Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diselenolodiselenole: a selenium containing fused heterocycle for conjugated systems†‡
Anjan
Bedi
,
Sashi
Debnath
and
Sanjio S.
Zade
*
Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Kolkata, Mohanpur 714246, India. E-mail: sanjiozade@iiserkol.ac.in
First published on 10th September 2014
Abstract
The synthesis of new conjugated building blocks, diselenolodiselenole (C4Se4) derivatives, is described for the first time. The structural and optoelectronic properties of C4Se4-derivatives are tuned by varying end-capping aromatic substituents. In cyclic voltammetry, all C4Se4-derivatives show two reversible oxidation peaks.
Organoselenium compounds have attracted considerable interest due to their wide range of applications in many fields.1 Chalcogenophene containing building blocks are being given great importance to synthesize new conjugated organic materials. Their applications in the state-of-the-art technologies including field-effect transistors, flexible light emitting diodes and organic photovoltaics are being extensively studied.2 Though the development in this field has grown rapidly in the last two decades, the conjugated electroactive building blocks with promising properties are limited.3 Conjugated chalcogenophene based materials with reversible redox activity have found applications in organic electronic devices.4
Though the applications of thiophene based electroactive small molecules or polymers in organic electronics have attracted considerable research attention, their selenium counterparts are sparsely reported.5–7 Partly, it can be ascribed to the difficulties in the synthesis of selenophene derivatives and their instabilities in the charged states. The advantages of replacement of sulfur by selenium in conjugated systems are manifold: (a) intermolecular Se⋯Se interactions lead to a wide bandwidth in organic conductors, which should facilitate intermolecular charge transfer, (b) selenium containing organoheteroles have lower oxidation and reduction potentials than that of sulfur containing heterocycles, (c) due to higher polarizability of Se than that of S, compounds with the selenium atom attached to the conjugated backbone possess more polarizability than their sulfur analogues, (d) selenium containing compounds should have a lower band gap than their sulfur counterparts and, consequently, their optoelectronic properties also differ.
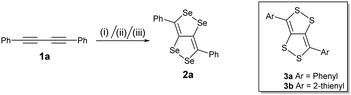
Blum and co-workers reported the synthesis of the first example of dithiolodithiole (C4S4) derivatives in 26% yield from 1,4-diphenylbutadiyne and elemental sulfur at 150 °C for 52 h.8 The growing interest in the C4S4 systems led to the development of a more facile synthetic strategy and a systematic study reported by Swager et al focusing on their interesting structural and optoelectronic properties.9 According to Hückel's rule, the C4S4 system is formally anti-aromatic in the ground state and non-aromatic in the excited state; thus these molecules can lead to interesting electronic properties. The structural and electronic properties of these systems can be further tuned by an atomistic approach by replacing S with Se, which may lead to a more interesting and important fused conjugated system (C4Se4). Here, we present for the first time the synthesis of diselenolodiselenole (C4Se4) derivatives, bicyclic heterocycles, as a new class of conjugated building blocks.
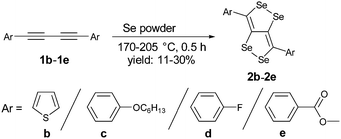
We have synthesized a series of compounds containing C4Se4 as the central conjugated system. The precursor diyne compounds 1a,101c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and 1e
11 and 1e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 were prepared by a previously reported procedure. Diynes 1b and 1d were synthesized by new synthetic methods (see ESI‡). The conversion of diynes to C4Se4 derivatives could proceed through the radical mechanism similar to the formation of C4S4 derivatives.9 However, the reaction of 1a with Se powder in the presence of solvent (1,2-dichloroethane (DCE)–o-dichlorobenzene (o-DCB)) and the radical initiator (azobisisobutyronitrile (AIBN)–2,2,6,6-tetramethylpiperidinyloxy (TEMPO)) at 190 °C in a pressure vessel with and without microwaves resulted in very low yields (Scheme 1, conditions I and II).
12 were prepared by a previously reported procedure. Diynes 1b and 1d were synthesized by new synthetic methods (see ESI‡). The conversion of diynes to C4Se4 derivatives could proceed through the radical mechanism similar to the formation of C4S4 derivatives.9 However, the reaction of 1a with Se powder in the presence of solvent (1,2-dichloroethane (DCE)–o-dichlorobenzene (o-DCB)) and the radical initiator (azobisisobutyronitrile (AIBN)–2,2,6,6-tetramethylpiperidinyloxy (TEMPO)) at 190 °C in a pressure vessel with and without microwaves resulted in very low yields (Scheme 1, conditions I and II).
Moreover, heating of elemental selenium with 1a nearly at the melting point of selenium without any solvents afforded the best yield of 23% for 2a (Scheme 1, condition III). Therefore, condition III was considered as a general procedure to prepare C4Se4 derivatives 2a–2e from diyne precursors (Scheme 2). The yields were obtained in the range of 10–30%. Though the strongly electron donating substituents on the phenyl ring were reasoned to cause a complex mixture of the product and low yield in the case of C4S4 derivatives,9 the reaction of di(p-hexyloxyphenyl) diacetylene (1c) afforded the highest yield (30%) in this series.
Crystals of 2a–2c were obtained by the slow evaporation method from their solution in dichloromethane (DCM). In the crystal structure of 2a, capped phenyl rings are twisted from the C4Se4 core by a dihedral angle of ∼53° (C8–C7–C6–C5 = 126.8(4)) (Fig. 1a and b), which is significantly higher (by ∼28°) than that of sulfur analogue 3a.9 Molecules of 2a form end-to-end dimers via intermolecular π–π interactions (C4–C5 = 3.91 Å and C4–C4 = 3.37 Å). Selenium atoms of the Se–Se bond of 2a form Se⋯Se interactions (Se1⋯Se2 = 3.64 Å) with the neighboring two molecules. This leads to the formation of a virtual (Se–Se⋯Se–Se)n polymeric chain along the c-axis, from which the phenyl rings are hanged like pendants (Fig. 1c), whereas in the crystal packing of 3a, face-to-face dimer formation was observed via S⋯S interactions.9 Interestingly, in the case of thiophene capped C4Se4 (2b) the torsional angle between the outer thiophene ring and the central C4Se4 unit is found to be only ∼10° (C1–C2–C3–C4 = 169.3(7)) (Fig. 2a and b). The nearly planar conjugated backbone of 2b exhibited resolute intermolecular interactions through heteroatoms.
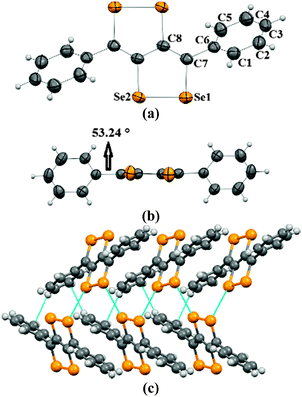 | ||
| Fig. 1 (a) ORTEP diagram of 2a, (b) torsional angle in 2a, and (c) packing of 2a. The ellipsoids are drawn at the 50% probability level in (a) and (b). | ||
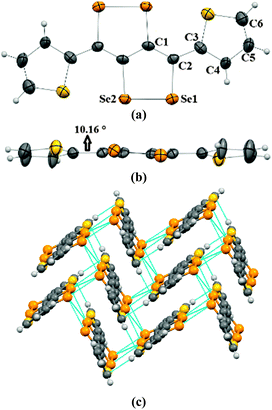 | ||
| Fig. 2 (a) ORTEP diagram of 2b, (b) torsional angle in 2b, and (c) packing of 2b. The ellipsoids are drawn at the 50% probability level in (a) and (b). | ||
In the crystal structure of 2b, a pair of Se atoms of each diselenole unit forms two perpendicular dimers by three different Se⋯Se interactions (Se1⋯Se1 = 3.648(1) and Se2⋯Se1 = 3.525(1), Se2⋯Se2 = 3.726(1)) with the diselenole unit of neighboring molecules (Fig. 2c). Thus four molecules connected by Se⋯Se and π⋯H–C interactions (C6–H6 = 2.81 Å) form a 2D brick-like structure in bulk. This 2D crystal packing with several nonbonding interactions could facilitate the intermolecular charge transport. In 2c the dihedral angle between the hexyloxy substituted phenyl ring and the central C4Se4 unit is ∼58° (C8–C3–C2–C1 = −122.4(5)) (Fig. S1, ESI‡).
DFT optimized structures (at B3LYP/6-31G(d)) of 2a and 2b showed dihedral angles of 47° and 0°, respectively, between the central C4Se4 unit and end-capping substituents. Corresponding values for C4S4 derivatives are 39° and 0°, respectively.
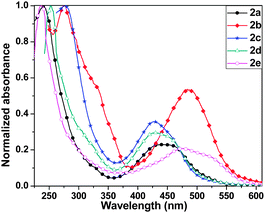
Compounds 2a–2e exhibited two sets of absorption peaks with λmax ranging from 236 to 277 nm and 427 to 484 nm, respectively (Fig. 3 and Table 1). Compound 2a showed λmax at 440 nm in solution, which was blue shifted compared to its C4S4 analogues. This may be ascribed to the large dihedral angle between the outer phenyl rings and the central C4Se4 unit that reduces the effective overlap between these two conjugated parts. A similar trend was observed in the absorption spectra of 2c and 2d. The absorption spectrum of 2e was red shifted compared to that of 2a, 2c and 2d. Eoptg of 2e is very close to its corresponding C4S4 analogue. It may be considered as a counterbalance of the blue shift in the absorption of 2e due to a larger dihedral angle than that of C4S4 by the red shift due to better electron donating properties of C4Se4 than that of C4S4 in the presence of the stronger electron accepting carboxylate substituted capped phenyl rings. The highest value of λmax for 2b among all C4Se4 derivatives is due to its nearly planar structures, which allow the effective end-to-end conjugation and enhance donor–acceptor properties.
| Compound | Yield (%) | λ max (nm) | E 1/2 vs. Ag/Ag+ (V) |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (eV) a (eV) |
HOMOb (eV) | LUMOc (eV) |
|---|---|---|---|---|---|---|
| a E optg = 1240/λonset. b E HOMO = −(4.44 + E1/2). c E LUMO = EHOMO + Eoptg. | ||||||
| 2a | 23 | 240, 440 | 0.35, 0.86 | 2.39 | −4.79 | −2.40 |
| 2b | 12 | 275, 484 | 0.47, 0.98 | 2.21 | −4.91 | −2.70 |
| 2c | 30 | 277, 428 | 0.25, 0.77 | 2.46 | −4.69 | −2.23 |
| 2d | 15 | 253, 427 | 0.49, 1.03 | 2.43 | −4.93 | −2.50 |
| 2e | 19 | 236, 475 | 0.50, 1.04 | 2.15 | −4.94 | −2.79 |
All C4Se4 derivatives 2a–2e exhibited two reversible oxidation potentials in the range of 0.30–0.54 V and 0.90–1.10 V, respectively, in cyclic voltammetry (CV) experiments (Fig. 4) similar to that of C4S4 derivatives.9 The variation in the oxidation potentials of 2a and 2c–2e could be understood on the basis of substituents on capped phenyl rings. Though thiophene is electron rich compared to benzene, 2b has higher oxidation potentials than those of 2a, and it is comparable with those of 2d and 2e. The planar structure of 2b could result in improved delocalization of the electrons of the C4Se4 unit throughout the conjugated backbone, which could decrease the electron density on the C4Se4 unit. The planarity of the conjugated core in 2b assisted the selenium atoms to exert the effect of its larger polarizability and less electronegativity on the extended conjugation. The first oxidation potential of C4Se4 derivatives is nearly in the same range as that of C4S4 derivatives, however, the difference between the first and second oxidation potentials is ∼0.1 V less than that of C4S4 derivatives.9
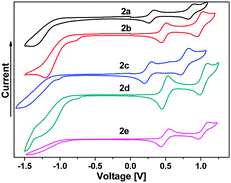 | ||
| Fig. 4 Electrochemical properties of compounds 2a–2e in 0.1 M TBAPF6 in dry DCM as solvent using a Pt-disk working electrode, a Pt-wire counter electrode and a Ag/AgCl reference electrode. | ||
Discussion of DFT calculated absorption spectra, HOMO–LUMO energy values, HOMO–LUMO gaps of 2a and 2b and comparison with C4S4 derivatives and experimental results is given in ESI‡ (Table S2, Fig. S2 and S3).
In summary, a new class of conjugated compounds, diselenolodiselenoles, was successfully synthesized simply by heating diaryl diynes with elemental selenium. The structural and optoelectronic properties of diselenolodiselenole derivatives can be tuned by the judicious choice of the capped aryl groups. The thiophene capped C4Se4 displayed a nearly planar structure with its absorption at the highest wavelength among the compounds in the present series. Therefore, it is a promising candidate to be exploited for application in organic electronics. Due to the presence of Se⋯Se interactions, diselenolodiselenole derivatives can arrange into interesting crystalline motifs. Thus, we have shown that the structural engineering and atomistic approach could be beneficial to synthesize meaningful building blocks for conjugated systems.
This work is supported by CSIR, India.
Notes and references
- (a) Chemistry of Organic Selenium and Tellurium Compounds, ed. Z. Rappoport, Wiley, Chichester, 2012, vol. 3, 2014, 4 Search PubMed; (b) A. J. Mukherjee, S. S. Zade, H. B. Singh and R. B. Sunoj, Chem. Rev., 2010, 110, 4357 CrossRef CAS PubMed.
- (a) Handbook of Organic Conductive Molecules and Polymers, ed. H. S. Nalwa, John Wiley & Sons, New York, 1997, vol. 1–4 Search PubMed; (b) A. Facchetti, Chem. Mater., 2011, 23, 733 CrossRef CAS.
- Handbook of Thiophene-based Materials: Applications in Organic Electronics and Photonics, ed. I. F. Perepichka and D. F. Perepichka, John Wiley & Sons, New York, 2009, vol. 1 and 2 Search PubMed.
- (a) P. M. Beaujuge and J. R. Reynolds, Chem. Rev., 2010, 110, 268 CrossRef CAS PubMed; (b) C. M. Amb, A. L. Dyer and J. R. Reynolds, Chem. Mater., 2011, 23, 397 CrossRef CAS.
- (a) A. Patra and M. Bendikov, J. Mater. Chem., 2010, 20, 422 RSC; (b) A. Patra, R. Kumar and S. Chand, Isr. J. Chem., 2014, 54, 621 CrossRef CAS; (c) J. Hollinger, D. Gao and D. S. Seferos, Isr. J. Chem., 2014, 54, 440 CrossRef CAS.
- (a) S. Das and S. S. Zade, Chem. Commun., 2010, 46, 1168 RSC; (b) S. Das, A. Bedi, G. Rama Krishna, C. M. Reddy and S. S. Zade, Org. Biomol. Chem., 2011, 9, 6963 RSC; (c) A. Bedi, S. P. Senanayak, K. S. Narayan and S. S. Zade, Macromolecules, 2013, 46, 5943 CrossRef CAS.
- A. Patra, Y. H. Wijsboom, S. S. Zade, M. Li, Y. Sheynin, G. Leitus and M. Bendikov, J. Am. Chem. Soc., 2008, 130, 6734 CrossRef CAS PubMed.
- J. Blum, Y. Badrieh, O. Shaaya, L. Meltser and H. Schumann, Phosphorus, Sulfur Silicon Relat. Elem., 1993, 79, 87 CrossRef CAS.
- D. J. Schipper, L. C. H. Moh, P. Müller and T. M. Swager, Angew. Chem., Int. Ed., 2014, 53, 5847 CrossRef CAS PubMed.
- I. D. Campbell and G. Eglinton, Org. Synth., 1965, 45, 39 CrossRef CAS.
- Y. Arakawa, S. Nakajima, R. Ishige, M. Uchimura, S. Kang, G.-i. Konishi and J. Watanabe, J. Mater. Chem., 2012, 22, 8394 RSC.
- G. Zhang, H. Yi, G. Zhang, Y. Deng, R. Bai, H. Zhang, J. T. Miller, A. J. Kropf, E. E. Bunel and A. Lei, J. Am. Chem. Soc., 2014, 136, 924 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to the memory of Professor Michael Bendikov. |
| ‡ Electronic supplementary information (ESI) available: Details of experimental procedures, characterization, and the crystallographic parameter table. CCDC 1011954, 1011956 and 1011963. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc05439c |
| This journal is © The Royal Society of Chemistry 2014 |