Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High-yield synthesis and crystal structure of a green Au30 cluster co-capped by thiolate and sulfide†
Huayan
Yang‡
a,
Yu
Wang‡
a,
Alison J.
Edwards
b,
Juanzhu
Yan
a and
Nanfeng
Zheng
*a
aState Key Laboratory for Physical Chemistry of Solid Surfaces and Department of Chemistry, Xiamen University, Xiamen 361005, China. E-mail: nfzheng@xmu.edu.cn; Web: http://chem.xmu.edu.cn/groupweb/nfzheng/index.asp Fax: +86 592 2183047; Tel: +86 592 2186821
bBragg Institute, Australian Nuclear Science and Technology Organization, New Illawarra Road, Lucas Heights, New South Wales 2234, Australia
First published on 24th September 2014
Abstract
A green gold-cluster, Au30S(StBu)18, was successfully prepared in high yield and crystallographically characterized. Each cluster consists of an Au22 core capped by a mixed layer of staple Au-thiolate units, bridging thiolates and a μ3-S2−.
Since the crystallographic structure determination of Au102(p-MBA)44 in 2007,1 thiolate-stabilized metal nanoclusters have attracted increasing research attention owing to their well-defined molecular structures.2–7 A series of atomically precise thiolated/selenolated Au nanoclusters have been synthesized and crystallographically characterized.8–13 In reported thiolated Au nanoclusters, staple Aux(SR)x+1 units are commonly observed in their surface protected layers. Numerous subsequent studies have demonstrated the influence of both the metal species in the core and capping ligands on the surface features of thiolated metal nanoclusters. No staple units were revealed on the surfaces of thiolated metal nanoclusters where metals other than Au were introduced. Ag44 and Au12Ag32 clusters reported to date do not include staple units.14–16 Non-staple units were proposed on the surface of Au41(S-Eind)12 where a bulky thiol is used.17 A μ3-like bonding of that ligand to Au was suggested. The potential for a diverse array of structure motifs beyond simple Aux(SR)x+1 staple units is envisaged to enrich the structures of thiolated metal nanoparticles and expand the range of surface properties beyond those of known thiolated-Au nanoclusters.17,18
Here we report the high-yield synthesis of Au30S(StBu)18 and its crystal structure. A trace amount of Na2S is used as the S2− source to explore incorporation of S2− on the surface of thiolated nanoclusters. The crystal structure analysis reveals that each cluster comprises an Au22 core capped by a mixed layer of staple Au-thiolate units, bridging thiolates and a μ3-S2−. As part of the surface protecting layer, S2− serves as a μ3-ligand coordinating to three Au atoms. The structure of Au30S(StBu)18 gives direct evidence that μ3-S2− readily acts as a surface protecting ligand to introduce a new surface structure giving potential for preparing more structurally diverse thiolated Au nanoclusters.
To incorporate S2− onto the surface of thiolated Au nanoclusters, Na2S was introduced deliberately in the synthesis of Au nanoclusters. In a typical synthesis of Au30S(StBu)18 nanoclusters (ESI†), HAuCl4 and tert-butylthiol (t-C4H9SH) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio) were combined in tetrahydrofuran (THF). After stirring for 15 min at 55 °C, aqueous solutions of NaBH4 and Na2S were added simultaneously into the mixture of HAuCl4 and t-C4H9SH. The ratio of HAuCl4
3 molar ratio) were combined in tetrahydrofuran (THF). After stirring for 15 min at 55 °C, aqueous solutions of NaBH4 and Na2S were added simultaneously into the mixture of HAuCl4 and t-C4H9SH. The ratio of HAuCl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaBH4
NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S was 50
Na2S was 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The reaction mixture turned dark-brown immediately and was kept under stirring at 55 °C for another hour. After the aqueous layer was removed, toluene and excess t-C4H9SH were added to the reaction mixture whose temperature was then raised to 60 °C. The colour of the solution gradually changed from dark-brown to dark-green in the following 6 h stirring at 60 °C. Brown sheet-like single crystals of Au30S(StBu)18 were recrystallized by diffusing hexane into the cluster solution in CH2Cl2 at 4 °C over 15 days. The crystals were readily redissolved in toluene to give a green solution.
1. The reaction mixture turned dark-brown immediately and was kept under stirring at 55 °C for another hour. After the aqueous layer was removed, toluene and excess t-C4H9SH were added to the reaction mixture whose temperature was then raised to 60 °C. The colour of the solution gradually changed from dark-brown to dark-green in the following 6 h stirring at 60 °C. Brown sheet-like single crystals of Au30S(StBu)18 were recrystallized by diffusing hexane into the cluster solution in CH2Cl2 at 4 °C over 15 days. The crystals were readily redissolved in toluene to give a green solution.
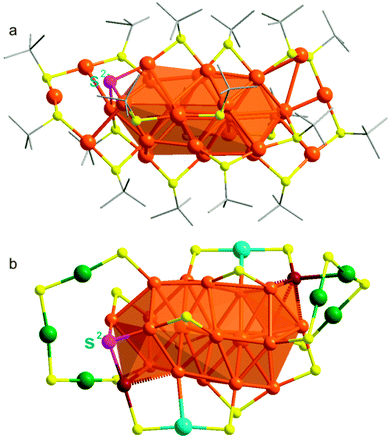
The molecular structure of Au30S(StBu)18 was determined by X-ray single crystal analysis.§ Au30S(StBu)18 clusters are crystallized in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Fig. S1, ESI†). As illustrated in Fig. 1 and Fig. S2 (ESI†), Au30S(StBu)18 is formulated as a neutral cluster with a rod-like Au22 core capped by a mixed layer of thiolate ligands, gold–thiolate complex units and S2−. The mixed surface capping layer consists of two Au3(SR)4 staple units, two Au(SR)2 units, six bridging thiolate SR ligands and one S2−. Although the Au-thiolate staple units1,8–12 and bridging thiolates10 have been previously revealed as important surface capping motifs in the reported structures of thiolated-Au nanoclusters, the presence of surface μ3-S2− motifs has not been previously observed in thiolated Au nanoclusters.
(Fig. S1, ESI†). As illustrated in Fig. 1 and Fig. S2 (ESI†), Au30S(StBu)18 is formulated as a neutral cluster with a rod-like Au22 core capped by a mixed layer of thiolate ligands, gold–thiolate complex units and S2−. The mixed surface capping layer consists of two Au3(SR)4 staple units, two Au(SR)2 units, six bridging thiolate SR ligands and one S2−. Although the Au-thiolate staple units1,8–12 and bridging thiolates10 have been previously revealed as important surface capping motifs in the reported structures of thiolated-Au nanoclusters, the presence of surface μ3-S2− motifs has not been previously observed in thiolated Au nanoclusters.
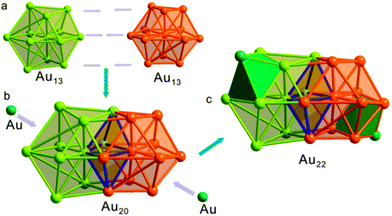
As depicted in Fig. 2, the Au22 core of Au30S(StBu)18 can be better described structurally as a rod-like Au20 unit face-capped by two Au atoms. Au20 is a bicuboctahedral unit consisting of two distorted interpenetrating Au13 cuboctahedrons, similar to the Au20 core of Au28(TBBT)20.10 In the core of Au30S(StBu)18, however, the Au20 unit is further face-capped by two Au atoms at either end, resulting in the formation of a rod-like Au22 core. The average Au–Au bond length of the Au20 bicuboctahedron is 2.89 Å which is comparable to the bond length in bulk gold (2.88 Å) and shorter than the bond length of the Au20 kernel of Au28 (2.92 Å).
The presence of two additional face-capping Au atoms (Aucap) in the core of Au30S(StBu)18 significantly modifies the surface binding structure when compared with that of Au28(TBBT)20.10 While the Au20 core in Au28(TBBT)20 is protected by four Au2(SR)3 staple units and eight bridging SR ligands, no comparable Au2(SR)3 staple units are revealed on the surface of Au30S(StBu)18 for which a diversity of surface motifs are identified. As shown in Fig. 1b and Fig. S3 (ESI†), each of the two Au3(SR)4 staple units is capping an end of the Au22 core with the terminal thiolates binding to Au atoms on the bicuboctahedral unit. The two Au(SR)2 units bind at the sides of the rod-like Au22 core with one thiolate coordinating to an Au atom on the bicuboctahedral unit and the other to a capping Au atom. Similar to the situation in [Au23(SC6H11)16]−,12 each of the two face-capping Au atoms in the core forms a linker between the Au3(SR)4 and Au(SR)2 units. The Au22 core is further bound by four bridging SR at its sides, two bridging SR ligands on both ends, and one μ3-S2− on one end. The average Au–S bond length/Au–S–Au bond angle are 2.311 Å/96.281° and 2.330 Å/97.337° in the Au3(SR)4 and Au(SR)2 units, respectively. Compared with those in staple units, the average Au–S–Au bond angle (92.22°) at the six bridging SR ligands is smaller and their average Au–S bond length (2.338 Å) is slightly longer.
Only one μ3-S2− is present on the surface of Au30S(StBu)18, rendering the cluster asymmetric due to its location at one end of the Au22 core. The μ3-S2− binds to two adjacent Au atoms on the bicuboctahedral Au20 unit and to one Aucap atom as described above. Such a coordination mode differentiates the binding structures of the two face-capping Au atoms in the Au22 core. While the Aucap at the end without μ3-S2− binding caps an Au4 square, the other Aucap only caps three Au atoms of the bicuboctahedral unit. Although observed in small Au clusters,19 the presence of μ3-S2− surface motifs has not been well reported in large thiolated Au nanoclusters. The unique μ3-coordinated sulfide on an Au cluster presents an opportunity to create custom-modified thiolated Au nanoclusters with the potential for targeted functionality and applications.
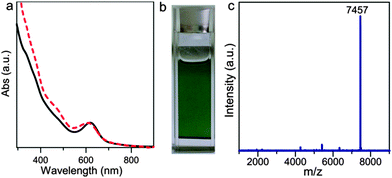
As illustrated in Fig. 3a, the UV-Vis spectrum of Au30S(StBu)18 in toluene displays one major absorption peak at 620 nm and two shoulder peaks around 375 nm and 475 nm (Fig. 3b). Such an optical absorption of Au30S(StBu)18 is very similar to that of the all-thiolate-protected Au30(S-tBu)18 cluster.20 Very recently, Dass and coworkers also obtained single crystals of Au30S(StBu)18 during the crystallization of chromatographically purified Au30(StBu)18.21 With the use of a trace amount of Na2S, we demonstrate a high-yield, high-purity synthesis of Au30S(StBu)18 in a one-pot method without purification. The crude reaction product of this synthesis displayed an identical UV-Vis absorption to that of single crystals of Au30S(StBu)18 dissolved in CH2Cl2 (Fig. 3a). To confirm the high purity of Au30S(StBu)18 in the reaction mixture, the mass spectra of both the crude product and the pure crystals were further analysed. A clean and strong peak with m/z of 7457 is clearly observed in both mass spectra (Fig. 3c and Fig. S4, ESI†). The peak is assigned to [Au30S(StBu)17]+ which corresponds to the loss of one StBu− group from the Au30S(StBu)18 cluster. These results demonstrate the high purity of Au30S(StBu)18 in the crude product. When the laser intensity used in the MALDI-MS measurements was increased, further loss of organic ligands from the cluster was observed (Fig. S5, ESI†). The high-yield production of Au30S(StBu)18 on introducing Na2S could be rationalized by the presence of μ3-S2− which helps to relieve the steric pressure caused by the adjacent five StBu groups. It should be noted that Au30S(StBu)18 is not luminescent while strongly absorbing in the UV-Vis region.
In conclusion, a new gold-nanocluster, Au30S(StBu)18, was successfully synthesized and structurally determined by single crystal analysis. The introduction of a trace amount of Na2S was found to be critical for achieving the high-yield synthesis of Au30S(StBu)18. While the core of Au30S(StBu)18 is an Au22 unit that can be described as a bicuboctahedral Au20 unit capped by two Au atoms, the surface layer of the cluster consists of two Au3(SR)4 staple units, two Au(SR)2 units, six bridging SR ligands and one μ3-S2−. The presence of μ3-S2− on a thiolated Au nanocluster provides opportunities to create and manipulate surface structures of thiolated Au nanoparticles.
We thank the Ministry of Science and Technology of China (2011CB932403) and the National Natural Science Foundation of China (21131005, 21420102001, 21390390, 21227001) for financial support.
Notes and references
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Science, 2007, 318, 430 CrossRef CAS PubMed.
- H. Qian, M. Zhu, Z. Wu and R. Jin, Acc. Chem. Res., 2012, 45, 1470–1479 CrossRef CAS PubMed.
- P. Maity, S. Xie, M. Yamauchi and T. Tsukuda, Nanoscale, 2012, 4, 4027–4037 RSC.
- D. Jiang, Nanoscale, 2013, 5, 7149–7160 RSC.
- H. Häkkinen, Nat. Chem., 2012, 4, 443–455 CrossRef PubMed.
- Y. Yu, Z. Luo, D. M. Chevrier, D. T. Leong, P. Zhang, D.-e. Jiang and J. Xie, J. Am. Chem. Soc., 2014, 136, 1246–1249 CrossRef CAS PubMed.
- R. Jin and K. Nobusada, Nano Res., 2014, 7, 285–300 CrossRef CAS PubMed.
- H. F. Qian, W. T. Eckenhoff, Y. Zhu, T. Pintauer and R. C. Jin, J. Am. Chem. Soc., 2010, 132, 8280–8281 CrossRef CAS PubMed.
- M. W. Heaven, A. Dass, P. S. White, K. M. Holt and R. W. Murray, J. Am. Chem. Soc., 2008, 130, 3754–3755 CrossRef CAS PubMed.
- C. J. Zeng, T. Li, A. Das, N. L. Rosi and R. C. Jin, J. Am. Chem. Soc., 2013, 135, 10011–10013 CrossRef CAS PubMed.
- M. Z. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2008, 130, 5883–5885 CrossRef CAS PubMed.
- A. Das, T. Li, K. Nobusada, C. Zeng, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2013, 135, 18264–18267 CrossRef CAS PubMed.
- Y. Song, S. Wang, J. Zhang, X. Kang, S. Chen, P. Li, H. Sheng and M. Zhu, J. Am. Chem. Soc., 2014, 136, 2963–2965 CrossRef CAS PubMed.
- H. Y. Yang, Y. Wang, H. Q. Huang, L. Gell, L. Lehtovaara, S. Malola, H. Häkkinen and N. F. Zheng, Nat. Commun., 2013, 4, 2422 Search PubMed.
- X. Zhang, H. Y. Yang, X. J. Zhao, Y. Wang and N. F. Zheng, Chin. Chem. Lett., 2014, 25, 839–843 CrossRef CAS PubMed.
- A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399–402 CrossRef CAS PubMed.
- J. Nishigaki, R. Tsunoyama, H. Tsunoyama, N. Ichikuni, S. Yamazoe, Y. Negishi, M. Ito, T. Matsuo, K. Tamao and T. Tsukuda, J. Am. Chem. Soc., 2012, 134, 14295–14297 CrossRef CAS PubMed.
- P. J. Krommenhoek, J. Wang, N. Hentz, A. C. Johnston-Peck, K. A. Kozek, G. Kalyuzhny and J. B. Tracy, ACS Nano, 2012, 6, 4903–4911 CrossRef CAS PubMed.
- Q.-M. Wang, Y.-A. Lee, O. Crespo, J. Deaton, C. Tang, H. J. Gysling, M. Concepción Gimeno, C. Larraz, M. D. Villacampa, A. Laguna and R. Eisenberg, J. Am. Chem. Soc., 2004, 126, 9488–9489 CrossRef CAS PubMed.
- D. Crasto and A. Dass, J. Phys. Chem. C, 2013, 117, 22094–22097 CAS.
- D. Crasto, S. Malola, G. Brosofsky, A. Dass and H. Häkkinen, J. Am. Chem. Soc., 2014, 136, 5000–5005 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and crystallographic data, and the MALDI mass spectra of pure Au30S(StBu)18 with the laser irradiation of increased power. CCDC 986161. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc01773k |
| ‡ H.Y. and Y.W. contributed equally to this work. |
| § The diffraction patterns measured for the compound are dominated by the scattering from the Au30S19 core with almost 3000 electrons whereas the less ordered tertiary butyl shell contains fewer than 600 electrons distributed in a considerably large volume. The structure analysis is complicated by the occurrence of twinning which appears to be an inherent property of crystals of this material. Review of the diffraction images confirmed this diagnosis and calculated precession layers demonstrated that additional smaller twin components may also be present. Residual electron density in the core region is ascribed to minor twin components which were not modeled. A large void in the structure, ∼22% of the cell volume, has a calculated electron content of ∼400 and is consistent with occupation by disordered solvent. A detailed description of the structure analysis is provided in the CIF. |
| This journal is © The Royal Society of Chemistry 2014 |