Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Proof-of-principle direct double cyclisation of a linear C15-precursor to a dibrominated bicyclic medium-ring ether relevant to Laurencia species†
D. Christopher
Braddock
* and
Dan-Tiberiu
Sbircea
Department of Chemistry, Imperial College London, London, SW7 2AZ, UK. E-mail: c.braddock@imperial.ac.uk; Fax: +44 (0)2075945805; Tel: +44 (0)2075945772
First published on 1st September 2014
Abstract
Bicyclic dibrominated C15 medium-ring ether hexahydrolaureoxanyne was produced directly from an acyclic model C15-epoxide when treated with NBS with water as the solvent.
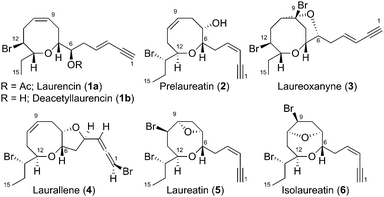
Since the original isolation of Laurencin (1a) in 1965,1 marine red algae of Laurencia species have provided a wide variety of C15-acetogenic halogenated diastereo- and constitutional isomeric monocyclic (C15H21BrO2) and bicyclic (C15H20Br2O2) medium-ring ethers that are oxygenated at both C-6 and C-7 (Fig. 1).2 Both the monocyclic and bicyclic metabolites have received considerable synthetic attention, with numerous necessarily different strategies used to forge the 7-, 8-, or 9-membered medium-ring, control the cis or trans α,α′-ether stereochemistry, install the requisite halogen(s), and – in the case of the bicyclic ethers – to fashion the second ring.3–5 Various recent studies have also been directed at the further understanding of their biogenesis,6 where the early pioneering work of Murai7 demonstrated enzymatic bromoetherifications of straight-chain co-isolated unsaturated C15-diols – laurediols (3E,6R,7R)-7a and (3Z,6S,7S)-7b8 – to monocyclic medium-ring ethers deacetyl laurencin 1b and prelaureatin 2 respectively, albeit in very low yields (Scheme 1, top).9 We have recently advanced an alternative biogenesis for the monocyclic (C15H21BrO2) medium-ring ethers from Laurencia species from (6S,7R)-epoxide 8via an intramolecular bromonium ion assisted epoxide ring-opening (IBIAERO) reaction with water functioning as the external nucleophile (Scheme 1, bottom, 8→B→O/O′→1b/2), and experimentally corroborated this with a model epoxide for the concurrent formation of 7-, 8- and 9-ring ethers corresponding to the halogenated medium-ring ethers of known metabolites from Laurencia species.10,11 The bicyclic metabolites are generally considered to originate by further bromoetherification of the residual unsaturation of the monocyclic compounds – the Z-configured medium-ring alkene or the pendant enyne – using the free alcohol of the original monocyclic compound located either at C-6 or C-7 as the nucleophile (Scheme 1, top).7 Several laboratory demonstrations of these later transformations have been successful, either as enzymatic-mediated bromoetherifications of naturally occurring monocycles,12 or as part of the synthetic strategy in a total synthesis of the bicyclic natural products.13 Interestingly, although bromocyclisation events had been postulated for both monocycle and bicycle formation, prior to our 2012 report10 and Snyder's recent elegant work,6b,c a non-enzymatic bromonium-ion induced cyclisation process to directly form medium-ring ether cores relevant to Laurencia species had not been reported. Moreover, to the best of our knowledge, there has been no report of a C15-dibrominated bicyclic medium-ring ether relevant to Laurencia species being formed directly from a linear unsaturated C15-precursor by two successive bromination events in the same pot. Herein we report on a successful strategy to effect such a transformation.
To investigate the proof-of-principle demonstration of a direct double cyclisation of a C15 unsaturated linear precursor to a bicyclic medium-ring ether relevant to Laurencia species we targeted hexahydroepoxide (6S*,7R*)-[H6]-8, with the aim that this would undergo an initial IBIAERO reaction via [H6]-B where water functions as both the solvent and the nucleophile (Scheme 2). The use of water in this manner thus guarantees a free hydroxyl group for any subsequent bromoetherification reaction (e.g., [H6]-1b→[H6]-3, Scheme 2) with a second equivalent of an electrophilic bromine source. While we had previously demonstrated successful IBIAERO reactions in water with NBS as the electrophilic bromine source,11 the attempted IBIAERO reaction of a model epoxide as a truncated C12 alcohol (inset, Scheme 2) under the same conditions had failed.10‡ We considered that hexahydroepoxide [H6]-8 offered distinct benefits compared to this earlier model and also to epoxide 8 for the proposed experiment: (i) the hydrophilic hexahydro chain may encourage folding of the substrate in water thus inherently facilitating the IBIAREO reaction; (ii) post-IBIAERO reaction, the only region of unsaturation will be located in the medium ring and – compared with the hypothetical use of the putative biosynthetic precursor itself, epoxide 8 – there can be no complicating bromoetherifications to form bromoallene adducts by cyclisation onto any C1–C4 enyne moiety; (iii) hexahydrobicyclic compounds of formulae C15H26O2Br2 are known in the literature as a consequence of the structural elucidation of the naturally occurring compounds via hydrogenation,14 providing data for identification of bicyclic products.
 | ||
| Scheme 2 Proposed proof-of-principle direct cyclisation of (6S*,7R*)-[H6]-8 to bicyclic medium ring ethers via IBIAERO reaction and subsequent bromoetherification of the remaining unsaturation. | ||
Accordingly, epoxide (6S*,7R*)-[H6]-8 was synthesised from bromide 12, itself prepared from (E)-2-penten-1-ol (9) via a known sequence10,15 with minor modifications. Subsequent copper-mediated coupling16 with hept-1-yne gave novel enediyne 13 (Scheme 3).† Chemoselective and stereoselective hydrogenation17 afforded (E,Z,Z)-doubly skipped triene 14. Epoxidation of triene 14 with DMDO18 was found to be entirely selective for the Z-olefins,19 giving a mixture of mono epoxides (6S*,7R*)-[H6]-8 and 15 which could be separated by chromatography.§¶||
With epoxide (6S*,7R*)-[H6]-8 in hand, it was treated with two equivalents of NBS – a water stable reagent – under high dilution conditions in water (Scheme 4).** Here, various dibromination adducts, bromohydrin regioisomers, and dibromotetrahydrofurans are expected to be formed by competing processes.10 In the event, as expected, a complex mixture was obtained that was subjected to extensive chromatography, where ‘non-polar’ components could be separated away from ‘polar’ components.†† Much to our delight, by further chromatography of the non-polar components, hexahydrolaureoxanyne [(±)-[H6]-3]12a was isolated as a bicyclic medium-ring ether with 1H NMR data identical to that previously reported,†‡‡ along with dibromoepoxides 16. Thus the desired proof-of-principle has been achieved. This also constitutes the first synthetic route to the laureoxanyne bicyclic medium-ring ether scaffold, and the isolated yield of (±)-[H6]-3 (2.5%) from (6S*,7R*)-[H6]-8 compares well with the reported enzymatic conversion of deacetyl laurencin 1b (obtained from natural laurencin 1a) into 3 (3%).12a
 | ||
| Scheme 4 Proof-of-principle direct double cyclisation of (6S*,7R*)-[H6]-8 into (±)-[H6]-3via IBIAERO reaction and subsequent bromoetherification of the remaining unsaturation (cf., Scheme 2). | ||
In conclusion, we have demonstrated the proof-of-principle direct cyclisation of a linear unsaturated C15-precursor into a C15-dibrominated bicyclic medium-ring ether relevant to Laurencia species – where hexahydrolaureoxanyne (±)-[H6]-3 has an identical bicyclic medium ring ether framework to laureoxanyne 3 – by two successive bromination events in the same pot. These studies are also consistent with epoxide (6S,7R)-8 acting as the biogenetic precursor10 for bromocyclisation to bicyclic medium-ring ethers of Laurencia species via IBIAERO reactions followed by subsequent bromoetherification events.
We thank the Dinu Patriciu Foundation for funding (to D.-T. S.).
Notes and references
- (a) T. Irie, M. Suzuki and T. Masamune, Tetrahedron Lett., 1965, 16, 1091–1099 CrossRef; (b) T. Irie, M. Suzuki and T. Masumune, Tetrahedron, 1968, 24, 4193–4205 CrossRef CAS.
- For comprehensive reviews see: (a) B.-G. Wang, J. B. Gloer, N.-Y. Ji and J.-C. Zhao, Chem. Rev., 2013, 113, 3632–3685 CrossRef CAS PubMed; (b) J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2014, 31, 160–258 RSC and earlier reviews in this series.
- For a comprehensive review of the synthesis of medium-ring ethers from Laurencia sp., see: K. Fujiwara, Top. Heterocycl. Chem., 2006, 5, 97–148 CAS . See also ref. 2a.
- For recent leading syntheses of C15Laurencia metabolites see: (a) G. Kim, T.-i. Sohn, D. Kim and R. S. Paton, Angew. Chem., 2014, 126, 276–280 CrossRef PubMed; (b) C. Recsei, B. Chan and C. S. P. McErlean, J. Org. Chem., 2014, 79, 880–887 CrossRef CAS PubMed; (c) J. Rodríguez-López, N. Ortega, V. S. Martín and T. Martín, Chem. Commun., 2014, 50, 3685–3688 RSC; (d) M. T. Holmes and R. Britton, Chem. – Eur. J., 2013, 19, 12649–12652 CrossRef CAS PubMed; (e) D. J. Shepherd, P. A. Broadwith, B. S. Dyson, R. S. Paton and J. W. Burton, Chem. – Eur. J., 2013, 19, 12644–12648 CrossRef CAS PubMed; (f) B. S. Dyson, J. W. Burton, T.-i. Sohn, B. Kim, H. Bae and D. Kim, J. Am. Chem. Soc., 2012, 134, 11781–11790 CrossRef CAS PubMed; (g) M. J. Kim, T.-i. Sohn, D. Kim and R. S. Paton, J. Am. Chem. Soc., 2012, 134, 20178–20188 CrossRef CAS PubMed and references cited therein.
- For two recent accounts of research in the arena see: (a) T. Martín, J. I. Padrón and V. S. Martín, Synlett, 2014, 12–32 Search PubMed; (b) D. Kim, Synlett, 2014, 33–57 Search PubMed.
- For recent representative examples see: (a) S. Keshipeddy, I. Martínez, B. F. Castillo II, M. D. Morton and A. R. Howell, J. Org. Chem., 2012, 77, 7883–7890 CrossRef CAS PubMed; (b) S. A. Snyder, A. P. Brucks, D. S. Treitler and I. Moga, J. Am. Chem. Soc., 2012, 134, 17714–17721 CrossRef CAS PubMed; (c) S. A. Snyder, D. S. Treitler, A. P. Brucks and W. Sattler, J. Am. Chem. Soc., 2011, 133, 15898–15901 CrossRef CAS PubMed.
- For a review see: A. Murai, in Comprehensive Natural Products Chemistry, ed. D. H. R. Barton, O. Meth-Cohn and K. Nakinishi, Elsevier, Oxford, 1999, vol. 1, pp. 303–324 and references cited therein Search PubMed.
- E. Kurosawa, A. Fukuzawa and T. Irie, Tetrahedron Lett., 1972, 13, 2121–2124 CrossRef.
- (a) Lactoperoxidase (LPO) mediated cyclisation of 7a into 1b (characterized as 1a after acetylation; 0.73% yield): A. Fukuzawa, M. Aye and A. Murai, Chem. Lett., 1990, 1579–1580 CrossRef CAS; (b) LPO mediated cyclisation of 7b into 2 (3%): A. Fukuzawa, Y. Takasugi, A. Murai, M. Nakamura and M. Tamura, Tetrahedron Lett., 1992, 33, 2017–2018 CrossRef CAS; (c) Bromoperoxidase (BPO) mediated cyclisation of 7a into 1b (0.015%) and 7b into 2 (‘trace’ amount): A. Fukuzawa, M. Aye, Y. Takasugi, M. Nakamura, M. Tamura and A. Murai, Chem. Lett., 1994, 2307–2310 CrossRef CAS.
- K. J. Bonney and D. C. Braddock, J. Org. Chem., 2012, 77, 9574–9584 CrossRef CAS PubMed and references cited therein.
- For an IBIAERO reaction with capture of the oxonium ion with an added external nucleophile, see: K. J. Bonney, D. C. Braddock, A. J. P. White and M. Yaqoob, J. Org. Chem., 2011, 76, 97–104 CrossRef CAS PubMed and references cited therein.
- (a) BPO mediated cyclisation of 1b into 3 (3%): A. Fukuzawa, M. Aye, M. Nakamura, M. Tamura and A. Murai, Tetrahedron Lett., 1990, 31, 4895–4898 CrossRef CAS . See ref. 9b for LPO mediated conversion of 1b into laureatin 5 (0.3%). See ref. 9c for BPO mediated conversion of 1b into laureoxanyne 3 (0.8%), and [1-2H]-2 into [1-2H]-5 (laureatin) (0.07%) and [1-2H]-6 (isolaureatin) (0.05%); (b) For a chemical conversion of [1-2H]-2 into [1-2H]-4 (12%) see: J. Ishihara, Y. Shimada, N. Kanoh, Y. Takasugi, A. Fukuzawa and A. Murai, Tetrahedron, 1997, 53, 8371–8382 CrossRef CAS.
- (a) For the formation of the bromoallene of laurallene 4 from (E)-prelaureatin (24%) see: M. T. Crimmins and E. A. Tabet, J. Am. Chem. Soc., 2000, 122, 5473–5476 CrossRef CAS ; for the formation of the tetrahydrofuran ring of (−)-isoprelaurefucin from a pre-existing oxepene (92%) see: ; (b) H. Lee, H. Kim, T. Yoon, B. Kim, S. Kim, H.-D. Kim and D. Kim, J. Org. Chem., 2005, 70, 8723–8729 CrossRef CAS PubMed ; the actual chemical conversion of the prelaureatin skeleton into laureatin and/or isolaureatin bicyclics has proved challenging: ; (c) H. Kim, H. Lee, D. Lee, S. Kim and D. Kim, J. Am. Chem. Soc., 2007, 129, 2269–2274 CrossRef CAS PubMed; (d) M. Sugimoto, T. Suzuki, H. Hagiwara and T. Hoshi, Tetrahedron Lett., 2007, 48, 1109–1112 CrossRef CAS PubMed.
- (a) Hexahydrolaureoxanyne ([H6]-3): ref. 12a; (b) hexahydrolaureatin ([H6]-5): T. Irie, M. Izawa and E. Kurosawa, Tetrahedron Lett., 1968, 2091–2096 CrossRef CAS; (c) hexahydroisolaureatin ([H6]-6): T. Irie, M. Izawa and E. Kurosawa, Tetrahedron Lett., 1968, 2735–2738 CrossRef CAS; (d) hexahydroisoprelaurefucin: M. Suzuki, K. Kurata, T. Suzuki and E. Kurosawa, Bull. Chem. Soc. Jpn., 1986, 59, 2953–2955 CrossRef CAS.
- (a) O. Loreau, A. Maret, D. Poullain, J. M. Chardigny, J. L. Sébédio, B. Beaufrère and J. P. Noël, Chem. Phys. Lipids, 2000, 106, 65–78 CrossRef CAS ; see also: ; (b) W. G. Young, L. Richards and J. Azorlosa, J. Am. Chem. Soc., 1939, 61, 3070–3074 CrossRef CAS; (c) F. P. Cossío, I. Ganboa and C. Palomo, Tetrahedron Lett., 1985, 26, 3041–3044 CrossRef; (d) L. M. Smith, R. G. Smith, T. M. Loehr, G. D. Daves Jr., G. E. Daterman and R. H. Wohleb, J. Org. Chem., 1978, 43, 2361–2366 CrossRef CAS; (e) B. Añorbe, V. S. Martin, J. M. Palazón and J. M. Trujillo, Tetrahedron Lett., 1986, 27, 4991–4994 CrossRef.
- N. P. Villalva-Servin, A. Laurent and A. G. Fallis, Can. J. Chem., 2004, 82, 227–239 CrossRef CAS.
- C. Oger, L. Balas, T. Durand and J.-M. Galano, Chem. Rev., 2013, 113, 1313–1350 CrossRef CAS PubMed.
- R. Murray and P. Singh, Org. Synth., 1997, 74, 91 CrossRef CAS.
- DMDO epoxidations of cis/trans-dialkylalkene pairs have been reported to have a ca. 10-fold greater reactivity for the former: A. L. Baumstark and P. C. Vasquez, J. Org. Chem., 1988, 53, 3437–3439 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterising data and 1H and 13C NMR spectra for all compounds; a comparison of 1H NMR data for (±)-[H6]-3 with the literature data. See DOI: 10.1039/c4cc06402j |
| ‡ We speculate that the truncated C12 epoxide suffers from an intramolecular hydrogen bond from the alcohol functional group reducing its nucleophilicity. |
| § 25% of a bis-epoxide was also observed. |
| ¶ Attempted epoxidation of 14 with mCPBA was unselective for the Z-olefins. |
| || 1H-13C and 1H-1H NMR correlation spectroscopy were used to distinguish between epoxides (6S*,7R*)-[H6]-8 and 15.† |
| ** In an experiment with 1 equivalent of NBS in water, (±)-[H6]-3 was isolated in 1.8% yield after extensive chromatography. |
| †† The ‘polar’ components were expected to contain regioisomeric bromohydrins and dibromohydrins by reference to our earlier work (ref. 10) and were not further characterised. |
| ‡‡ The medium-ring bicyclic structure of [H6]-3 is also supported by a characteristic NOESY cross-peak between H7 and H9 as previously reported (as an nOe) for 3 (ref. 12a).† |
| This journal is © The Royal Society of Chemistry 2014 |