Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Zirconium complexes of bipyrrolidine derived salan ligands for the isoselective polymerisation of rac-lactide†
Matthew D.
Jones
*a,
Stuart L.
Hancock
a,
Paul
McKeown
ab,
Pascal M.
Schäfer
a,
Antoine
Buchard
a,
Lynne H.
Thomas
a,
Mary F.
Mahon
*c and
John P.
Lowe
a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: mj205@bath.ac.uk; Fax: +44 (0)1225 386231; Tel: +44 (0)1225 384908
bDoctoral Training Centre in Sustainable Chemical Technologies, University of Bath, Bath BA2 7AY, UK
cBath Chemical Crystallography Unit, Department of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: m.f.mahon@bath.ac.uk
First published on 4th November 2014
Abstract
Herein we report the synthesis and characterisation of a series of Zr(IV) 2,2′-bipyrrolidine–salan derived complexes and their exploitation for the ring opening polymerisation of rac-lactide to afford highly isotactically enriched polymers.
Poly(lactic acid) is unequivocally a success story in modern sustainable chemistry.1 This is in no small part due to the desirable properties of the resultant polymer-namely biodegradability and the fact that the monomer is sourced from annually renewable raw materials.2 Poly(lactic acid) (PLA) is currently commercially produced using tin(II) octanoate as the initiator. However, there is a desire to prepare new initiators that are faster and able to control the microstructure of the resultant polymer.3 The stereochemistry of PLA dramatically affects the properties of the material-for example isotactic PLLA (or PDLA) has a melting point of ca. 170 °C, moreover for stereoblock/gradient PLA with isotactic sequences of L- and D-lactide the Tm increases to 180–220 °C.4 There are many impressive examples in the literature reporting the stereoselective polymerisation of rac-lactide (rac-LA).3,5 Compared to the number of initiators that are active for the production of heterotactic PLA, isoselective initiators are rare. The metal centres that are typically used for the production of isotactic PLA from rac-LA are Zn(II),6 Al(III)5c,7 and Y(III).3,8 Du and co-workers have recently reported a series of Zn(II)–amido-oxazolinate complexes for the ring opening polymerisation (ROP) of rac-LA, which afforded high isoselectivity in toluene at 50 °C. Full conversion was achieved after 30 min with a Pm (probability of isotactic enchainment) up to 0.8.6 When the temperature was lowered to 23 °C this could be increased to 0.91, but full conversion was only reached in 44 hrs at this temperature. Williams has recently shown that Yttrium phosphasalen complexes can produce PLA with a Pm of up to 0.84, very rapidly.3 Examples of Al(III) include the early work of Spassky, Feijen and Nomura with salen derived complexes.7a–d Whilst there have been many elegant examples of the utility of group 4 complexes for the ROP of rac-LA, the vast majority produce atactic or heterotactic PLA.9 However, Davidson and co-workers have shown that C2-symmetric Zr(IV) amine-bis(phenolates) are capable of producing moderate isotacticity (Pm = 0.7).10 More recently Kol and Okuda have prepared a series of Zr(IV) complexes with ONSO ligands, which depending upon the flexibility of the backbone, produce either heterotactically (Pr up to 0.87) or isotactically inclined PLA (Pm up to 0.67).11 Group 4 initiators have the added advantage of being easy to prepare, relatively moisture stable and can be trialled under the industrially preferred melt conditions.
In this paper we report the preparation of three Zr(IV) complexes based on a 2,2′-bispyrrolidine and their exploitation in the ROP of rac-LA. Examples based on such ligands are rare in the literature12 and this is only the second example of the use of this meso-ligand in catalysis13 (Scheme 1).
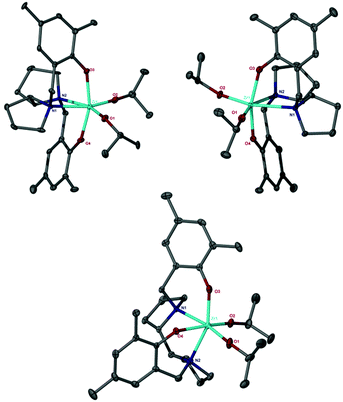
The ligands were prepared by modified Mannich reactions involving either enantiopure (R,R or S,S) or meso variants of 2,2′-bispyrrolidine.12c All ligands have been characterised by NMR spectroscopy, HR-MS and the solid-state structure has been determined for the meso ligand (3H2); see ESI† for full details. All Zr(IV) complexes were characterised by single crystal X-ray diffraction. The metric data for the complexes are similar and are in agreement with literature reported salan complexes.10 The R,R and S,S ligands coordinate to Zr(IV) in a fac–fac fashion with the R,R enantiomer forming the Δ-isomer and the S,S enantiomer the Λ-isomer exclusively in the solid-state (Fig. 1). Zr(1)(OiPr)2 crystallises in the chiral space group P41 whereas Zr(3)(OiPr)2 in P43. This is in agreement with Kol and co-workers for related Ti(IV) complexes where the ligands lead to a predetermined chirality at the metal centre.14 The 1H NMR spectra of complexes Zr(1/2)(OiPr)2 were indicative of the solid-state structure being maintained in solution, see ESI.† The ligands are locked in position as indicated by discrete doublets for the methylene CH2 moieties, furthermore there are no exchange peaks observed in the NOESY/EXSY spectrum at 298 K (CDCl3).
In the solid state Zr(3)(OiPr)2 crystallises in the monoclinic P21/c space group, with both enantiomers (the Λ and Δ forms) present, arbitrarily the Δ-isomer is shown in Fig. 1. Compared to the chiral ligands a different coordination mode of the salan (fac–mer) was observed, this is presumably due to the syn relationship of the hydrogen atoms pertaining to the C–C bond between the five membered rings (cf. antiperiplanar for the chiral complexes). When this complex was recrystallised from toluene, the room temperature solution state NMR spectrum (in either CDCl3 or d8-THF) was complicated, with clearly more than one species present in solution. The ground state energies of various isomers have been studied via DFT methods.† Unsurprisingly the observed structure is the most thermodynamically stable, however the trans isomer is only 8.3 kcal mol−1 higher. Fortunately, when Zr(3)(OiPr)2 was recrystallised in hexane the product gave an NMR spectrum that was consistent with the fac–mer isomer, with discrete doublets for the methylene CH2 moieties being observed along with two observed resonances for the methine isopropoxides and four resonances in the aryl region. Room temperature NOESY/EXSY measurements showed the presence of exchange peaks, indicating that in solution an equilibrium exists which potentially is Δ-isomer ⇔ Λ-isomer.† Presumably, these enantiomers are interconverting in solution at room temperature giving rise to the exchange peaks, which were absent in the chiral complexes Zr(1/2)(OiPr)2.
The majority of examples of the polymerisation of rac-LA using group 4 initiators use sublimation methods to purify the monomer. In this study we have simply used recrystallised monomer to mimic more industrially relevant conditions (entries 4 and 11 use sublimed monomer as a comparison). The selectivity and dispersity appear to be relatively similar for the sublimed monomer compared to recrystallised, although similar conversions are achieved in a slightly shorter time frame for the sublimed monomer. The solution polymerisations of rac-LA are incredibly well controlled with low dispersities obtained. For Zr(3)(OiPr)2 in the melt (entries 3 and 4) the dispersity of the polymer is slightly higher indicating a degree of transesterification.
Interestingly, the Zr(3)(OiPr)2 yielded PLA with a high isotactic bias (Pm = 0.86 in solution), which to the best of our knowledge is the highest reported isotactic initiator in the literature to date utilising a group 4 initiator. Analysis of this highly isotactic polymer prepared in solution via DSC showed there to be a major endothermic peak at 190 °C, indicative of stereoblock isotactic PLA.4b Moreover, in the case of Zr(3)(OiPr)2 the sis tetrad is significantly smaller than the sii, iis and isi tetrads indicating isotactic PLA of a blocky nature.5g,15 Furthermore, the MALDI-ToF analysis has a repeat unit of 144 g mol−1, this coupled with the low Mw/Mn (1.05) indicates a controlled polymerisation with little transesterification occurring. The expected H- and -OiPr end groups from the coordination insertion mechanism were also observed. The solution state kinetics of the polymerisation were investigated with Zr(3)(OiPr)2 at room temperature in CDCl3 (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 LA
1 LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Init), for rac-LA kapp = 4.3 × 10−3 min−1 was observed, Zr(3)(OiPr)2 polymerises L-LA significantly faster with a kapp = 5.9 × 10−3 min−1, Fig. 2. Both Zr(1–2)(OiPr)2 complexes were inactive for the solution state polymerisation of rac-LA in CDCl3 at room temperature. However, the chiral complexes were active at 70 °C in toluene – with ca. 50% conversion being achieved after 4 and 8 hours respectively, with a strong isotactic bias observed and Tm = 178 °C, from DSC (Table 1, entry 5) and Tm = 176 °C, from DSC (Table 1, entry 7). In the case of Zr(1–2)(OiPr)2 the mechanism of polymerisation is presumably enantiomorphic site control. There does appear to be a slight difference in selectivity with reaction time (entries 5 vs. 7 and 6 vs. 8), at low conversion (ca. 10%) Pm = 0.75 similar to the 4 h run (entries 5 and 6). Only ca. 50% conversion of rac-LA could be achieved in solution with the chiral complexes compared to 85% with the meso complex in the same timeframe (N.B. the Zr(3)(OiPr)2 test was at 20 °C cf. 70 °C for Zr(1/2)(OiPr)2). The polymerisation was investigated further with L-LA, where Λ-Zr(2)(OiPr)2 was active (kapp = 4.1 × 10−4 min−1 after 37 h, conversion to PLLA = 58% Mn = 6900, Mw/Mn = 1.07) and Δ-Zr(1)(OiPr)2 was slow (kapp = 0.97 × 10−4 min−1 after 37 hrs, conversion to PLLA = 18%) Fig. 3.16
Init), for rac-LA kapp = 4.3 × 10−3 min−1 was observed, Zr(3)(OiPr)2 polymerises L-LA significantly faster with a kapp = 5.9 × 10−3 min−1, Fig. 2. Both Zr(1–2)(OiPr)2 complexes were inactive for the solution state polymerisation of rac-LA in CDCl3 at room temperature. However, the chiral complexes were active at 70 °C in toluene – with ca. 50% conversion being achieved after 4 and 8 hours respectively, with a strong isotactic bias observed and Tm = 178 °C, from DSC (Table 1, entry 5) and Tm = 176 °C, from DSC (Table 1, entry 7). In the case of Zr(1–2)(OiPr)2 the mechanism of polymerisation is presumably enantiomorphic site control. There does appear to be a slight difference in selectivity with reaction time (entries 5 vs. 7 and 6 vs. 8), at low conversion (ca. 10%) Pm = 0.75 similar to the 4 h run (entries 5 and 6). Only ca. 50% conversion of rac-LA could be achieved in solution with the chiral complexes compared to 85% with the meso complex in the same timeframe (N.B. the Zr(3)(OiPr)2 test was at 20 °C cf. 70 °C for Zr(1/2)(OiPr)2). The polymerisation was investigated further with L-LA, where Λ-Zr(2)(OiPr)2 was active (kapp = 4.1 × 10−4 min−1 after 37 h, conversion to PLLA = 58% Mn = 6900, Mw/Mn = 1.07) and Δ-Zr(1)(OiPr)2 was slow (kapp = 0.97 × 10−4 min−1 after 37 hrs, conversion to PLLA = 18%) Fig. 3.16
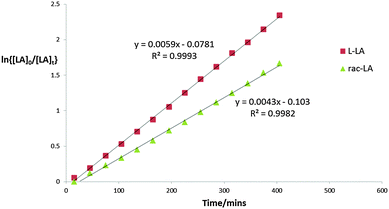 | ||
Fig. 2 Semi-logarithmic plots of the polymerisation of L-LA and rac-LA ([LA]0 = 0.56 mol dm−3) [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Init] = 100 [Init] = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Init = Zr(3)(OiPr)2. 1, Init = Zr(3)(OiPr)2. | ||
| Entry | Initiator | Time (h) | Conv.d (%) | M n | M w /M n | P m |
|---|---|---|---|---|---|---|
| a Conditions: [M]/[I] = 300, 130 °C, solvent free. b Conditions: [M]/[I] = 100, toluene, T = 70 °C. c Conditions: [M]/[I] = 100, CDCl3, T = 20 °C. d As determined via1H NMR spectroscopy. e Determined from GPC (in THF) referenced to polystyrene. f P m is the probability of isotactic enchainment, calculated from the 1H homonuclear decoupled NMR spectra. N.B. entries 4 and 11 are using sublimed lactide as a comparison, all others use recrystallised lactide. | ||||||
| 1 | Zr(1)(OiPr)2a | 0.17 | 55 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1.13 | 0.70 |
| 2 | Zr(2)(OiPr)2a | 0.17 | 45 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
1.09 | 0.71 |
| 3 | Zr(3)(OiPr)2a | 0.17 | 90 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.21 | 0.66 |
| 4 | Zr(3)(OiPr)2a | 0.1 | 89 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
1.17 | 0.65 |
| 5 | Zr(1)(OiPr)2b | 4 | 48 | 6200 | 1.08 | 0.75 |
| 6 | Zr(2)(OiPr)2b | 4 | 51 | 7250 | 1.07 | 0.75 |
| 7 | Zr(1)(OiPr)2b | 8 | 54 | 8000 | 1.07 | 0.80 |
| 8 | Zr(2)(OiPr)2b | 8 | 60 | 9600 | 1.06 | 0.80 |
| 9 | Zr(3)(OiPr)2c | 8 | 85 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.05 | 0.86 |
| 10 | Zr(3)(OiPr)2c | 4 | 45 | 7650 | 1.05 | 0.85 |
| 11 | Zr(3)(OiPr)2c | 6 | 79 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.04 | 0.84 |
| 12 | Zr(3)(OiPr)2b | 8 | 94 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.22 | 0.75 |
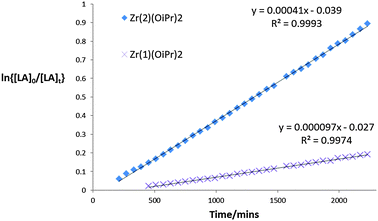 | ||
Fig. 3 Semi-logarithmic plots of the polymerisation of L-LA ([LA]0 = 0.56 mol dm−3) [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Init] = 100 [Init] = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Init = Zr(1 or 2)(OiPr)2. 1, Init = Zr(1 or 2)(OiPr)2. | ||
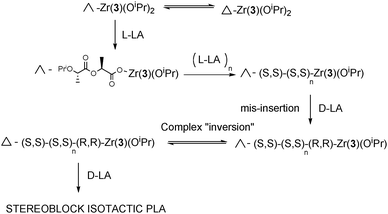
Both Zr(1/2)(OiPr)2 are “locked’, in solution as single enantiomers (Δ-Zr(1)(OiPr)2 and Λ-Zr(2)(OiPr)2 respectively). The data clearly show a strong preference for Λ-Zr(2)(OiPr)2 to specifically polymerise L-LA compared to Δ-Zr(1)(OiPr)2. It is thus hypothesised if we consider the polymerisation of rac-LA with the chiral complex Δ-Zr(1) when an L-LA inserts a significantly less active catalytic species will form (the same is true when D-LA inserts into Δ-Zr(2)). Essentially this is an enantiomorphic site controlled mechanism. As discussed previously Zr(3)(OiPr)2 is fluxional in solution, Δ-Zr(3)(OiPr)2 ⇔ Λ-Zr(3)(OiPr)2, and initially the chirality of Δ/Λ-Zr(3)(OiPr)2 controls which monomer is inserted in a site controlled manner (Δ → D-LA, Λ → L-LA). However, we tentatively suggest that if the “wrong” insertion occurs the complex can convert to the other form and now the chirality of the metal and chain end complement each other again, Scheme 2, and propagation continues leading to stereoblock isotactic PLA. It is also interesting to note that the rate of ROP of L-lactide with Zr(3)(OiPr)2 is significantly faster than that of Λ-Zr(2)(OiPr)2, this may well be due to the difference in coordination of the ligand around the Zr(IV) centre.
Work is currently on-going to investigate the mechanism in more detail, the applicability of the initiators with other cyclic esters and for co-polymer formation. Encouragingly, Zr(3)(OiPr)2 is active for the ROP of rac-butyrolactone ([M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Init] 300
[Init] 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, T = 80 °C solvent free; conversion = 75%, Mn = 19
1, T = 80 °C solvent free; conversion = 75%, Mn = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Mw/Mn = 1.10 after 4 hours to produce atactic polymer).
500, Mw/Mn = 1.10 after 4 hours to produce atactic polymer).
Zirconium(IV) complexes have been prepared and screened for the polymerisation of rac-LA. Stereoblock isotactic PLA is prepared with the meso salan complex Zr(3)(OiPr)2, which is significantly faster than either of the chiral complexes Zr(1/2)(OiPr)2. It is hypothesised that this reflects the fluxionality of Zr(3)(OiPr)2. We wish to thank the EPSRC (EP/G03768X/1), the EPSRC UK National Service for Computational Chemistry Software (CHEM752), Corbion for lactide and support of the CDT at Bath and the University of Bath for funding.
Notes and references
- (a) R. Auras, B. Harte and S. Selke, Macromol. Biosci., 2004, 4, 835–864 CrossRef CAS PubMed; (b) R. J. Pounder and A. P. Dove, Polym. Chem., 2010, 1, 260–271 RSC; (c) M. Vert, Biomacromolecules, 2005, 6, 538–546 CrossRef CAS PubMed.
- S. Slomkowski, S. Penczek and A. Duda, Polym. Adv. Technol., 2014, 25, 436–447 CrossRef CAS.
- C. Bakewell, C. Thi-Phuong-Anh, N. Long, X. F. Le Goff, A. Auffrant and C. K. Williams, J. Am. Chem. Soc., 2012, 134, 20577–20580 CrossRef CAS PubMed.
- (a) M. J. Stanford and A. P. Dove, Chem. Soc. Rev., 2010, 39, 486–494 RSC; (b) K. Fukushima and Y. Kimura, Polym. Int., 2006, 55, 626–642 CrossRef CAS.
- (a) D. C. Aluthge, B. O. Patrick and P. Mehrkhodavandi, Chem. Commun., 2013, 49, 4295–4297 RSC; (b) B. Gao, R. Duan, X. Pang, X. Li, Z. Qu, Z. Tang, X. Zhuang and X. Chen, Organometallics, 2013, 32, 5435–5444 CrossRef CAS; (c) P. Hormnirun, E. L. Marshall, V. C. Gibson, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 2004, 126, 2688–2689 CrossRef CAS PubMed; (d) N. Nomura, R. Ishii, M. Akakura and K. Aoi, J. Am. Chem. Soc., 2002, 124, 5938–5939 CrossRef CAS PubMed; (e) T. M. Ovitt and G. W. Coates, J. Am. Chem. Soc., 1999, 121, 4072–4073 CrossRef CAS; (f) A. Pilone, K. Press, I. Goldberg, M. Kol, M. Mazzeo and M. Lamberti, J. Am. Chem. Soc., 2014, 136, 2940–2943 CrossRef CAS PubMed; (g) H. Wang and H. Ma, Chem. Commun., 2013, 49, 8686–8688 RSC; (h) Z. Y. Zhong, P. J. Dijkstra and J. Feijen, J. Am. Chem. Soc., 2003, 125, 11291–11298 CrossRef CAS PubMed.
- S. Abbina and G. Du, ACS Macro Lett., 2014, 3, 689–692 CrossRef CAS PubMed.
- (a) G. Montaudo, M. S. Montaudo, C. Puglisi, F. Samperi, N. Spassky, A. LeBorgne and M. Wisniewski, Macromolecules, 1996, 29, 6461–6465 CrossRef CAS; (b) N. Nomura, R. Ishii, Y. Yamamoto and T. Kondo, Chem. – Eur. J., 2007, 13, 4433–4451 CrossRef CAS PubMed; (c) N. Spassky, M. Wisniewski, C. Pluta and A. LeBorgne, Macromol. Chem. Phys., 1996, 197, 2627–2637 CrossRef CAS; (d) M. Wisniewski, A. LeBorgne and N. Spassky, Macromol. Chem. Phys., 1997, 198, 1227–1238 CrossRef CAS; (e) P. Hormnirun, E. L. Marshall, V. C. Gibson, R. I. Pugh and A. J. P. White, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15343–15348 CrossRef CAS PubMed.
- (a) C. Bakewell, R. H. Platel, S. K. Cary, S. M. Hubbard, J. M. Roaf, A. C. Levine, A. J. P. White, N. J. Long, M. Haaf and C. K. Williams, Organometallics, 2012, 31, 4729–4736 CrossRef CAS; (b) A. Alaaeddine, C. M. Thomas, T. Roisnel and J.-F. Carpentier, Organometallics, 2009, 28, 1469–1475 CrossRef CAS; (c) R. Heck, E. Schulz, J. Collin and J.-F. Carpentier, J. Mol. Catal. A: Chem., 2007, 268, 163–168 CrossRef CAS.
- (a) A. Sauer, A. Kapelski, C. Fliedel, S. Dagorne, M. Kol and J. Okuda, Dalton Trans., 2013, 42, 9007–9023 RSC; (b) A. L. Zelikoff, J. Kopilov, I. Goldberg, G. W. Coates and M. Kol, Chem. Commun., 2009, 6804–6806 RSC; (c) A. J. Chmura, M. G. Davidson, C. J. Frankis, M. D. Jones and M. D. Lunn, Chem. Commun., 2008, 1293–1295 RSC.
- A. J. Chmura, M. G. Davidson, M. D. Jones, M. D. Lunn, M. F. Mahon, A. F. Johnson, P. Khunkamchoo, S. L. Roberts and S. S. F. Wong, Macromolecules, 2006, 39, 7250–7257 CrossRef CAS.
- (a) J.-C. Buffet, A. N. Martin, M. Kol and J. Okuda, Polym. Chem., 2011, 2, 2378–2384 RSC; (b) A. Stopper, J. Okuda and M. Kol, Macromolecules, 2012, 45, 698–704 CrossRef CAS; (c) A. Stopper, K. Press, J. Okuda, I. Goldberg and M. Kol, Inorg. Chem., 2014, 53, 9140–9150 CrossRef CAS PubMed.
- (a) X. Gu, Y. Zhang, Z.-J. Xu and C.-M. Che, Chem. Commun., 2014, 50, 7870–7873 RSC; (b) R. Mayilmurugan, P. Traar, J. A. Schachner, M. Volpe and N. C. Moesch-Zanetti, Eur. J. Inorg. Chem., 2013, 3664–3670 CrossRef CAS; (c) M. Miller and E. Y. Tshuva, Eur. J. Inorg. Chem., 2014, 1485–1491 CrossRef CAS; (d) E. Sergeeva, J. Kopilov, I. Goldberg and M. Kol, Inorg. Chem., 2009, 48, 8075–8077 CrossRef CAS PubMed; (e) E. Sergeeva, K. Press, I. Goldberg and M. Kol, Eur. J. Inorg. Chem., 2013, 3362–3369 CrossRef CAS.
- V. A. Yazerski, P. Spannring, D. Gatineau, C. H. M. Woerde, S. M. Wieclawska, M. Lutz, H. Kleijn and R. J. M. K. Gebbink, Org. Biomol. Chem., 2014, 12, 2062–2070 CAS.
- E. Sergeeva, J. Kopilov, I. Goldberg and M. Kol, Chem. Commun., 2009, 3053–3055 RSC.
- T. M. Ovitt and G. W. Coates, J. Polym. Sci., Polym. Chem. Ed., 2000, 38, 4686–4692 CrossRef CAS.
- It was found using D-LA it was necessary to sublime the monomer prior to use (N.B. for L-LA this was not the case). Using sublimed D-LA for Zr(1)(OiPr)2kapp = 7.5 × 10−4 min−1 Zr(2)(OiPr)2kapp = 0.7 × 10−4 min−1. These results clearly show that Zr(1)(OiPr)2 has a much stronger preference for D-LA and Zr(2)(OiPr)2 has a stronger preference for L-LA.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental data. CCDC 1027438–1027441. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc07871c |
| This journal is © The Royal Society of Chemistry 2014 |