Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oscillatory carbonylation using alkyne-functionalised poly(ethylene glycol)†
Lynn
Donlon
and
Katarina
Novakovic
*
Department of Chemical Engineering and Advanced Materials, Newcastle University, Merz Court, Newcastle, NE1 7RU, UK. E-mail: katarina.novakovic@ncl.ac.uk; Fax: +44 (0)191 208 5748; Tel: +44 (0)191 208 3683
First published on 17th June 2014
Abstract
In this study palladium-catalysed oscillatory carbonylation has been achieved using mono alkyne-terminated poly(ethylene glycol) methyl ether substrates. Reproducible, synchronised oscillations in pH and solution turbidity have been recorded over several days. A reaction network accounting for the observed phenomena has been proposed.
Reactions that display oscillatory behaviour have received increasing attention over the past decade. In particular, the coupling of reactions that show autonomous changes in reagent concentrations (e.g. H+) to responsive materials has prospective applications in pulsatile drug delivery as well as in micro-actuators and pumps.1–3 To date, efforts have focused on the catalytic component of oscillatory reactions (e.g. ruthenium bi- or terpyridyl complexes in the case of the well-known Belousov–Zhabotinsky reaction), using autonomous redox behaviour to drive self-assembly4,5 or trigger a volume phase transition when bound to a hydrogel substrate.6,7
In this work we demonstrate that an alkyne functionalised polymer can act as a substrate in oscillatory chemical reactions. The oscillatory system upon which the current study is based is the palladium-catalysed oxidative carbonylation of phenylacetylene (PCPOC reaction),8–16 a chemical oscillator in which complex products are synthesised from simple starting materials with high selectivity.12,13 Large oscillations in pH (1–6) coupled with synchronised oscillations in reaction heat output have been reported over several days.14,15 By replacing the acetylene substrate with a soluble polymeric analogue, mono alkyne-terminated poly(ethylene glycol) methyl ether (PEGA), reproducible oscillations in both pH and solution turbidity are observed at catalyst concentrations two orders of magnitude lower than routinely used. Lower catalyst concentrations result in a transparent solution, permitting the measurement of solution turbidity and giving insight into cycling of the catalyst between oxidation states.
PEGA (2000 and 5000 Da), and methyl 4-pentynoate (4MP, a ‘monomeric’ analogue of PEGA) were synthesised from commercially available starting materials according to literature procedures.17–19 For each oscillatory run a catalyst solution consisting of palladium(II) iodide and potassium iodide in methanol was equilibrated to 20 °C and a baseline pH value established. Solutions were then purged with carbon monoxide and air for 15 min and treated with methanolic solutions of PEGA. CO/air purging was maintained throughout each experiment resulting in a semi-batch set-up. pH values were monitored continuously using an aqueous pH probe connected to HEL WinISO software. Detailed discussion of experimental procedures is given in the ESI.†
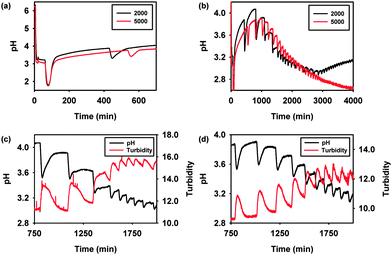
Initial purging of catalyst solutions with CO/air led to a sharp fall in pH to ∼pH 3. Upon addition of PEGA, pH remained relatively constant during an induction period, after which a second sharp fall was noted, Fig. 1a. pH then continued to rise until the onset of periodic oscillations which lasted for several days, Fig. 1b. Reactions were carried out using PEGA of 2000 and 5000 Da. Oscillatory pH behaviour was reproducibly recorded in both cases confirming that polymer bound substrates are viable in oscillatory systems. Some variations in oscillatory behaviour were observed during repeat runs (Fig. S1, ESI†), however in all cases well defined, periodic oscillations of the same shape were recorded. Due to the batch set-up, oscillations decrease in both period and amplitude as the reaction progresses, Fig. 1b. Oscillations recorded using PEGA2000 initially show higher amplitude and period than those recorded using PEGA5000, however within 3–4 cycles oscillatory behaviour becomes similar irrespective of PEGA molecular weight. Periods between 4 and 6 h and amplitudes between 0.4 and 0.7 pH units are initially observed for PEGA2000, decreasing to ∼1 h and 0.04 pH units as oscillations begin to subside. In the case of PEGA5000 periods are initially 3–4 h with amplitudes ∼0.4 pH units, decreasing to ∼50 min and 0.05 pH units towards the end of the oscillatory regime.
The pH behaviour of four alternative substrates, 4MP, PEG 2000 mono methyl ether (PEG 2000-OMe), a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar mixture of 4MP and PEG 2000-OMe (to mimic reaction conditions should PEGA undergo cleavage of the ester linkage), and phenylacetylene were studied under identical reaction conditions to those reported in Fig. 1. Although some fluctuations in pH were noted in all cases oscillations were not observed (Fig. S2, ESI†).
1 molar mixture of 4MP and PEG 2000-OMe (to mimic reaction conditions should PEGA undergo cleavage of the ester linkage), and phenylacetylene were studied under identical reaction conditions to those reported in Fig. 1. Although some fluctuations in pH were noted in all cases oscillations were not observed (Fig. S2, ESI†).
The absence of oscillations using non-polymeric analogues under identical reaction conditions suggests that the alkyne functionality remains attached to the PEG backbone throughout the reaction and that the macromolecular nature of the substrate is essential to achieve an oscillatory regime under the conditions reported in this study. This theory is further supported by GC-MS analysis of the reaction products which shows no evidence for either free 4MP or associated carbonylation products that may be expected.12,13
Palladium-catalysed carbonylation reactions involving small molecules, such as phenylacetylene, have been shown to proceed in either an oscillatory or non-oscillatory manner, with significant differences observed in both selectivity and conversion. Operation in a non-oscillatory mode generates a complex mixture of products, including mono- and di-esters, anhydrides and lactones.12,13,20 Oscillatory carbonylation of phenylacetylene generates z-2-phenylbut-2-enedioic acid dimethyl ester with >75% selectivity however at least 4 minor products are also observed.12,13 Detailed studies to characterise the products of oscillatory reactions using PEGA are ongoing and the product mixture obtained is simply referred to as PEGP.
In addition to pH oscillations, synchronised oscillations in solution turbidity were noted during all PEGA oscillatory runs (Fig. 1c and d). We suggest that turbidity oscillations occur due to cycling of the palladium catalyst between oxidation states, in particular Pd(II)/Pd(0). However this redox process may involve a complex mechanism including palladium(I) compounds,9,10 therefore changes in experimentally recorded turbidity are not readily attributed to a particular species or oxidation sate of the palladium catalyst.
A simplified reaction network that accounts for the experimentally recorded behaviour is proposed in Scheme 1. Two autocatalytic reactions have been postulated (eqn (1) and (4), Scheme 1). The reaction in eqn (1) is assumed to be autocatalytic in HI which accounts for the sharp rise in hydrogen ion concentration as well as PEGP product formation and catalyst reduction. The reaction presented in eqn (2) accounts for formation of iodine, which is subsequently responsible for regeneration of the palladium catalyst (eqn (3) and (4)). Eqn (2) also accounts for hydrogen ion consumption. Catalyst recycling is postulated to proceed in both a non-catalytic and autocatalytic manner, eqn (3) and (4) respectively. Autocatalysis supports the recorded oscillatory behaviour of solution turbidity whilst eqn (3) allows for catalyst regeneration in the event of full consumption of PdI2. Eqn (5), although experimentally recorded as a slow and reversible process,21 is included as it accounts for the initial formation of hydrogen ions required to initiate the reaction given in eqn (1). Reversibility is acknowledged by eqn (6). Due to the excess of CH3OH, O2 and CO their concentrations were considered constant15,21–24 and were not included in the reaction rates.
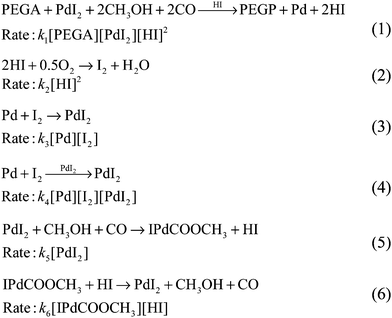 | ||
| Scheme 1 Reaction network proposed to account for the experimentally recorded oscillations with PEGA. Concentrations of CH3OH, O2 and CO are in excess, therefore considered constant and were not included in the reaction rates. This assumption is experimentally confirmed15,21–24 and is valid for this particular system. | ||
The reaction network given in Scheme 1 is validated in a simulation study using BatchCAD kinetic fitting and simulation software. In this software all reaction rate constants are expressed in min−1 regardless of the difference in kinetic laws. Clearly the units differ to reflect the order of the reaction and for order n the rate coefficient has units of mol1−n Ln−1 min−1. An example of the results is given in Fig. 2. Estimated rate constants, Table 1, are not a unique set of solutions and should be taken as an indication of the plausibility of the proposed model. To probe the validity of the model oxidation of HI in methanol (eqn (2)) was studied separately under the conditions reported for oscillatory runs (same reactor, reaction volume and rate of air flow). The rate of HI oxidation recoded experimentally is in good agreement with that used in the simulation study.
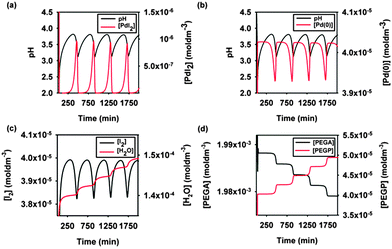 | ||
| Fig. 2 Example simulation study in BatchCAD using the reaction network given in Scheme 1. Initial conditions: [PdI2] = 4.05 × 10−5 M; [PEGA] = 2.03 × 10−3 M. Rate constants (min−1): k1 = 6 × 1012, k2 = 2 × 103, k3 = 1 × 10−7, k4 = 2 × 107, k5 = 3 × 10−4, k6 = 1 × 102. Results are validated using both adaptive Euler and adaptive Runge–Kutta integrators. | ||
| Rate constant | Magnitude | Units |
|---|---|---|
| k 1 | 6 × 1012 | mol−3 L3 min−1 |
| k 2 | 2 × 103 | mol−1 L1 min−1 |
| k 3 | 1 × 10−7 | mol−1 L1 min−1 |
| k 4 | 2 × 107 | mol−2 L2 min−1 |
| k 5 | 3 × 10−4 | min−1 |
| k 6 | 1 × 102 | mol−1 L1 min−1 |
Simulated pH data (Fig. 2a and b) correlate well with that recorded experimentally (Fig. 1). The model also illustrates oscillations in both Pd(II) and Pd(0), (Fig. 2a and b, respectively), however these do not synchronise directly with experimentally recorded turbidity due to the complexity of the underlying mechanism. In addition subtle changes in solution colour that were visually observed during experiments may be due to oscillations in the concentration of I2 as indicated by the simulation study (Fig. 2c). Simulation results also indicate step-wise formation of products (water, Fig. 2c; PEGP, Fig. 2d) and consumption of reactant (PEGA, Fig. 2d). This observation is in agreement with calorimetry studies of the oscillatory phenylacetylene carbonylation system in which a staircase function of heat of reaction is recorded.13
We have demonstrated both experimentally and in a modelling study, that oscillatory pH behaviour can be reproducibly achieved via palladium-catalysed carbonylation of alkyne-terminated PEG substrates. Future work will explore a multitude of alkyne functionalised polymers as substrates for oscillatory systems, opening up exciting possibilities in the area of fully autonomous oscillatory materials.
This work was supported by Engineering and Physical Sciences Research Council grant EP H003908/1.
Notes and references
- R. Yoshida and T. Takahashi, J. Am. Chem. Soc., 1996, 118, 5134–5135 CrossRef CAS.
- C. J. Crook, A. Smith, R. A. L. Jones and A. J. Ryan, Phys. Chem. Chem. Phys., 2002, 4, 1367–1369 RSC.
- J. R. Howse, P. Topham, C. J. Crook, A. J. Gleeson, W. Bras, R. A. L. Jones and A. J. Ryan, Nano Lett., 2006, 6, 73–77 CrossRef CAS PubMed.
- H. Zhou, E. Liang, X. Ding, Z. Zheng and Y. Peng, Chem. Commun., 2012, 48, 10553–10555 RSC.
- T. Ueki, Y. Takasaki, K. Bundo, T. Ueno, T. Sakai, Y. Akagi and R. Yoshida, Soft Matter, 2014, 10, 1349–1355 RSC.
- R. Yoshida, Adv. Mater., 2010, 22, 3463–3483 CrossRef CAS PubMed.
- Y. Shiraki and R. Yoshida, Angew. Chem., Int. Ed., 2012, 51, 6112–6116 CrossRef CAS PubMed.
- A. V. Malashkevich, L. G. Bruk and O. N. Temkin, J. Phys. Chem. A, 1997, 101, 9825–9827 CrossRef CAS.
- S. N. Gorodskii, A. N. Zakharov, A. V. Kulik, L. G. Bruk and O. N. Temkin, Kinet. Catal., 2001, 42, 280–293 CrossRef.
- O. N. Temkin and L. G. Bruk, Kinet. Catal., 2003, 44, 661–667 CrossRef.
- S. N. Gorodskii, E. S. Kalenova, L. G. Bruk and O. N. Temkin, Russ. Chem. Bull. Int. Ed., 2003, 52, 1534–1543 CrossRef CAS.
- C. Grosjean, K. Novakovic, S. K. Scott, A. Whiting, M. J. Willis and A. R. Wright, J. Mol. Catal. A: Chem., 2008, 284, 33–39 CrossRef CAS PubMed.
- K. Novakovic, C. Grosjean, S. K. Scott, A. Whiting, M. J. Willis and A. R. Wright, Phys. Chem. Chem. Phys., 2008, 10, 749–753 RSC.
- K. Novakovic, C. Grosjean, S. K. Scott, A. Whiting, M. J. Willis and A. R. Wright, Chem. Phys. Lett., 2007, 435, 142–147 CrossRef CAS PubMed.
- K. Novakovic, A. Mukherjee, M. J. Willis, A. R. Wright and S. K. Scott, Phys. Chem. Chem. Phys., 2009, 11, 9044–9049 RSC.
- L. Donlon, J. Parker and K. Novakovic, React. Kinet., Mech. Catal., 2014, 112, 1–13 CrossRef CAS.
- H. Shimatahira, S. Fusazaki, I. Ikeda and Y. Ozoe, Bioorg. Med. Chem. Lett., 2011, 21, 1598–1600 CrossRef PubMed.
- A. J. Inglis, P. Pierrat, T. Muler, S. Bräse and C. Barner-Kowollik, Soft Matter, 2010, 6, 82–84 RSC.
- J. A. Opsteen and J. C. M. van Hest, Chem. Commun., 2005, 57–59 RSC.
- B. Gabriele, G. Salerno and M. Costa, Top. Organomet. Chem., 2006, 18, 239–272 CrossRef CAS.
- J. Parker and K. Novakovic, Ind. Eng. Chem. Res., 2013, 52, 2520–2527 CrossRef CAS.
- K. Novakovic and J. Parker, Int. J. Chem. Eng., 2011, 2011, 1–11 CrossRef PubMed.
- S. P. Tonner, M. S. Wainwright, D. L. Trimm and N. W. Cant, J. Chem. Eng. Data, 1983, 28, 59–61 CrossRef CAS.
- M. Quaranta, M. Murkovic and I. Klimant, Analyst, 2013, 138, 6243–6245 RSC.
Footnote |
† Electronic supplementary information (ESI) available: Experimental details; repeat runs showing oscillatory pH behaviour for PEGA2000 and PEGA5000; pH behaviour for additional substrates PhAc, 4MP, PEG2000-OMe and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar mixture of 4MP and PEG2000-OMe. See DOI: 10.1039/c4cc01548g 1 molar mixture of 4MP and PEG2000-OMe. See DOI: 10.1039/c4cc01548g |
| This journal is © The Royal Society of Chemistry 2014 |