Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Elucidating proline dynamics in spider dragline silk fibre using 2H–13C HETCOR MAS NMR†
Xiangyan
Shi
,
Jeffery L.
Yarger
* and
Gregory P.
Holland
*
Department of Chemistry and Biochemistry, Magnetic Resonance Research Center, Arizona State University, Tempe, AZ 85287-1604, USA. E-mail: greg.holland@asu.edu; jyarger@gmail.com
First published on 24th March 2014
Abstract
2H–13C HETCOR MAS NMR is performed on 2H/13C/15N-Pro enriched A. aurantia dragline silk. Proline dynamics are extracted from 2H NMR line shapes and T1 in a site-specific manner to elucidate the backbone and side chain molecular dynamics for the MaSp2 GPGXX β-turn regions for spider dragline silk in the dry and wet, supercontracted states.
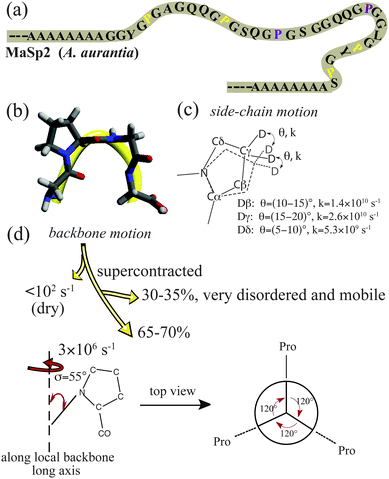
Orb-weaving dragline silk fibre possesses outstanding mechanical properties – a combination of stiffness, toughness and extensibility.1,2 Two proteins, major ampullate spidroin 1 and major ampullate spidroin 2 (MaSp1 and MaSp2), are the primary components of dragline silk from most orb-weaving spiders, including A. aurantia (a common garden spider collected in California).3 MaSp1 and 2 are composed of highly repetitive amino acid motifs with unique secondary structures.2,4–8 Solid-state NMR revealed that poly-Ala (typically 5–8 Ala units long) and poly-(Gly-Ala) motifs found in both MaSp1 and 2 proteins are arranged into β-sheet structures in spider silk fibres, and are believed to be the primary source of fibre strength and stiffness.4,5,8 Gly-Gly-X domains form disordered 31-helical structures and are primarily found in MaSp1.4,5,8 While Gly-Pro-Gly-XX (GPGXX) domains are exclusively found in MaSp2 and form disordered type II β-turn structures, thought to be partially responsible for dragline spider silk's extensibility.6 When exposed to water, spider dragline silk shrinks up to 50% in length and swells in diameter.9,10 This phenomenon is known as supercontraction and is accompanied by a decrease in fibre stiffness and loss of molecular structural order along the fibre axis.9–12 Previous studies suggest that silk supercontraction correlates to its Pro content.13 Pro residues reside exclusively in the GPGXX motif.3 Understanding the dynamics of proline, and hence the GPGXX domain in MaSp2, will provide insight into the supercontraction mechanism and the silk's extensibility.
Deuterium (2H) NMR line shapes and spin–lattice relaxation times (T1) provide extensive information about molecular dynamics and geometry.14 One-dimensional (1D) 2H experiments exhibit poor resolution caused by the large quadrupolar interaction and small chemical shift dispersion. Furthermore, labelling 2H at specific groups is difficult or impossible for natural biopolymers.15 Our research group has recently developed a two-dimensional (2D) 2H–13C heteronuclear correlation (HETCOR) magic angle spinning (MAS) NMR technique for extracting site-specific 2H line shapes for systems with multiple isotope labelled sites.16 The HETCOR NMR experiment was accomplished using cross-polarization (CP)-MAS under carefully calibrated experimental conditions. Further, a method was developed to indirectly measure 2H T1 through 2H–13C CP-MAS in a site-specific manner and was successfully applied to several model systems.16A. aurantia dragline silk was chosen for this study, because it contains a high abundance of MaSp2, and therefore it is more Pro-rich compared to the other dragline silks.17 In present work, 2H–13C HETCOR MAS experiments were performed on dragline silk collected from spiders fed with a U-[2H7,13C5,15N]-Pro aqueous solution. Pro molecular dynamics were probed for both dry (native material) and supercontracted (wet) dragline spider silks.
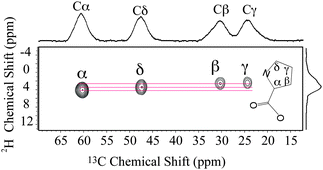
1D liquid-state 2H NMR shows that Pro side-chain CD2 groups and backbone CD were labelled by 2H for the silk samples (Fig. S1, ESI†). The 2H J-splitting patterns indicate that all the 2H labelled groups were also enriched with 13C. This selective amino acid labelling with 2H–13C pairs is ideal for 2D HETCOR MAS NMR experiments for dynamic studies. HETCOR MAS NMR experiments were performed on this dragline silk. A 2D NMR experiment with a small spectral window and rotor-synchronized sampling in the 2H dimension was performed to obtain a 2H–13C chemical shift correlation spectrum (Fig. 1). As shown in the projection of the spectrum, 13C signals were assigned to each Pro group based on the chemical shifts reported in previous studies.6 The 2H–13C correlation was only observed between directly bonded spin pairs, illustrating the site-specific nature of the HETCOR MAS NMR experiment.
2H line shapes were extracted from a 2D NMR experiment with a 2H spectral window larger than the deuterium quadrupolar interaction. The spectrum and extracted 2H line shapes are displayed in Fig. 2. Pro side-chain molecular motion can be described by each CD2 undergoing a two-site reorientation. To interpret this motion for each site, 2H line shape simulations were conducted and compared with the experimental data (see ESI†). It reveals that each deuterium on the side-chain undergoes fast two-site reorientation at an angle of 10–15°, 15–20° and 5–10° for Pro 2Hβ, 2Hγ and 2Hδ, respectively (Fig. 2(b)). The corresponding reorientation rates are greater than 108 s−1. Pro residues in spider dragline silk (GPGXX motif) have much smaller reorientation angles when compared to crystalline proline.16,19 A static MAS pattern was observed for Pro 2Hα, illustrating the rigidity of the Pro backbone environment (<102 s−1) in dry spider dragline silk.
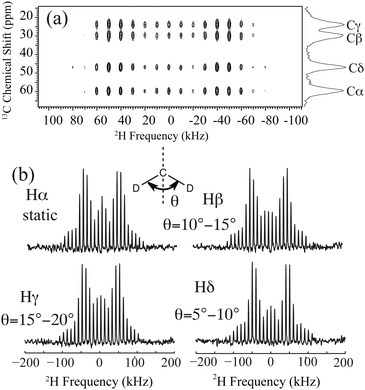 | ||
| Fig. 2 (a) 2H–13C HETCOR MAS NMR spectrum for U-[2H7,13C5,15N]-Pro labelled A. aurantia dragline silk. (b) Pro 2H line shapes extracted from the 2D spectrum and the proposed dynamics for each site. Pro side-chain dynamics is described by each CD2 undergoing fast reorientation between two sites separated by an angle θ. The angles are extracted from comparing experimental 2H line shapes with simulations (see ESI†). | ||
The molecular dynamics of the Pro side-chain was determined to be >108 s−1, however, 2H line shape cannot provide the exact motional rate. 2H T1 is another NMR tool for characterizing the molecular motion on the picosecond – nanosecond timescale. In the current work, site-specific 2H T1 was indirectly measured through 13C-detected 2H–13C CP-MAS experiments. 2H T1's were determined to be 613 ms, 569 ms, 573 ms and 561 ms for Pro 2Hα, 2Hβ, 2Hγ and 2Hδ, respectively (Fig. S3, ESI†). The quadrupolar interaction is the dominant 2H T1 relaxation mechanism and has been hypothesized as the only relevant relaxation source when investigating 2H dynamics for various systems.7,20,21 If only the 2H quadrupolar interaction is considered, a two-site reorientation rate of 1.4 × 1010 s−1, 2.6 × 1010 s−1 and 5.3 × 109 s−1 is determined from the corresponding deuterium T1 for Pro 2Hβ, 2Hγ and 2Hδ, respectively (see ESI† for calculation detail). When the silk is wet and supercontracted, 2H–13C CP signal was undetectable with reasonable NMR experimental time because of inefficient CP. This indicates that the Pro-containing motifs interact strongly with water molecules when the silk is supercontracted and exhibit mobility that completely averages the 2H–13C dipolar interaction that facilitates CP.
1D 2H solid-echo (Fig. 3) and one-pulse (Fig. S4, ESI†) MAS NMR experiments were conducted to probe Pro dynamics in the wet, supercontracted silk. These experiments illustrate that the large Pro 2H spinning sideband (SSBs) pattern observed in dry silk are reduced to a central peak accompanied by one set of weak SSBs when the silk is wet. Significant signal loss was observed for the supercontracted silk in fully relaxed 2H solid-echo and one-pulse MAS NMR spectra (Fig. 3a and Fig. S4, ESI†) compared to the dry silk. For the wet supercontracted silk, the central peak cannot be fit by one peak possessing a Lorentzian, Gaussian or combined lineshape. Instead, fits of 2H 1D data indicate the existence of two components, a broad (∼3.8 kHz FWHM) and narrow peak (∼400 Hz FWHM) (Fig. 3b and Fig. S4, ESI†). The broad component is indicative of microsecond dynamics for a Pro deuterium population. This dynamical process could be the motion of the Pro local backbone as no additional bond on the side-chain is available for the CD2 undergoing another motion besides the fast two-site reorientations. This backbone motion can be described by a simple model where the entire Pro residue undergoes three site reorientation along an external axis with a rate of 3 × 106 s−1 (Fig. 3c). The axis is considered the long axis of the local protein backbone. The observed 2H signal loss is due to the short T2 of the broad component, a consequence of molecular dynamics in the microsecond regime. In contrast, the narrow component obtained from the fit corresponds to a Pro population that becomes extremely mobile due to strong interactions with water. This Pro mobile population accounts for 30–35% based on the signal loss of 2H 1D data of supercontracted silk compared to dry silk and the peak deconvolution (Fig. 3b and Fig. S4, ESI†). Thus, 65–70% of the Pro residues undergo 3 × 106 s−1 backbone motions when silk is wet and supercontracted. As the protein exhibits much higher mobility for supercontracted (wet) silk, the exact dynamical time scale for the Pro side-chain could not be extracted from T1, that is no longer dominated by the 2H quadrupolar interaction.
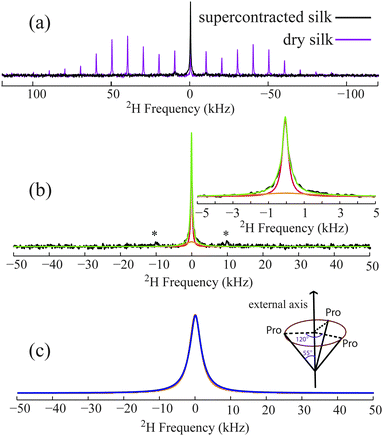 | ||
| Fig. 3 (a) 2H solid-echo MAS NMR spectra for U-[2H7,13C5,15N]-Pro labelled A. aurantia dragline silk in the dry and supercontracted (wet) state. (b) 2H solid-echo MAS spectrum of supercontracted (wet) silk (black, same one as show in (a) and its fit (green)). The central peak region is expanded and shown on the upper right. A broad (orange) and narrow (red) component was used for the fit. The asterisks indicate the spinning sidebands. (c) Experimental (orange) and simulated 2H line shape (blue). The experimental line shape is the broad component extracted from the fit (shown in orange in b). Simulations were performed using SPINEVOLUTION18 with a model of the entire Pro residue undergoing a three-site reorientation along an external axis with a rate of 3 × 106 s−1 (the schematic representation of the motion is show on the upper right). | ||
Pro residues exist exclusively in the MaSp2 protein and within the GPGXX motif. From the primary protein sequence, the GPGXX motifs typically repeat 3–5 times and are sandwiched between the poly-Ala and poly-(Gly-Ala) domains that form rigid β-sheets.3,8 Hence, it is reasonable to propose that the GPGXX motifs close to β-sheet regions (within two repeats) exhibit microsecond motions when the silk is supercontracted, because the dynamics are likely restricted by the flanking rigid β-sheet crystalline domains. In contrast, hydrated GPGXX motifs located further away from the β-sheet regions (>2 repeat units) are less constrained by the crystalline regions and undergo much faster, near isotropic motion. Combining the silk protein primary sequence with the molecular motion revealed by 2H NMR, a model is proposed for Pro side-chain and the local protein backbone dynamics in dry and wet, supercontracted silk (Fig. 4).
In the current work, molecular dynamics for the GPGXX regions in spider dragline silk were investigated with 2H–13C HETCOR MAS NMR. In native (dry) dragline silk, the local backbone dynamics appears static (<102 s−1), while the side-chains undergo fast two-site reorientations in the >109 s−1 regime. For supercontracted (wet) silk, two different molecular motions are observed for the GPGXX units in MaSp2 from 2H MAS NMR: 65–70% of the GPGXX regions exhibit 3 × 106 s−1 backbone motion, while the rest behave near isotropic. These two Pro populations are proposed to be within two units of the β-sheet domains and greater than two units from these rigid regions, respectively.
This work was supported by grants from AFOSR (FA9550-14-1-0014), DURIP (FA2386-12-1-3031 DURIP 12RSL231) and NSF (DMR-1264801). We would also like to thank Dr Brian Cherry for help with NMR instrumentation and student training.
Notes and references
- J. M. Gosline, M. W. Denny and M. E. Demont, Nature, 1984, 309, 551–552 CrossRef CAS.
- R. V. Lewis, Chem. Rev., 2006, 106, 3762–3774 CrossRef CAS PubMed.
- J. Gatesy, C. Hayashi, D. Motriuk, J. Woods and R. Lewis, Science, 2001, 291, 2603–2605 CrossRef CAS PubMed.
- J. D. van Beek, S. Hess, F. Vollrath and B. H. Meier, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 10266–10271 CrossRef CAS PubMed.
- G. P. Holland, J. E. Jenkins, M. S. Creager, R. V. Lewis and J. L. Yarger, Chem. Commun., 2008, 5568–5570 RSC.
- J. E. Jenkins, M. S. Creager, E. B. Butler, R. V. Lewis, J. L. Yarger and G. P. Holland, Chem. Commun., 2010, 46, 6714–6716 RSC.
- A. H. Simmons, C. A. Michal and L. W. Jelinski, Science, 1996, 271, 84–87 CAS.
- G. P. Holland, M. S. Creager, J. E. Jenkins, R. V. Lewis and J. L. Yarger, J. Am. Chem. Soc., 2008, 130, 9871–9877 CrossRef CAS PubMed.
- R. W. Work, J. Exp. Biol., 1985, 118, 379–404 Search PubMed.
- Y. Liu, Z. Shao and F. Vollrath, Nat. Mater., 2005, 4, 901–905 CrossRef CAS PubMed.
- Z. Shao, F. Vollrath, J. Sirichaisit and R. J. Young, Polymer, 1999, 40, 2493–2500 CrossRef CAS.
- Z. T. Yang, O. Liivak, A. Seidel, G. LaVerde, D. B. Zax and L. W. Jelinski, J. Am. Chem. Soc., 2000, 122, 9019–9025 CrossRef CAS.
- Y. Liu, A. Sponner, D. Porter and F. Vollrath, Biomacromolecules, 2008, 9, 116–121 CrossRef CAS PubMed.
- D. A. Torchia, Annu. Rev. Biophys. Bioeng., 1984, 13, 125–144 CrossRef CAS PubMed.
- X. Shi, J. L. Yarger and G. P. Holland, Anal. Bioanal. Chem., 2013, 405, 3997–4008 CrossRef CAS PubMed.
- X. Shi, J. L. Yarger and G. P. Holland, J. Magn. Reson., 2013, 226, 1–12 CrossRef CAS PubMed.
- A. E. Brooks, H. B. Steinkraus, S. R. Nelson and R. V. Lewis, Biomacromolecules, 2005, 6, 3095–3099 CrossRef CAS PubMed.
- M. Veshtort and R. G. Griffin, J. Magn. Reson., 2006, 178, 248–282 CrossRef CAS PubMed.
- S. K. Sarkar, P. E. Young and D. A. Torchia, J. Am. Chem. Soc., 1986, 108, 6459–6464 CrossRef CAS.
- D. A. Torchia and A. Szabo, J. Magn. Reson., 1982, 49, 107–121 CAS.
- L. S. Batchelder, C. H. Niu and D. A. Torchia, J. Am. Chem. Soc., 1983, 105, 2228–2231 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, liquid-state 2H spectrum of hydrolyzed A. aurantia dragline silk, simulated 2H quadrupole line shapes, 13C-detected Pro 2H T1 inversion recovery curves, calculating molecular motional rate using 2H T1, 2H one-pulse spectrum and its fit. See DOI: 10.1039/c4cc00971a |
| This journal is © The Royal Society of Chemistry 2014 |