Open Access Article
Open Access ArticleEnantioselective intramolecular propargylic amination using chiral copper–pybox complexes as catalysts†
Masashi
Shibata
,
Kazunari
Nakajima
and
Yoshiaki
Nishibayashi
*
Institute of Engineering Innovation, School of Engineering, The University of Tokyo, Yayoi, Bunkyo-ku, Tokyo, 113-8656, Japan. E-mail: ynishiba@sogo.t.u-tokyo.ac.jp; Fax: +81 3 5841-1175
First published on 29th May 2014
Abstract
Intramolecular propargylic amination of propargylic acetates bearing an amino group at the suitable position in the presence of chiral copper–pybox complexes proceeds enantioselectively to give optically active 1-ethynyl-isoindolines (up to 98% ee). The method described in this communication provides a useful synthetic approach to the enantioselective preparation of nitrogen containing heterocyclic compounds with an ethynyl group at the α-position.
Heterocycles containing a nitrogen atom, such as pyrrolidines, tetrahydroquinolines and isoindolines, and their derivatives are widely found in many natural products and biologically active compounds.1 In addition to classical synthetic approaches to obtain these heterocycles, a variety of preparative methods catalyzed by transition metal complexes have been reported including their asymmetric version for the optically active heterocycles.1,2
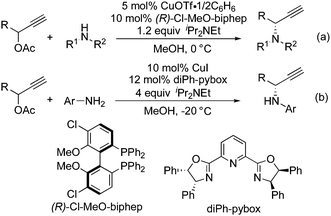
In continuation of our study on the development of transition metal-catalyzed propargylic substitution reactions of propargylic alcohol derivatives with various nucleophiles including their enantioselective versions,3,4 we have recently disclosed the copper-catalyzed propargylic amination of propargylic esters with amines via copper–allenylidene complexes as key reactive intermediates.5–7 In our reaction system, (R)-Cl-MeO-biphep was found to work as an effective ligand toward the propargylic amination with secondary amines such as N-methylaniline (Scheme 1(a)),5 in contrast to van Maarseveen's reaction system, where the propargylic amination with primary amines was achieved by using diPh-pybox as a chiral ligand (Scheme 1(b)).6 Based on these research backgrounds, we envisaged the application of this reaction system to the preparation of heterocycles containing a nitrogen atom via an intramolecular cyclization of propargylic esters bearing an amine moiety at a suitable position. In fact, we have succeeded in obtaining chiral 1-ethynyl-isoindolines in good to high yields with up to 98% ee. Preliminary results are described here.
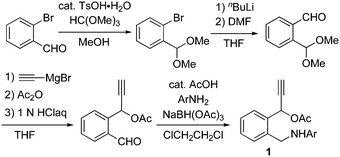
We have designed 1-phenylpropargylic acetates bearing an aminomethyl group at the ortho-position of the benzene ring 1, which were prepared via four steps from 2-bromobenzaldehyde, as shown in Scheme 2. After the protection of the original formyl group in 2-bromobenzaldehyde, the introduction of another formyl group and sequential ethynylation of the formyl group gave 1-(2-formylphenyl)prop-2-yn-1-yl acetate in a good yield. Then, reductive amination with various aniline derivatives led to the formation of 1 in high yields.
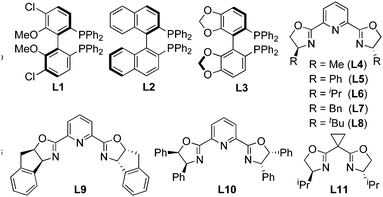
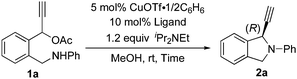
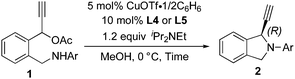
Treatment of 1-(2-((phenylamino)methyl)phenyl)prop-2-yn-1-yl acetate (1a) in methanol at room temperature for 14 h in the presence of 5 mol% of CuOTf·1/2(C6H6) and 10 mol% of (R)-Cl-MeO-biphep8 (L1) gave 1-ethynyl-2-phenylisoindoline (2a) in 17% yield with 57% ee (Table 1, entry 1). Typical results are shown in Table 1. The use of related diphosphines such as (R)-binap9 (L2) and (R)-segphos10 (L3) as chiral ligands afforded only low yields of 2a (Table 1, entries 2 and 3). When pyboxs were used as chiral ligands under the same reaction conditions, the intramolecular amination proceeded smoothly to give 2a in good to high yields with a high enantioselectivity. The use of a larger amount (2 equiv. to Cu atom) of pyboxs slightly increased the enantioselectivity in all cases. (S)-Me-pybox11 (L4) was found to work as an effective chiral ligand to achieve the highest enantioselectivity, i.e. 89% ee (Table 1, entry 4) although the use of related pyboxs such as Ph-pybox11 (L5), iPr-pybox11 (L6), and Bn-pybox11 (L7) gave high enantioselectivities (85% ee, 82% ee, and 80% ee, respectively) (Table 1, entries 5–7). Other pyboxs such as tBu-pybox11 (L8), indan-pybox11 (L9), and diPh-pybox6 (L10) did not work as effective ligands, with only low to moderate enantioselectivities (56% ee, 50% ee, and 23% ee, respectively) (Table 1, entries 8–10). When a bis(oxazoline) ligand12 (L11) was used as a chiral ligand, the amination did not occur smoothly, affording 2a with only a low enantioselectivity (Table 1, entry 11). A higher enantioselectivity was observed when the cyclic amination was carried out at a lower reaction temperature by using L4 and L5 as chiral ligands. The highest enantioselectivity was achieved at 0 °C and −10 °C by using L4 (Table 1, entries 12 and 13). A slightly lower enantioselectivity was observed in the reaction at 0 °C by using L5 as a chiral ligand (Table 1, entry 14).
| Entry | Ligand | Time (h) | Yield of 2ab (%) | eec (%) |
|---|---|---|---|---|
| a Reactions of 1a (0.2 mmol) in the presence of CuOTf·1/2(C6H6) (0.01 mmol), ligand (0.02 mmol), and iPr2NEt (0.24 mmol) were carried out in MeOH at room temperature. b Isolated yield. c Determination by HPLC. d The opposite absolute configuration (1S) was found. e At 0 °C. f At −10 °C. | ||||
| 1 | L1 | 14 | 17 | 57d |
| 2 | L2 | 4 | 19 | 55d |
| 3 | L3 | 20 | 2 | 17d |
| 4 | L4 | 4 | 80 | 89 |
| 5 | L5 | 4 | 83 | 85 |
| 6 | L6 | 4 | 81 | 82 |
| 7 | L7 | 8 | 87 | 80 |
| 8 | L8 | 20 | 25 | 56 |
| 9 | L9 | 4 | 49 | 50 |
| 10 | L10 | 4 | 82 | 23 |
| 11 | L11 | 4 | 34 | 20d |
| 12e | L4 | 8 | 87 | 93 |
| 13f | L4 | 20 | 87 | 93 |
| 14e | L5 | 8 | 91 | 90 |
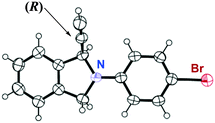
Intramolecular cyclic amination of various propargylic acetates bearing an aminomethyl group was investigated by using L4 and L5 as chiral ligands. Typical results are shown in Table 2. The presence of a substituent such as a methyl, fluoro, or bromo group at the para-position of the benzene ring in the amino group decreased the reactivity, a longer reaction time (20–30 h) being necessary to obtain the corresponding 1-ethynyl-isoindolines in high yields with a high enantioselectivity (Table 2, entries 1–6). The highest enantioselectivity was achieved in the reaction of 1d as a substrate by using L5 (Table 2, entry 8). After one recrystallization of crude cyclic product, the enantiomerically pure 2d was isolated and its absolute configuration (1R) was determined by X-ray analysis (Fig. 1).13
| Entry | 1, Ar | Ligand | Time (h) | Yield of 2b (%) | eec (%) |
|---|---|---|---|---|---|
| a Reactions of 1 (0.2 mmol) in the presence of CuOTf·1/2(C6H6) (0.01 mmol), L4 or L5 (0.02 mmol), and iPr2NEt (0.24 mmol) were carried out in MeOH at 0 °C. b Isolated yield. c Determination by HPLC. | |||||
| 1 | 1a, C6H5 | L4 | 8 | 87 | 93 |
| 2 | 1a, C6H5 | L5 | 8 | 91 | 90 |
| 3 | 1b, 4-MeC6H4 | L4 | 20 | 79 | 92 |
| 4 | 1b, 4-MeC6H4 | L5 | 20 | 70 | 92 |
| 5 | 1c, 4-FC6H4 | L4 | 30 | 79 | 95 |
| 6 | 1c, 4-FC6H4 | L5 | 30 | 77 | 88 |
| 7 | 1d, 4-BrC6H4 | L4 | 30 | 89 | 93 |
| 8 | 1d, 4-BrC6H4 | L5 | 30 | 89 | 96 |
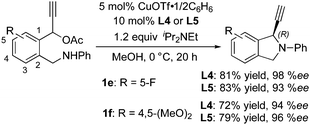
Next, we investigated the nature of substituents on the aromatic scaffold linking the propargylic acetate. Typical results are shown in Scheme 3. The introduction of a fluoro group at the 5-position and two methoxy groups at the 4- and 5-positions substantially increased the enantioselectivity under the same reaction conditions.
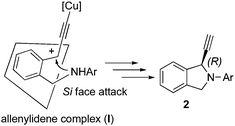
As described in our previous work, the intermolecular propargylic amination proceeded via copper–allenylidene complex (I),5,6,14 which was generated from the copper–pybox complex with the propargylic acetate. At present, we consider that the intramolecular amination also proceeds via a similar reaction pathway. The absolute configuration at the propargylic position in 2 indicates that the intramolecular attack of an amino group on the cationic γ-carbon in I occurs from the Si face (Fig. 2).
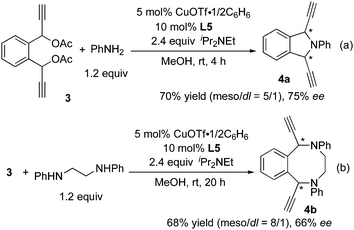
The successful results of the intramolecular cyclic amination prompted us to investigate double propargylic amination including sequential inter- and intra-molecular amination (Scheme 4). The reaction of 1,1′-(1,2-phenylene)-bis(prop-2-yne-1,1-diyl) diacetate (3) with aniline in methanol at room temperature in the presence of 5 mol% of CuOTf·1/2(C6H6) and 10 mol% of L5 proceeded smoothly to give 1,3-di(ethynyl)-2-phenylisoindoline (4a) in 70% yield as a mixture of two diastereoisomers (meso-isomer/DL-isomer = 5/1) (Scheme 4(a)). The minor DL-isomer was obtained with 75% ee. On the other hand, the reaction of 3 with N,N′-diphenylethane-1,2-diamine under the same reaction conditions afforded 1,6-diethynyl-2,5-diphenyl-1,2,3,4,5,6-hexahydrobenzo[f][1,4]diazocine (4b) in 68% yield as a mixture of two diastereoisomers (meso-isomer/DL-isomer = 8/1) (Scheme 4(b)). The minor DL-isomer was obtained with 66% ee. The low selective formation of DL-isomers in the both reaction systems indicates that the first intermolecular amination of 3 took place with only a low enantioselectivity. This low selectivity was not surprising based on the results found by van Maarseveen and co-workers for the intermolecular amination with primary aniline by using Ph-pybox.6
In summary, we have disclosed the copper-catalyzed intramolecular propargylic amination of propargylic acetates bearing an amine moiety at a suitable position to give optically active 1-ethynyl-isoindolines. In the present reaction system, copper–pybox complexes have been found to work as effective catalysts toward the propargylic amination (up to 98% ee). We believe that the present method provides a useful synthetic approach to the enantioselective preparation of optically active nitrogen containing heterocyclic compounds with an ethynyl group at the α-position with a high enantioselectivity as an application of the copper-catalyzed propargylic amination. Further studies on the transition metal-catalyzed propargylic substitution reactions are currently in progress.
Notes and references
- For reviews, see: (a) M. Chrzanowska and M. D. Rozwadowska, Chem. Rev., 2004, 104, 3341 CrossRef CAS PubMed; (b) Y.-G. Zhou, Acc. Chem. Res., 2007, 40, 1357 CrossRef CAS PubMed; (c) S. Anas and H. B. Kagan, Tetrahedron: Asymmetry, 2009, 20, 2193 CrossRef CAS PubMed; (d) M. Ahamed and M. H. Todd, Eur. J. Org. Chem., 2010, 5935 CrossRef CAS; (e) V. Sridharan, P. A. Suryavanshi and J. C. Menéndez, Chem. Rev., 2011, 111, 7157 CrossRef CAS PubMed.
- For enantioselective synthesis of isoindolines, see: (a) Y. Sato, T. Nishimata and M. Mori, J. Org. Chem., 1994, 59, 6133 CrossRef CAS; (b) Y. Sato, T. Nishimata and M. Mori, Heterocycles, 1997, 44, 443 CrossRef CAS PubMed; (c) D. Enders, A. A. Narine, F. Toulgoat and T. Bisschops, Angew. Chem., Int. Ed., 2008, 47, 5661 CrossRef CAS PubMed; (d) C. Wang, X.-H. Chen, S.-M. Zhou and L.-Z. Gong, Chem. Commun., 2010, 46, 1275 RSC; (e) S. Takizawa, N. Inoue, S. Hirata and H. Sasai, Angew. Chem., Int. Ed., 2010, 49, 9725 CrossRef CAS PubMed; (f) R. Morán-Ramallal, V. Gotor-Fernández, P. Laborda, F. J. Sayago, C. Cativiela and V. Gotor, Org. Lett., 2012, 14, 1696 CrossRef PubMed.
- For our recent reviews, see: (a) Y. Miyake, S. Uemura and Y. Nishibayashi, ChemCatChem, 2009, 1, 342 CrossRef CAS; (b) Y. Nishibayashi, Synthesis, 2012, 489 CrossRef CAS PubMed.
- For recent reviews by other research groups, see: (a) R. J. Detz, H. Hiemstra and J. H. van Maarseveen, Eur. J. Org. Chem., 2009, 6263 CrossRef CAS; (b) C.-H. Ding and X.-L. Hou, Chem. Rev., 2011, 111, 1914 CrossRef CAS PubMed; (c) O. Debleds, E. Gayon, E. Vrancken and J.-M. Campagne, Beilstein J. Org. Chem., 2011, 7, 866 CrossRef CAS PubMed; (d) E. B. Bauer, Synthesis, 2012, 1131 CrossRef CAS PubMed.
- For our recent papers of enantioselective propargylic amination, see: (a) G. Hattori, H. Matsuzawa, Y. Miyake and Y. Nishibayashi, Angew. Chem., Int. Ed., 2008, 47, 3781 CrossRef CAS PubMed; (b) G. Hattori, A. Yoshida, Y. Miyake and Y. Nishibayashi, J. Org. Chem., 2009, 74, 7603 CrossRef CAS PubMed; (c) G. Hattori, Y. Miyake and Y. Nishibayashi, ChemCatChem, 2010, 2, 155 CrossRef CAS; (d) G. Hattori, K. Sakata, H. Matsuzawa, Y. Tanabe, Y. Miyake and Y. Nishibayashi, J. Am. Chem. Soc., 2010, 132, 10592 CrossRef CAS PubMed; (e) A. Yoshida, M. Ikeda, G. Hattori, Y. Miyake and Y. Nishibayashi, Org. Lett., 2011, 13, 592 CrossRef CAS PubMed; (f) A. Yoshida, G. Hattori, Y. Miyake and Y. Nishibayashi, Org. Lett., 2011, 13, 2460 CrossRef CAS PubMed.
- For recent papers of enantioselective propargylic amination reported by van Maarseveen and co-workers, see: (a) R. J. Detz, M. M. E. Delville, H. Hiemstra and J. H. van Maarseveen, Angew. Chem., Int. Ed., 2008, 47, 3777 CrossRef CAS PubMed; (b) R. J. Detz, Z. Abiri, R. le Griel, H. Hiemstra and J. H. van Maarseveen, Chem. – Eur. J., 2011, 17, 5921 CrossRef CAS PubMed.
- For recent papers of enantioselective propargylic amination reported by other research groups, see: (a) C. Zhang, Y.-H. Wang, X.-H. Hu, Z. Zheng, J. Xu and X.-P. Hu, Adv. Synth. Catal., 2012, 354, 2854 CrossRef CAS; (b) T. Mino, H. Taguchi, M. Hashimoto and M. Sakamoto, Tetrahedron: Asymmetry, 2013, 24, 1520 CrossRef CAS PubMed.
- (R)-Cl-MeO-biphep = (R)-5,5′-dichloro-6,6′-dimethoxy-2,2′-bis(diphenylphosphino)-1,1′-biphenyl: (a) R. R. Huddleston, H.-Y. Jang and M. J. Krische, J. Am. Chem. Soc., 2003, 125, 11488 CrossRef CAS PubMed; (b) H.-Y. Jang, F. W. Hughes, H. Gong, J. Zhang, J. S. Brodbelt and M. J. Krische, J. Am. Chem. Soc., 2005, 127, 6174 CrossRef CAS PubMed; (c) J. U. Rhee and M. J. Krische, J. Am. Chem. Soc., 2006, 128, 10674 CrossRef CAS PubMed; (d) E. Skucas, J. R. Kong and M. J. Krische, J. Am. Chem. Soc., 2007, 129, 7242 CrossRef CAS PubMed.
- (R)-binap = (R)-2,2′-bis(diphenylphosphino)-1,1′-binaphthyl: R. Noyori and H. Takaya, Acc. Chem. Res., 1990, 23, 345 CrossRef CAS and references therein.
- (R)-segphos = (R)-5,5′-bis(diphenylphosphino)-4,4′-bi-1,3-benzodioxole: H. Shimizu, I. Nagasaki, K. Matsumura, N. Sayo and T. Saito, Acc. Chem. Res., 2007, 40, 1385 CrossRef CAS PubMed and references therein.
- pybox = Pyridine-2,6-bis(oxazolines): G. Desimoni, G. Faita and P. Quadrelli, Chem. Rev., 2003, 103, 3119 CrossRef CAS PubMed.
- For a recent review on bis(oxazoline) ligands, see: G. Desimoni, G. Faita and K. A. Jørgensen, Chem. Rev., 2006, 106, 3561 CrossRef CAS PubMed.
- See ESI† for Experimental details.
- (a) The copper–allenylidene complexes have not yet been isolated and characterized until now although silver- and gold–allenylidene complexes have already been reported by Bertrand and Hashmi, respectively; (b) M. Asay, B. Donnadieu, W. W. Schoeller and G. Bertrand, Angew. Chem., Int. Ed., 2009, 48, 4796 CrossRef CAS PubMed; (c) M. M. Hansmann, F. Rominger and A. S. K. Hashmi, Chem. Sci., 2013, 4, 1552 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 989441. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc01676a |
| This journal is © The Royal Society of Chemistry 2014 |