Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Postsynthetic modification of an amino-tagged MOF using peptide coupling reagents: a comparative study†
Henrik
Hintz
and
Stefan
Wuttke
*
Department of Chemistry and Center for NanoScience (CeNS), University of Munich (LMU), Butenandtstraße 11 (E), 81377 München, Germany. E-mail: stefan.wuttke@cup.uni-muenchen.de
First published on 18th June 2014
Abstract
The suitability of four peptide coupling reagents for postsynthetic modification (PSM) of amino-tagged metal–organic frameworks (MOFs) with carboxylic acids was investigated. Mild reaction conditions at room temperature allow effective covalent attachment of drugs and biomolecules inside the pores of MOFs with moderate chemical stability.
MOFs are crystalline hybrid materials synthesized via self-assembly. They consist of inorganic connectors (metal ions or clusters) and linkers (organic ligands) bound together by covalent and ionic interactions.1 Because of their unique properties – high Brunauer–Emmett–Teller (BET) surface area (up to 7000 m2 g−1) and tunable porosity – MOFs are promising candidates for many applications such as gas storage, gas separation, catalysis, chemical sensing and drug delivery.2 For the synthesis of MOFs for these applications efficient and flexible pathways for chemical functionalization are in great demand.
One approach for incorporation of chemical function into a MOF structure is carried out by pre-modification of the linker before MOF synthesis. However, this pathway is not suitable for incorporation of linker with large and sophisticated functionalities into the MOF structure because of their mutual interference during the MOF formation.3 Moreover, it is not possible to use thermally labile, metal coordinating or solubility lowering functions tagged at the MOF linker.4 Therefore, this procedure is often restricted to small functions like amino, hydroxyl or halocarbons.
A procedure which overcomes the limitations for chemical functionalization described above is postsynthetic modification (PSM). In this approach the organic linker is covalently modified after the MOF has been already formed.5 MOFs with amino-functionalized linker have been used more extensively for PSM than other reactive groups because of their successful incorporation in various structures.4 Acid anhydrides are predominantly used for PSM of these MOFs because of the resistance of often used MOF structures like IRMOF-3 (isoreticular MOF-3, Zn4O(bdc-NH2)3 where bdc-NH2 stands for 2-aminobenzene-1,4-dicarboxylate)6 towards PSM reaction conditions and by-products.7 Indeed, anhydrides are much larger molecules compared to their carboxylic acid analogue which leads to a strong decrease in PSM yield with increasing anhydride chain length for microporous MOF structures.8 One way to reduce reactant size is the application of acid chlorides,9 but in the majority of cases the MOF scaffold would decompose during PSM because of the hydrochloric acid by-product. On the other hand, carboxylic acids react only under strong reaction conditions with amino functions at temperatures above 160 °C.10 Furthermore, in order to have enough space for the incorporation of sophisticated large molecules mesoporous structures have to be employed. However, these structures have lower stability compared to microporous MOFs and hence soft PSM approaches are needed. This is even more important for the covalent incorporation of biological molecules or other sensitive compounds.
In the last century peptide coupling reagents were developed for the unification of carboxylic acid and amino function of amino acids under mild conditions. These reagents generate with the carboxylic acid function an activated compound (acid halide, mixed anhydride or an active ester) which subsequently reacts with the amino function to an amide (Fig. 1).11 In 2009 the mild reaction conditions of peptide coupling reagents were employed for the first time in MOF chemistry for PSM of MIL-101(Fe)-NH2 (Materials from Institute Lavoisier structure number 101 with the empirical formula Fe3OCl(H2O)2(bdc-NH2)3) with the prodrug ethoxysuccinato-cisplatin using 1,1′-carbonyldiimidazole (CDI).12 In 2010 N,N′-dicyclohexylcarbodiimide (DCC) was used to activate the carboxylate outer surface of IRMOF-3 for the subsequent conjugation of green fluorescent protein without loss of structural integrity.13 One year later MIL-68(In)-NH2 (with the empirical formula In(OH)(bdc-NH2))14 was postfunctionalized with fluorenylmethyloxycarbonyl (Fmoc) protected proline and alanine using bromotripyrrolidinophosphonium hexafluorophosphate (PyBroP®) with 4-(dimethylamino)pyridine (DMAP) as base additive.15 PSM yields were about 10% in both cases.15
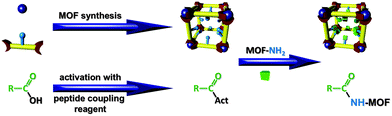 | ||
| Fig. 1 Schematic representation of amino-MOF PSM with activated carboxylic acids generated by peptide coupling reagents. | ||
These examples proof the general adaptability of peptide coupling reagents for MOF PSM. However, reaction conditions and coupling educts differ for every publication. The lack on comparative studies about effectivity of different coupling reagents in PSM leads to the question which coupling reagent to use in order to postfunctionalize an amino-tagged MOF efficiently. Therefore, in this communication four reactive and structurally diverse peptide coupling reagents were employed for a comparative study about synthesis efficiency in PSM of MOFs: (1) N,N′-diisopropylcarbodiimide (DIC) belongs like DCC to the group of carbodiimides. In contrast to DCC the urea side-product of DIC coupling is much better soluble e.g. in dichloromethane16 which could facilitate purification of the MOF structure after PSM. (2) CDI was published as a reagent for peptide coupling in 1958.17 As described above it was already used for PSM with good yields.12 (3) Halophosphonium type reagents were developed to generate acid halides in a much milder way compared to classic reagents for production of acid halides like thionyl chloride. One member of this group with high coupling efficiency is PyBroP®, published in 1990.18 (4) A prominent group of peptide coupling reagents are aminium/uronium type compounds. An effective member of this group used for peptide coupling is N,N,N′,N′-tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate (HBTU), first mentioned in literature for this purpose in 1978.19 After comparing these peptide coupling reagents, we will demonstrate the coupling efficiency for drugs and a small biomolecule.
The comparative study for efficiency of the four peptide coupling reagents was carried out with MIL-101(Al)-NH2 (with the empirical formula Al3OCl(H2O)2(bdc-NH2)3).20 This structure has large pentagonal (12 Å diameter) and hexagonal windows (16 Å diameter) whereby the activated carboxylic acids get access to the interior of the structure.20 Furthermore, the MOF lattice has moderate chemical stability which allows to evaluate the mildness of the coupling reactions. Acetic acid was chosen as carboxylic acid for the study because of its small size minimizing steric hindrance effects. Structures of the activated acids are displayed in Table S2 in ESI,† their projection diameter are compared to the MIL-101(Al)-NH2 window sizes in Fig. S1–S3 in ESI.†
Suitable synthesis conditions for PSM with peptide coupling reagents were evaluated employing PyBroP®. First tentative experiments revealed instability of MIL-101(Al)-NH2 towards DMAP which is a commonly used base for peptide coupling. Therefore, N,N-diisopropylethylamine (Hünig's base) was employed for deprotonation of acetic acid. To determine suitable parameter for reaction time and base amount MIL-101(Al)-NH2 was postfunctionalized with acetic acid employing PyBroP® as well as different amounts of Hünig's base in dichloromethane up to 48 h. PSM yields were measured from 1H-NMR spectra after sample digestion (Fig. S4 in ESI†). X-ray diffraction (XRD) measurements reveal high crystallinity for all samples (Fig. S5 in ESI†). Highest yields were obtained for 6 eq. Hünig's base (referred to 1 eq. amino functions in the MOF structure) and 2 d reaction time.
Acetic acid PSM procedures for MIL-101(Al)-NH2 employing DIC, PyBroP®, HBTU and CDI are presented in Scheme 1. MIL-101(Al)-NH2 (1 eq.) was postfunctionalized with 5.2 eq. DIC. For coupling with PyBroP®, HBTU and CDI only 3 eq. coupling reagent was used. The very low increase in PSM yield for these three coupling reagents when switching to 5 eq. (few percent points) does not justify the increase in costs for coupling reagent and acid. Peptide coupling reactions were carried out each at following five synthesis conditions: N,N-dimethylformamide (DMF) at room temperature for 2 d and at 50 °C for 7 h, dichloromethane at room temperature for 2 d and reflux for 7 h, and in chloroform at reflux for 7 h. All reactions were repeated once again.
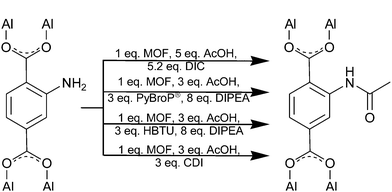 | ||
| Scheme 1 PSM reaction conditions for MIL-101(Al)-NH2 with acetic acid employing peptide coupling reagents. AcOH stands for acetic acid and DIPEA for Hünig's base. | ||
In Fig. 2 PSM yields of MIL-101(Al)-NH2 samples postfunctionalized with acetic acid employing PyBroP®, HBTU, CDI and DIC are displayed. These yields were determined from 1H-NMR spectra after sample digestion in CsF–D2O–DMSO-d6. For all reagents PSM yields of reactions carried out in dichloromethane or chloroform were superior to those obtained by PSM in DMF which might be because of solvation effects. Best results were obtained for DIC in dichloromethane at room temperature (51% yield) followed by PyBroP® and HBTU with around 30% yield for reaction in chloroform at reflux. Moderate yields were obtained for CDI reactions (less than 15% yield).
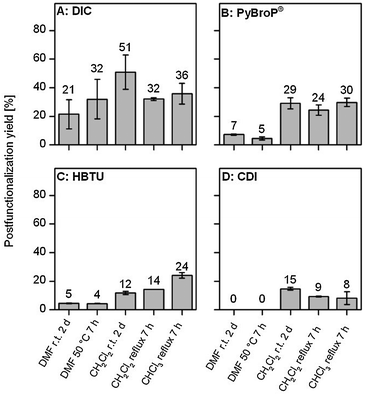 | ||
| Fig. 2 Yields for PSM of MIL-101(Al)-NH2 with acetic acid employing peptide coupling reagents. The error bars express the mean deviation of two independent synthesis with different MOF batches. | ||
For all samples XRD patterns with nearly unchanged crystallinity were obtained (Fig. S6 and S7 in ESI†). Therefore, all coupling reagents are suitable for PSM of MIL-101(Al)-NH2. The XRD patterns of the blank samples incubated without coupling reagent reveal strong damages of the MOF lattice because of the free acid (Fig. S8 in ESI†). Thus, reaction of the carboxylic acid with the coupling reagent before MOF addition prevented the material from acid induced degradation.
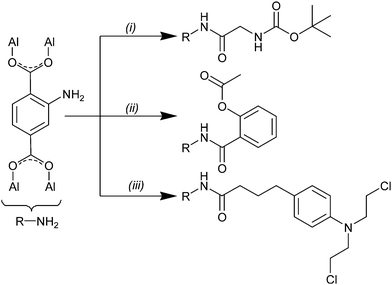
After finding efficient synthesis conditions for PSM with the peptide coupling reagent DIC, its performance was further evaluated for coupling of larger molecules from life science and pharmacy. The activated acids of the tert-butoxycarbonyl protected amino acid glycine (Boc-Gly-OH) and the analgesic acetylsalicylic acid (also known as the registered trade name Aspirin) easily fit through the hexagonal windows of MIL-101(Al)-NH2 (Fig. S1 in ESI†). The activated acid of the larger chemotherapy drug chlorambucil only fits with its short side through the pore openings of MIL-101(Al)-NH2 (Fig. S1 in ESI†). PSM procedures of these three acids are shown in Scheme 2. Each experiment from Scheme 2 was repeated twice. After synthesis the crude products were purified by Soxhlet extraction for 8 h with ethanol.
Apart from a small ethanol residue MOF samples were successfully cleaned by Soxhlet extraction. No signals from coupling reagent DIC or side-products were observed in the NMR spectra (Fig. S9 and S10 in ESI†). For PSM with Boc-Gly-OH a yield of (41.1 ± 0.8)% was measured by NMR which indicates high suitability of DIC for amino acid coupling. PSM yield for acetylsalicylic acid was (6.4 ± 0.2)% which might be referred to a lower reactivity of the aromatic acid with DIC compared to Boc-Gly-OH. However, this yield signifies a statistical distribution of about one modified amino function per MOF cage (derivation can be found in ESI†). PSM yield for coupling with chlorambucil was (2.0 ± 0.2)% which is more than twice the amount expected of only the external surface of this MOF would have been postfunctionalized (derivation can be found in ESI†). Successful PSM was also proven by mass spectrometry (Fig. S11 in ESI†). It is remarkable that the crystallinity of the MOF material is fully maintained throughout the inclusion process; the diffraction lines in the XRD patterns of the postfunctionalized samples are coincident with those of the starting material (Fig. S12 in ESI†). Only a decrease in the Bragg intensities of PSM with acetylsalicylic acid and chlorambucil can be found which might be because of the covalent attachment of the large molecules to the crystalline framework and thus affects its electron density. Nitrogen sorption results (Fig. S13 and S14 and Table S3 in ESI†) show that permanent porosity is retained for all PSM samples. For MOF samples postfunctionalized with Boc-Gly-OH (88 ± 12)% of the BET surface (compared to the surface of the unfunctionalized material) was preserved. Due to the fact that a decrease of about 50% surface area during PSM is typical for MOFs which undergo PSM2 (even by attachment of very small molecules) this result underlines the high potential of PSM with peptide coupling reagents. For the larger molecules acetylsalicylic acid and chlorambucil values of (40 ± 12)% and (54 ± 20)% were calculated.
In conclusion, we presented the first comparative study about the efficiency of peptide coupling reagents for PSM of MOFs in this work. Acetic acid was coupled with amino functionalized MIL-101(Al)-NH2 employing DIC, CDI, HBTU or PyBroP®. Best PSM yields were achieved with DIC, a peptide coupling reagent which is applied for the first time for PSM with MOFs. Purification by Soxhlet extraction led to highly pure material without coupling reagent or by-product impurity. Furthermore, this coupling reagent was used for the covalent attachment of drugs and biomolecules. The results reveal the advantages of using mild reaction conditions at room temperature: retaining the crystallinity and porosity of the MOF and covalent postmodification of thermally and/or chemically sensitive molecules is possible. Thus, covalent attachment of molecules with a peptide coupling reagent is a very convenient way for PSM of amino-tagged MOFs.
The authors are grateful for financial support from the Center for NanoScience Munich (CeNS) and Prof. Thomas Bein (LMU).
Notes and references
- S. Kitagawa, R. Kitaura and S.-i. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS PubMed.
- O. K. Farha, I. Eryazici, N. C. Jeong, B. G. Hauser, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, S. T. Nguyen, A. Ö. Yazaydın and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 15016 CrossRef CAS PubMed; G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC; A. Corma, H. García and F. X. Llabrés i Xamena, Chem. Rev., 2010, 110, 4606 CrossRef PubMed; P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y. K. Hwang, V. Marsaud, P.-N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2009, 9, 172 CrossRef PubMed; L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef PubMed.
- Z. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315 RSC.
- S. M. Cohen, Chem. Rev., 2012, 112, 970 CrossRef CAS PubMed.
- S. M. Cohen, Chem. Sci., 2010, 1, 32 RSC.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469 CrossRef CAS PubMed.
- S. J. Garibay, Z. Wang, K. K. Tanabe and S. M. Cohen, Inorg. Chem., 2009, 48, 7341 CrossRef CAS PubMed.
- K. K. Tanabe, Z. Wang and S. M. Cohen, J. Am. Chem. Soc., 2008, 130, 8508 CrossRef CAS PubMed.
- A. Modrow, D. Zargarani, R. Herges and N. Stock, Dalton Trans., 2012, 41, 8690 RSC.
- B. S. Jursic and Z. Zdravkovski, Synth. Commun., 1993, 23, 2761 CrossRef CAS.
- E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606 RSC.
- K. M. L. Taylor-Pashow, J. Della Rocca, Z. Xie, S. Tran and W. Lin, J. Am. Chem. Soc., 2009, 131, 14261 CrossRef CAS PubMed.
- S. Jung, Y. Kim, S.-J. Kim, T.-H. Kwon, S. Huh and S. Park, Chem. Commun., 2011, 47, 2904 RSC.
- M. Savonnet, D. Farrusseng, C. Pinel, D. Bazer-Bachi, N. Bats and V. Lecocq, US Pat., US 2012/0296095 A1, 2012 Search PubMed.
- J. Canivet, S. Aguado, G. Bergeret and D. Farrusseng, Chem. Commun., 2011, 47, 11650 RSC.
- S.-Y. Han and Y.-A. Kim, Tetrahedron, 2004, 60, 2447 CrossRef CAS PubMed.
- G. W. Anderson and R. Paul, J. Am. Chem. Soc., 1958, 80, 4423 CrossRef CAS.
- J. Coste, D. Le-Nguyen and B. Castro, Tetrahedron Lett., 1990, 31, 205 CrossRef CAS.
- V. Dourtoglou, J.-C. Ziegler and B. Gross, Tetrahedron Lett., 1978, 19, 1269 CrossRef.
- M. Hartmann and M. Fischer, Microporous Mesoporous Mater., 2012, 164, 38 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cc02650k |
| This journal is © The Royal Society of Chemistry 2014 |