Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diffusion of vaporous guests into a seemingly non-porous organic crystal†
Simon A.
Herbert
a,
Agnieszka
Janiak‡
a,
Praveen K.
Thallapally
*b,
Jerry L.
Atwood
*c and
Leonard J.
Barbour
*a
aDepartment of Chemistry and Polymer Science, University of Stellenbosch, Matieland 7602, Stellenbosch, South Africa. E-mail: ljb@sun.ac.za
bPacific Northwest National Laboratory, PO Box 999, Richland, WA 99359, USA. E-mail: praveen.thallapally@pnnl.gov
cDepartment of Chemistry, University of Missouri-Columbia, 125 Chemistry Building, 601 S. College Avenue, Columbia, MO 65211, USA. E-mail: AtwoodJ@missouri.edu
First published on 7th October 2014
Abstract
The tetragonal apohost phase of p-tert-butyltetramethoxythiacalix[4]arene absorbs hydrochloric acid and iodine. These guest molecules occupy different sites in the solid-state structure – either within the small intrinsic voids of the macrocycle or within the interstitial spaces between the host molecules. This study illustrates the dynamic deformation of the host, providing strong mechanistic insight into the diffusion of guests into this seemingly non-porous material.
In recent years much attention has been devoted to unravelling the mechanisms governing diffusion of guest molecules into crystalline materials. These studies have also raised questions about the fundamental nature of porosity, especially with regard to transient pores.1 The effective design of new porous materials for practical applications relies on our understanding of the factors that underpin the creation of molecular space, as well as mechanics of diffusion processes.
A range of different design strategies have been used to create porous crystalline materials. The major classes of these materials include metal–organic frameworks (MOFs),2,3 covalent organic frameworks (COFs),4 and molecular crystals4–6 where the “awkward” shapes of the molecules overcome their close packing tendencies.6–10 Although significant efforts are being made to design materials with ever increasing accessible void space,11,12 there is also great interest in materials that appear not to possess conventional channels or even pores, but are nevertheless permeable to guests. Indeed, transient porosity has been observed in MOFs,13,14 organic salts15 and in low molecular weight purely organic materials.16–18
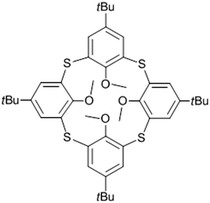
The incorporation of volatile mineral acids (hydrochloric or hydrobromic) into crystalline materials has thus far been limited to metal–organic materials, accompanied by a concomitant acid–base gas-solid reaction.19–26 The reaction in these reports involves either protonation of a pyridyl metal-coordinated group,19–23 or protonation of a zwitterionic system.24–26 Perhaps as might be anticipated, significant structural strain is associated with these transformations, leading to characterization of the products by means of powder X-ray diffraction19–25 or atomic force microscopy26 rather than by single-crystal diffraction. Interestingly, all of these reports are associated with seemingly non-porous materials, implying that the acid–base reaction provides a strong incentive for the guest uptake. We have previously reported the diffusion of water into a seemingly non-porous hydrophobic crystal composed of a particular apohost phase of p-tert-butyltetramethoxythiacalix[4]arene (1, Fig. 1).27 As a continuation of this work we were interested in determining if incorporation of HCl by 1 would also be possible, and if inclusion of the acid could be achieved without utilizing a formal acid–base reaction.
Crystal growth by means of sublimation produced a mixture of phases comprising a monoclinic form 1a (space group C2/c) and the desired “porous” tetragonal form 1b (space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m). In both of these forms the calixarene assumes the 1,3-alternate conformation and both structures therefore possess small vacant pockets within the protective shell of the macrocycle (endo- or intrinsic28 pores). However, the parallel pillars of calixarenes in phase 1b also form isolated hydrophobic pockets in the interstitial spaces between the columns (i.e. extrinsic pores28). Such additional voids are not present in the more efficiently packed phase 1a.
21m). In both of these forms the calixarene assumes the 1,3-alternate conformation and both structures therefore possess small vacant pockets within the protective shell of the macrocycle (endo- or intrinsic28 pores). However, the parallel pillars of calixarenes in phase 1b also form isolated hydrophobic pockets in the interstitial spaces between the columns (i.e. extrinsic pores28). Such additional voids are not present in the more efficiently packed phase 1a.
Two subtly different (i.e. symmetry-independent) calixarene molecules (1b1 and 1b2, see Fig. 2) are present in 1b. In the case of 1b1 the molecule possesses ![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) symmetry and the two endo voids are thus identical. The second molecule (1b2) possesses 2mm symmetry, which does not require identical geometries for the two voids – two distal aromatic rings are tilted at a slightly greater angle relative to each other than their corresponding neighbors. This asymmetry of the two molecular extremities creates two endo voids that differ slightly in volume. Potential guest molecules are therefore presented with a choice of three distinct voids to occupy in phase 1b.
symmetry and the two endo voids are thus identical. The second molecule (1b2) possesses 2mm symmetry, which does not require identical geometries for the two voids – two distal aromatic rings are tilted at a slightly greater angle relative to each other than their corresponding neighbors. This asymmetry of the two molecular extremities creates two endo voids that differ slightly in volume. Potential guest molecules are therefore presented with a choice of three distinct voids to occupy in phase 1b.
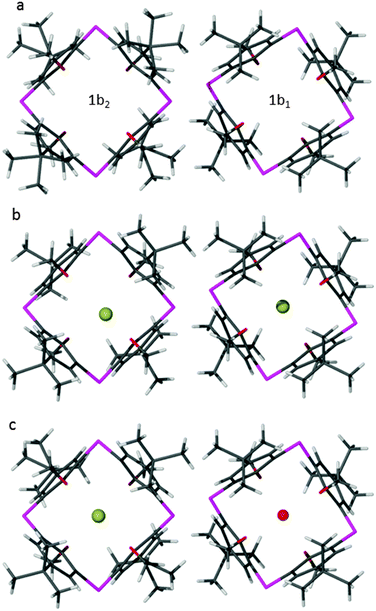 | ||
| Fig. 2 Calixarene molecules in the structures of (a) 1b, (b) 1bHCl and (c) 1bHCl·H2O viewed along [001]. Symmetry-imposed disorder has been suppressed. | ||
Exposure of 1b to dry HCl vapor for one week yielded 1bHCl, which was characterized by single crystal X-ray diffraction (space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m). The structure possesses significant electron density associated with the incorporation of HCl into the endo cavities. Energy-dispersive X-ray (EDX) spectroscopic analysis of 1bHCl confirmed the incorporation of chloride into 1b while similar analysis of 1a (which was also present during exposure to HCl vapor) failed to produce any peaks associated with chloride (ESI†).
21m). The structure possesses significant electron density associated with the incorporation of HCl into the endo cavities. Energy-dispersive X-ray (EDX) spectroscopic analysis of 1bHCl confirmed the incorporation of chloride into 1b while similar analysis of 1a (which was also present during exposure to HCl vapor) failed to produce any peaks associated with chloride (ESI†).
Similar to the inclusion of H2O into 1b, HCl ultimately occupies two of the three unique endo pores, and could be modelled at 18% site occupancy in the two symmetry-related pockets of 1b1 and also exclusively in the larger pocket of 1b2 with 50% site occupancy. In 1b1 the closest Cl⋯O distance to a methoxy oxygen atom is 3.277(11) Å, and the corresponding value in 1b2 is 3.041(6) Å. These distances are in good agreement with literature values for Cl⋯Oether interactions (3.23(6) Å), in contrast to the shorter distance expected for an Owater⋯Oether interaction (2.93(8) Å).29
In order to probe the effect of water on the diffusion of HCl into 1b, a crystal was placed in a sealed container containing wet HCl vapor for one week to yield 1bHCl·H2O. Single crystal X-ray diffraction analysis indicates selective occupancy of the distinct endo voids: the two symmetry-related cavities 1b1 each contain H2O molecules with a site occupancy of 22%, whereas cavity 1b2 appears to be occupied by HCl (20% occupancy). The identities of the guests were inferred from their different distances to the methoxy oxygen atoms (2.947(18) and 3.138(12) Å, respectively).
Although both 1bHCl and 1bHCl·H2O display non-stoichiometric guest occupancy, efforts to increase the occupancy by extended exposure resulted in significant degradation of single-crystal quality without any increase in occupancy being observed. Owing to phase impurity of the apohost resulting from sublimation growth, it is difficult to monitor the change in guest occupancy with time using techniques such as powder X-ray diffraction or gravimetric analysis.
We have already speculated27 that rotation of the tert-butyl groups guarding the interstitial pockets facilitates the movement of the guests through the lattice. In order to probe this conjecture further, crystals of 1b were exposed to vapors consisting of larger guest molecules; is it possible that a hydrophobic guest might occupy the interstitial voids, thereby confirming at least the notion that these can constitute a pathway for diffusion?
Iodine is a relatively large, linear and non-polar compound with a sufficiently high vapor pressure to make it suitable for diffusion experiments. Exposure of 1a and 1b to anhydrous iodine vapor rapidly yields visual confirmation that 1b is capable of iodine uptake (see ESI† for video file). Single crystal X-ray diffraction analysis of 1bI2 (P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m) shows significant electron density in the interstitial pockets (Fig. 3). The peaks could be modelled as iodine (20% site occupancy) with an I–I bond length of 2.682(3) Å. As observed for HCl, prolonged exposure of 1b to iodine vapor failed to increase the guest occupancy.
21m) shows significant electron density in the interstitial pockets (Fig. 3). The peaks could be modelled as iodine (20% site occupancy) with an I–I bond length of 2.682(3) Å. As observed for HCl, prolonged exposure of 1b to iodine vapor failed to increase the guest occupancy.
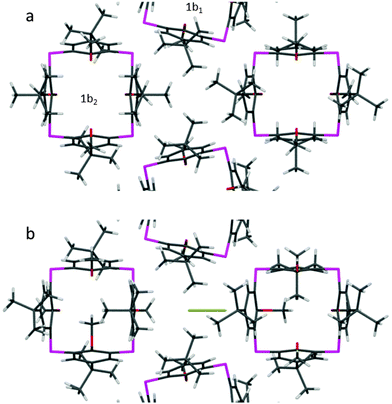 | ||
| Fig. 3 Corresponding structures of (a) 1b and (b) 1bI2 viewed along [001]. Symmetry-imposed disorder has been suppressed for clarity. | ||
The influence of water on iodine uptake was also explored to determine whether the inclusion of iodine would also be suppressed by competition with H2O. Crystals of 1b were placed in a vial containing both water and iodine vapors for 10 days. The resulting crystal structure 1bI2·H2O (P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m) was compared to that of 1bI2; both structures yielded site occupancy factors of 20% for iodine, but 1bI2·H2O shows additional incorporation of water into both endo pockets 1b1 (2 × 25%) and 1b2 (25%). It therefore appears that the exo and endo voids possess different enough environments that both the polar H2O and the non-polar iodine can diffuse through the lattice simultaneously, each ultimately being unaffected by the other's presence.
21m) was compared to that of 1bI2; both structures yielded site occupancy factors of 20% for iodine, but 1bI2·H2O shows additional incorporation of water into both endo pockets 1b1 (2 × 25%) and 1b2 (25%). It therefore appears that the exo and endo voids possess different enough environments that both the polar H2O and the non-polar iodine can diffuse through the lattice simultaneously, each ultimately being unaffected by the other's presence.
Although guest uptake by 1b does not alter the space group, it is interesting to compare the structure of 1bI2 with that of 1bHCl. In the former, one of the methoxy methyl groups of 1b1 rotates upon guest uptake to occupy a second position that projects into the calixarene cavity. This is most likely a steric requirement to accommodate the iodine molecule within the exo cavity, and it allows a linear head-on arrangement of iodine relative to the oxygen atom of the inwardly oriented methoxy group (see Fig. S3, ESI†). Similar arrangements have been observed in other structures (CSD average I⋯Oether = 2.84(8) Å), as compared to 2.911(4) observed in 1bI2. This orientation is probably due to electronic stabilization between I2 molecules interacting with the lone pairs of the oxygen atom.30 A second large scale distortion of the geometry of 1 in 1bI2 relative to that in 1b is that two of the symmetry related distal aromatic rings in 1b1 assume more “upright” positions in the former, i.e. they are pushed inwards by the interstitial guest.
The competition experiments between H2O and HCl or I2 raises some interesting questions: how are guest molecules able to sense the most suitable position to occupy and then how are they able organize themselves optimally? The mixed occupancy that results when H2O and HCl are in competition implies similar affinities for these two guests by the host sites. Preferential occupancy of 1b2 by HCl seems to stem from size selectivity, which also suggests the ability of the guest to move between endo cavities. The interplay between H2O and I2 is apparently different since no reduction in the occupancy of iodine occurs in the presence of water. This implies that the water molecules must spend most of their time in the endo pores, perhaps rapidly traversing from one pore to the next, whereas iodine only associates with the intermolecular pores.
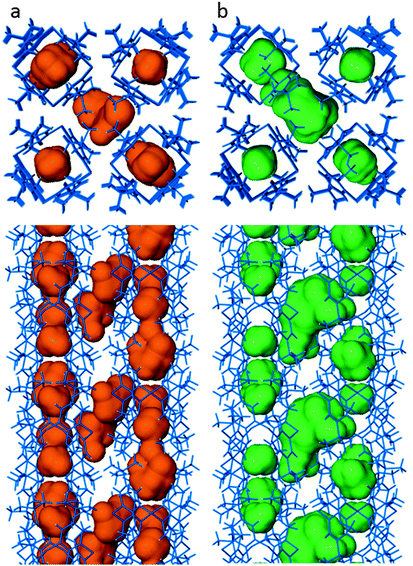
Since sorption occurs under relatively mild conditions, the drive for 1b to fill its voids with suitable guests must be an energetically favorable process. The different environments offered by the endo and exo pockets must facilitate favorable interactions that allow the guests to enter and traverse the material. Mechanistically, the clathrate 1bI2 illustrates the inherent flexibility incorporated into the structure of 1b. A map of the guest-accessible space associated with the alternative conformation of 1 adopted by 1bI2 in order to incorporate the guest provides an impression of the size and shape that the cavities in 1b can assume when host flexibility is taken into account (Fig. 4). While no permanent channels are apparent in either 1b or 1bI2 (even when using a probe radius of 1.2 Å), the rocking motion of the aryl rings of 1b1 would most likely result in the formation of transient channels that allow guest transport. Although the interstitial pocket of 1b is also hydrophobic in nature, the inversion of the methoxy group provides a temporary polar site that may help to facilitate the diffusion of polar guests through the structure.
The fact that the occupancy of the guests reaches a non-stoichiometric limit, even after prolonged exposure to the guest vapors, may be due to a self-terminating process or insufficient partial pressure of the guest. It is evident that guest diffusion requires deformation of the host structure, but for this to occur there must be some free space. Guest uptake reduces the amount of available space, thus inhibiting the ability of the host to deform – hence guest occupancy may reach a critical limit beyond which further uptake is precluded.
In summary, we have demonstrated two contrasting examples of guest sorption by a seemingly non-porous and purely organic structure; void occupancy is based on size, polarity and inter-guest competition. Deformation of the host structure to incorporate iodine provides insight into a possible “breathing” mechanism that allows guest transport through the crystal. Although we have not focused on the release of HCl from 1bHCl, we show that it is possible to trap the guest without the requirement for an acid–base reaction. Indeed, the use of HCl in synthetic laboratories and industrial applications is widespread, with estimates of global production exceeding 20 million tonnes annually. However, its use as an anhydrous reagent is often complicated by its highly hygroscopic and volatile nature. It is likely that certain reactions and applications would greatly benefit from an easily handled solid material capable of controllably releasing HCl in an anhydrous fashion, specifically where catalytic quantities are required. As a proof of concept, this study represents a possible stepping stone towards creating a smart material capable of slowly/controllably releasing HCl (or other guests) as a reagent.
SH, AJ and LJB thank the National Research Foundation for financial support. JLA thanks the National Science Foundation for and PKT thanks the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering (Award No. KC020105-FWP12152).
Notes and references
- L. J. Barbour, Chem. Commun., 2006, 1163 RSC.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- B. Chen, S. Xiang and G. Qian, Acc. Chem. Res., 2010, 43, 1115 CrossRef CAS PubMed.
- S.-Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548 RSC.
- N. B. McKeown, J. Mater. Chem., 2010, 20, 10588 RSC.
- J. Tian, P. K. Thallapally and B. P. McGrail, CrystEngComm, 2012, 14, 1909 RSC.
- S. J. Dalgarno, P. K. Thallapally, L. J. Barbour and J. L. Atwood, Chem. Soc. Rev., 2007, 36, 236 RSC.
- T. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner and A. I. Cooper, Nat. Mater., 2009, 8, 973 CrossRef CAS PubMed.
- P. K. Thallapally, S. J. Dalgarno and J. L. Atwood, J. Am. Chem. Soc., 2006, 128, 15060 CrossRef CAS PubMed.
- M. E. Davis, Nature, 2002, 417, 813 CrossRef CAS PubMed.
- O. K. Farha, I. Eryazici, N. C. Jeong, B. G. Hauser, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, S. T. Nguyen, A. O. Yazaydin and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 15016 CrossRef CAS PubMed.
- A. I. Cooper, Angew. Chem., Int. Ed., 2012, 51, 7892 CrossRef CAS PubMed.
- S. A. Dalrymple and G. K. H. Shimizu, Chem. Commun., 2006, 956 RSC.
- Z. Huang, P. S. White and M. Brookhart, Nature, 2010, 465, 598 CrossRef CAS PubMed.
- P. Metrangolo, Y. Carcenac, M. Lahtinen, T. Pilati, K. Rissanen, A. Vij and G. Resnati, Science, 2009, 323, 1461 CrossRef CAS PubMed.
- K. E. Jelfs, X. Wu, M. Schmidtmann, J. T. A. Jones, J. E. Warren, D. J. Adams and A. I. Cooper, Angew. Chem., Int. Ed., 2011, 50, 10653 CrossRef CAS PubMed.
- J. L. Atwood, L. J. Barbour, A. Jerga and B. L. Schottel, Science, 2002, 298, 1000 CrossRef CAS PubMed.
- G. D. Enright, K. A. Udachin, I. L. Moudrakovski and J. A. Ripmeester, J. Am. Chem. Soc., 2003, 125, 9896 CrossRef CAS PubMed.
- C. J. Adams, H. M. Colquhoun, P. C. Crawford, M. Lusi and A. G. Orpen, Angew. Chem., Int. Ed., 2007, 46, 1124 CrossRef CAS PubMed.
- G. M. Espallargas, A. J. Florence, J. van de Streek and L. Brammer, CrystEngComm, 2011, 13, 4400 RSC.
- G. M. Espallargas, J. van de Streek, P. Fernandes, A. J. Florence, M. Brunelli, K. Shankland and L. Brammer, Angew. Chem., Int. Ed., 2010, 49, 8892 CrossRef PubMed.
- G. Mínguez Espallargas, M. Hippler, A. J. Florence, P. Fernandes, J. van de Streek, M. Brunelli, W. I. F. David, K. Shankland and L. Brammer, J. Am. Chem. Soc., 2007, 129, 15606 CrossRef PubMed.
- I. J. Vitorica-Yrezabal, R. A. Sullivan, S. L. Purver, C. Curfs, C. C. Tang and L. Brammer, CrystEngComm, 2011, 13, 3189 RSC.
- D. Braga, G. Cojazzi, D. Emiliani, L. Maini and F. Grepioni, Chem. Commun., 2001, 2272 RSC.
- D. Braga, L. Maini, M. Mazzotti, K. Rubini, A. Masic, R. Gobetto and F. Grepioni, Chem. Commun., 2002, 2296 RSC.
- G. Kaupp, M. R. Naimi-Jamal, L. Maini, F. Grepioni and D. Braga, CrystEngComm, 2003, 5, 474 RSC.
- P. K. Thallapally, G. O. Lloyd, J. L. Atwood and L. J. Barbour, Angew. Chem., Int. Ed., 2005, 44, 3848 CrossRef CAS PubMed.
- M. J. Bojdys, M. E. Briggs, J. T. A. Jones, D. J. Adams, S. Y. Chong, M. Schmidtmann and A. I. Cooper, J. Am. Chem. Soc., 2011, 133, 16566 CrossRef CAS PubMed.
- Cambridge Structural Database, version 5.34. F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380 CrossRef PubMed.
- G. Maroulis, C. Makris, U. Hohm and D. Goebel, J. Phys. Chem. A, 1997, 101, 953 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the synthesis of 1, along with general experimental protocols and detailed descriptions of the crystallographic procedures and structures. CCDC 973280 (1HCl), 973281 (1HCl·H2O), 973282 (1I2) and 973283 (1I2·H2O). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc07366e |
| ‡ AJ is currently on leave from Adam Mickiewicz University, Poznan, Poland. |
| This journal is © The Royal Society of Chemistry 2014 |