscopy (Scheme 1). The relative intensities of the signals due to the Me2Si(Nt-Bu)2 and the Et groups within each set of resonances in the 1H NMR spectra were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. In contrast, reactions of 1 with Me2Se2 and Et2Te2 at 25 °C only yielded [Me2Si(Nt-Bu)2]Ge(SeMe)24 and [Me2Si(Nt-Bu)2]Ge(TeEt)27. Fractional crystallisation of the reaction mixtures gave [Me2Si(Nt-Bu)2]Ge(EEt)2 (E = S 2, Se 3), which are the expected products from the insertion reaction of 1 into the E–E bond, and the digermanes [Me2Si(Nt-Bu)2]GeEEt2 (E = S 5, Se 6). Attempts to grow single crystals of 7, which slowly decomposes in solution into [Me2Si(Nt-Bu)2]Ge(μ-Te) 8 and TeEt2 (ESI‡), failed.24
1, respectively. In contrast, reactions of 1 with Me2Se2 and Et2Te2 at 25 °C only yielded [Me2Si(Nt-Bu)2]Ge(SeMe)24 and [Me2Si(Nt-Bu)2]Ge(TeEt)27. Fractional crystallisation of the reaction mixtures gave [Me2Si(Nt-Bu)2]Ge(EEt)2 (E = S 2, Se 3), which are the expected products from the insertion reaction of 1 into the E–E bond, and the digermanes [Me2Si(Nt-Bu)2]GeEEt2 (E = S 5, Se 6). Attempts to grow single crystals of 7, which slowly decomposes in solution into [Me2Si(Nt-Bu)2]Ge(μ-Te) 8 and TeEt2 (ESI‡), failed.24
Single-crystals of 2–6 were obtained from solutions in hexane upon storage at −30 °C, whereas 8 was obtained from a solution of 7 in C6D6 after 72 h (Fig. 2 and 3). The bond lengths and angles within the SiN2Ge ring in 2–6 are almost identical to those of 1 (Table 1). The Ge–E bond lengths agree with the calculated (S 2.24 Å; Se 2.37 Å)25 and experimental values for Ge–E single bonds26 but are longer than those of Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E double bonds (calc: S 2.05 Å; Se 2.18 Å27).2d,15b The Ge–Se bond lengths in [MeC(NCy)2]Ge(SePh)2 (2.3522(5), 2.4009(5) Å) are slightly elongated.19 The Ge–Ge bond lengths (2.4727(6) 5, 2.4921(5) Å 6) are comparable to those observed in digermanes (Table 2).28
E double bonds (calc: S 2.05 Å; Se 2.18 Å27).2d,15b The Ge–Se bond lengths in [MeC(NCy)2]Ge(SePh)2 (2.3522(5), 2.4009(5) Å) are slightly elongated.19 The Ge–Ge bond lengths (2.4727(6) 5, 2.4921(5) Å 6) are comparable to those observed in digermanes (Table 2).28
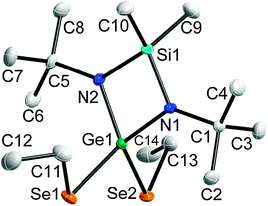 | ||
| Fig. 2 Solid state structure of 3. Non-H-atoms shown as thermal ellipsoids at 50% probability levels, H atoms omitted for clarity. | ||
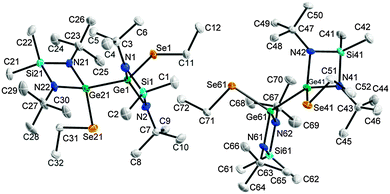 | ||
| Fig. 3 Solid state structure of 6 (2 independent molecules). Non-H-atoms shown as thermal ellipsoids at 50% probability levels, H atoms omitted for clarity. | ||
| 1lt | 2 | 3 | 4 | |
|---|---|---|---|---|
| a Special position. Equal values are symmetry equivalent and labeling may differ. b Structural parameters of the “ht” version of 1 are almost identical (ESI). | ||||
| Ge(1)–N(1) | 1.8574(13) | 1.8400(7) | 1.8444(19) | 1.8451(9) |
| Ge(1)–N(2) | 1.8584(13) | 1.8410(7) | 1.8411(18) | 1.8451(9) |
| Ge(1)–E(1) | — | 2.2072(2) | 2.3355(4) | 2.3439(2) |
| Ge(1)–E(2) | — | 2.2070(2) | 2.3371(4) | 2.3439(2) |
| N(1)–Ge(1)–N(2) | 81.33(6) | 82.93(3) | 82.74(8) | 82.89(6) |
| E(1)–Ge(1)–E(2) | — | 101.15(1) | 102.05(2) | 102.53(1) |
| N(1)–Si(1)–N(2) | 88.82(6) | 88.70(3) | 88.80(9) | 89.00(6) |
| 5 | 6 | |
|---|---|---|
| Ge(1)–N(1) | 1.855(3) | 1.846(3) |
| Ge(1)–N(2) | 1.845(4) | 1.851(3) |
| Ge(1)–E(1) | 2.2271(13) | 2.3700(5) |
| Ge(1)–Ge(2) | 2.4727(6) | 2.4921(5) |
| N(1)–Ge(1)–N(2) | 82.05(16) | 81.75(13) |
| N(1)–Si(1)–N(2) | 88.69(17) | 88.40(14) |
The ratio of the Ge(III) and Ge(IV) species formed in the reactions with Et2E2 (E = S, Se) depends on the reaction temperature and the molar ratio of the starting reagents. 2 and 3 were formed in equimolar ratios at 70 °C. 5 was formed in 36% yield together with 2 in the reaction of 1 and Et2S2 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio at −30 °C, while 6 was obtained in less than 25% yield together with 3. Equimolar amounts of 1 and Et2S2 reacted at −30 °C to 2 and 5, while the reaction of 1 with 0.5 equivalents of Et2S2 at 70 °C gave 2 and unreacted 1. The reaction of 5 with Et2S2 failed to give 2 even after heating to 70 °C for 1 h, hence proving that 5 is no reaction intermediate in the formation of 2 (Fig. S22, ESI‡) In contrast, 5 was quantitatively converted at 100 °C in solution into 1 and 2 by disproportionation reaction (Fig. S11, ESI‡).
1 molar ratio at −30 °C, while 6 was obtained in less than 25% yield together with 3. Equimolar amounts of 1 and Et2S2 reacted at −30 °C to 2 and 5, while the reaction of 1 with 0.5 equivalents of Et2S2 at 70 °C gave 2 and unreacted 1. The reaction of 5 with Et2S2 failed to give 2 even after heating to 70 °C for 1 h, hence proving that 5 is no reaction intermediate in the formation of 2 (Fig. S22, ESI‡) In contrast, 5 was quantitatively converted at 100 °C in solution into 1 and 2 by disproportionation reaction (Fig. S11, ESI‡).
The formation of digermanes 5 and 6 is without precedence in germylene chemistry. 1H NMR spectroscopy studies on the reactions of 1 with isolated 2 and 3 did not show the formation of 5 and 6, hence excluding the formation of 5 and 6 by insertion of the germylene [Me2Si(Nt-Bu)2]Ge 1 into the Ge–E bond of initially formed 2 and 3. Since digermenes R2Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) GeR2 are known to react with water, alcohols, carboxylic acids, CCl4, CHCl3 or HN3 in a 1,2 fashion with the formation of the corresponding digermanes,291H NMR spectra were recorded at −60 °C and +60 °C to investigate whether 1 formed a temperature-dependent germylene–digermene equilibrium in solution. However, only a single set of resonances was observed. In addition, reactions of 1 with 2-methylbutadiene, a trapping agent for transient and stable germylenes and digermenes,31 at 25 °C and −60 °C only yielded the germylene products (germacyclopentene) as was reported previously,32 whereas no sign of the digermane reaction products was observed. A possible explanation for the formation of 5 and 6 is the presence of a loosely bound dimer in solution, held together by weak dispersion forces. Computational studies are currently being performed in order to address this hypothesis.
GeR2 are known to react with water, alcohols, carboxylic acids, CCl4, CHCl3 or HN3 in a 1,2 fashion with the formation of the corresponding digermanes,291H NMR spectra were recorded at −60 °C and +60 °C to investigate whether 1 formed a temperature-dependent germylene–digermene equilibrium in solution. However, only a single set of resonances was observed. In addition, reactions of 1 with 2-methylbutadiene, a trapping agent for transient and stable germylenes and digermenes,31 at 25 °C and −60 °C only yielded the germylene products (germacyclopentene) as was reported previously,32 whereas no sign of the digermane reaction products was observed. A possible explanation for the formation of 5 and 6 is the presence of a loosely bound dimer in solution, held together by weak dispersion forces. Computational studies are currently being performed in order to address this hypothesis.
Oxidative addition reactions of dichalcogenanes to germylene 1 at elevated temperature yielded the expected Ge(IV) species, whereas the reactions at low temperatures proceeded with the predominant formation of digermanes 5 and 6, in which the Ge atoms adopted the formal oxidation state of +III. These findings indicate that 1 forms a loosely bound dimer in solution.
Notes and references
- (a) C. Boehme and G. Frenking, J. Am. Chem. Soc., 1996, 118, 2039 CrossRef CAS; (b) M. Z. Kassaee, S. Soleimani-Amiri, M. Ghambarian, F. Boazar and E. Motamedi, THEOCHEM, 2008, 849, 37 CrossRef CAS PubMed; (c) A. Bundhun, P. Ramasami and H. F. Schaefer III, J. Phys. Chem. A, 2009, 113, 8080 CrossRef CAS PubMed.
- (a) W. P. Neumann, Chem. Rev., 1991, 91, 311 CrossRef CAS; (b) N. Tokitoh and R. Okazaki, Coord. Chem. Rev., 2000, 210, 251 CrossRef CAS; (c) O. Kühl, Coord. Chem. Rev., 2004, 248, 411 CrossRef PubMed; (d) S. Nagendran and H. W. Roesky, Organometallics, 2008, 27, 457 CrossRef CAS; (e) Y. Mizuhata, T. Sasamori and N. Tokitoh, Chem. Rev., 2009, 109, 3479 CrossRef CAS PubMed.
- (a) M. Veith, M. Grosser and V. Huch, Z. Anorg. Allg. Chem., 1984, 513, 89 CrossRef CAS; (b) J. K. West and L. Stahl, Organometallics, 2012, 31, 2042 CrossRef CAS.
- R. D. Sweeder, K. A. Miller, F. A. Edwards, J. Wang, M. M. Banaszak Holl and J. W. Kampf, Organometallics, 2003, 22, 5054 CrossRef CAS.
- P. Bleckmann, R. Minkwitz, W. P. Neumann, M. Schriewer, M. Thibud and B. Watta, Tetrahedron Lett., 1984, 25, 2467 CrossRef CAS.
- (a) L. Lange, B. Meyer and W.-W. du Mont, J. Organomet. Chem., 1987, 329, C17 CrossRef CAS; (b) P. Jutzi, H. Schmidt, B. Neumann and H.-G. Stammler, Organometallics, 1996, 15, 741 CrossRef CAS; (c) B. Gehrhus, P. Hitchcock and M. F. Lappert, Angew. Chem., Int. Ed., 1997, 36, 2514 CrossRef CAS; (d) R. H. Walker, K. A. Miller, S. L. Scott, Z. T. Cygan, J. M. Bartolin, J. W. Kampf and M. M. Banaszak Holl, Organometallics, 2009, 28, 2744 CrossRef CAS.
- M. Schriewer and W. P. Neumann, J. Am. Chem. Soc., 1983, 105, 897 CrossRef CAS.
- M. Weidenbruch, Eur. J. Inorg. Chem., 1999, 373 CrossRef CAS.
- T. Fjeldberg, A. Haaland, B. E. R. Schilling, M. F. Lappert and A. J. Thorne, J. Chem. Soc., Dalton Trans., 1986, 1551 RSC.
- P. J. Davidson, D. H. Harris and M. F. Lappert, J. Chem. Soc., Dalton Trans., 1976, 2268 RSC.
- P. B. Hitchcock, M. F. Lappert, S. J. Miles and A. J. Thorne, J. Chem. Soc., Chem. Commun., 1984, 480 RSC.
- P. Jutzi, A. Becker, H. G. Stammler and B. Neumann, Organometallics, 1991, 10, 1647 CrossRef CAS.
- (a) D. H. Harris and M. F. Lappert, J. Chem. Soc., Chem. Commun., 1974, 895 RSC; (b) R. W. Chorley, P. B. Hitchcock, M. F. Lappert and W.-P. Leung, Inorg. Chim. Acta, 1992, 198, 203 CrossRef.
- M. Veith and M. Grosser, Z. Naturforsch., B: J. Chem. Sci., 1982, 37, 1375 Search PubMed.
- (a) G. Pfister-Guillouzo and C. Guimon, Phosphorus and Sulphur, 1985, 23, 197 CAS; (b) P. B. Hitchcock, H. A. Jasim, M. F. Lappert, W.-P. Leung, A. K. Rai and R. E. Taylor, Polyhedron, 1991, 10, 1203 CrossRef CAS; (c) D. Ellis, P. B. Hitchcock and M. F. Lappert, J. Chem. Soc., Dalton Trans., 1992, 3397 RSC.
- (a) N. Tokitoh, T. Matsumoto, K. Manmaru and R. Okazaki, J. Am. Chem. Soc., 1993, 115, 8855 CrossRef CAS; (b) T. Matsumoto, N. Tokitoh and R. Okazaki, Angew. Chem., Int. Ed. Engl., 1994, 33, 2316 CrossRef; (c) N. Tokitoh, T. Matsumoto and R. Okazaki, J. Am. Chem. Soc., 1997, 119, 2337 CrossRef CAS; (d) R. K. Siwatch, D. Yadav, G. Mukherjee, G. Rajaraman and S. Nagendran, Inorg. Chem., 2014, 53, 5073 CrossRef CAS PubMed.
- (a) N. Tokitoh, T. Matsumoto and R. Okazaki, Tetrahedron Lett., 1992, 33, 2531 CrossRef CAS; (b) T. Matsumoto, N. Tokitoh and R. Okazaki, Organometallics, 1995, 14, 1008 CrossRef CAS.
- T. Chen, W. Hunks, P. S. Chen, G. T. Stauf, T. M. Cameron, C. Xu, A. G. DiPasquale and A. L. Rheingold, Eur. J. Inorg. Chem., 2009, 2047 CrossRef CAS.
- S. R. Foley, G. P. A. Yap and D. S. Richeson, J. Chem. Soc., Dalton Trans., 2000, 1663 RSC.
- S. R. Foley and D. S. Richeson, J. Chem. Soc., Chem. Commun., 2000, 1391 RSC.
- S. Schulz, Chem. – Eur. J., 2010, 16, 6416 CrossRef CAS PubMed.
- (a) M. Wagner, C. Dietz, M. Bouška, L. Dostál, Z. Padělková, R. Jambor and K. Jurkschat, Organometallics, 2013, 32, 4973 CrossRef CAS; (b) P. Simon, R. Jambor, A. Růžička and L. Dostál, Organometallics, 2013, 32, 239 CrossRef CAS; (c) P. Simon, R. Jambor, A. Růžička and L. Dostál, J. Organomet. Chem., 2013, 740, 98 CrossRef CAS PubMed.
- S. Gondzik, S. Schulz, D. Bläser and C. Wölper, J. Chem. Soc., Chem. Commun., 2014, 50, 1189 RSC.
- M. Veith, M. Notzel, L. Stahl and V. Huch, Z. Anorg. Allg. Chem., 1994, 620, 1264 CrossRef CAS.
- P. Pyykkö and M. Atsumi, Chem. – Eur. J., 2009, 15, 186 CrossRef PubMed.
- CCDC search: Ge
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S cn(S) = 1, hits (bond(s) 95(198), range 2.05–2.387, mean 2.146(52)); Ge–S–Ge hits (bond(s) 113(586), range 2.11–2.472, mean 2.238(38)); Ge–S–C cn(S) = 2, hits (bond(s) 107(246), range 2.115–2.412, mean 2.241(43)); Ge
S cn(S) = 1, hits (bond(s) 95(198), range 2.05–2.387, mean 2.146(52)); Ge–S–Ge hits (bond(s) 113(586), range 2.11–2.472, mean 2.238(38)); Ge–S–C cn(S) = 2, hits (bond(s) 107(246), range 2.115–2.412, mean 2.241(43)); Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se cn(S(e)) = 1, hits (bond(s) 63(117), range 2.172–2.426, mean 2.271(54)); Ge–Se–Ge, hits (bond(s) 49(302), 2.304–2.459, mean 2.374(24)); Ge–Se–C cn(S(e)) = 2, hits (bond(s) 20(40), 2.314–2.511, mean 2.379(51)).
Se cn(S(e)) = 1, hits (bond(s) 63(117), range 2.172–2.426, mean 2.271(54)); Ge–Se–Ge, hits (bond(s) 49(302), 2.304–2.459, mean 2.374(24)); Ge–Se–C cn(S(e)) = 2, hits (bond(s) 20(40), 2.314–2.511, mean 2.379(51)). - P. Pyykkö and M. Atsumi, Chem. – Eur. J., 2009, 15, 12770 CrossRef PubMed.
- (a) M. Hölbling, S. L. Masters, M. Flock, J. Baumgartner, K. Hassler, H. E. Robertson and D. A. Wann, Inorg. Chem., 2008, 47, 3023 CrossRef PubMed; (b) K. V. Zaitsev, A. A. Kapranov, A. V. Churakov, O. Kh. Poleshchuk, Y. F. Oprunenko, B. N. Tarasevich, G. S. Zaitseva and S. S. Karlov, Organometallics, 2013, 32, 6500 CrossRef CAS.
- (a) B. Pampuch, W. Saak and M. Weidenbruch, J. Organomet. Chem., 2006, 691, 3540 CrossRef CAS PubMed; (b) W. J. Leigh, F. Lollmahomed, C. R. Harrington and J. M. McDonald, Organometallics, 2006, 25, 5424 CrossRef CAS; (c) L. A. Huck and W. J. Leigh, Organometallics, 2007, 26, 1339 CrossRef CAS; (d) K. L. Hurni, P. A. Rupar, N. C. Payne and K. M. Baines, Organometallics, 2007, 26, 5569 CrossRef CAS; (e) K. Mochida, T. Kayamori, M. Wakasa, H. Hayashi and M. P. Egorov, Organometallics, 2000, 19, 3379 CrossRef CAS; (f) M. S. Samuel, M. C. Jennings and K. M. Baines, Organometallics, 2001, 20, 590 CrossRef CAS; (g) H. Schäfer, W. Saak and M. Weidenbruch, J. Organomet. Chem., 2000, 604, 211 CrossRef.
- R. Boese and M. Nussbaumer, in situ Crystallisation Techniques, in Organic Crystal Chemistry, ed. D. W. Jones, Oxford University Press, Oxford, England, 1994, p. 20 Search PubMed.
- (a) N. Tokitoh and W. Ando, in Reactive Intermediate Chemistry, ed. R. A. Moss, M. S. Platz and M. Jones, Jr., Wiley-Interscience, New York, 2004, pp. 651–715 Search PubMed; (b) W. P. Neumann, Chem. Rev., 1991, 91, 311 CrossRef CAS; (c) M. Nag and P. P. Gaspar, Organometallics, 2009, 28, 5612 CrossRef CAS; (d) K. Mochida, T. Kayamori, M. Wakasa, H. Hayashi and M. P. Egorov, Organometallics, 2000, 19, 3379 CrossRef CAS.
- M. Veith, A. Rammo, S. Faber and B. Schillo, Pure Appl. Chem., 1999, 71, 401 CrossRef CAS.
Footnotes |
| † Dedicated to Prof. H. J. Frohn on the occasion of his 70th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental details, NMR spectra of 2–8; bond lengths and angles of 1–6. CCDC 1009920 (1lt), 1009921 (1ht), 1009925 (2), 1009924 (3), 1009923 (4), 1009922 (5), 1019789 (6) and 1010028 (8). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc07921c |
| This journal is © The Royal Society of Chemistry 2014 |