Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
5,5′-Bis-(trinitromethyl)-3,3′-bi-(1,2,4-oxadiazole): a stable ternary CNO-compound with high density†
M. A.
Kettner
and
T. M.
Klapötke
*
Department of Chemistry, Ludwig-Maximilian University of Munich, Butenandtstr. 5-13, Haus D, 81377 Munich, Germany. E-mail: tmk@cup.uni-muenchen.de; Web: http://www.chemie.uni-muenchen.de/ac/klapoetke/ Fax: +49 (0)89-2180 77492
First published on 17th January 2014
Abstract
5,5′-Bis-(trinitromethyl)-3,3′-bi-(1,2,4-oxadiazole) is a new ternary CNO-compound. It has been synthesized by nitration of diammonium 5,5′-bis-(dinitromethanide)-3,3′-bi-(1,2,4-oxadiazole) with nitronium tetrafluoroborate. Single crystal X-ray diffraction studies show a remarkable high density. Thermal stability and sensitivities of the new compound were determined by differential scanning calorimetry (DSC) and standardized drop hammer and friction tests.
The challenges in the development of the field of energetic materials include – besides better environmental acceptance – the search for materials with preferably high densities, which result in higher performance values.1 This and other requirements have been fulfilled for secondary explosives by various high nitrogen containing materials such as bi-tetrazole2 and bi-triazole3 derivatives (Scheme 1). However, the field of energetic oxidizers for propellants requires in particular a high oxygen content for providing oxygen to the actual fuel for a complete combustion.1 Furthermore, oxidizers are the main ingredient in composite propellants (up to 70 wt%),4 hence a high density results in space-saving effects. It is also desired that the compounds do not explode, but burn fast in an open flame test. It has been shown that novel oxidizers including one or more trinitromethyl moieties do not explode.5 In this communication a hydrogen atom free bi-(1,2,4-oxadiazole) with two trinitromethyl moieties attached at its 5-position is presented. 5,5′-Bis-(trinitromethyl)-3,3′-bi-(1,2,4-oxadiazole) 2 (TNM2-BOD) is analogous to 5,5′-bis-(trinitromethyl)-3,3′-bi-(1,2,4-triazole) synthesized by Petrie et al. in 2012 (Scheme 1),6 just by replacing the NH-group by an oxygen atom (Scheme 1).
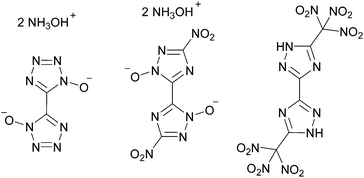 | ||
| Scheme 1 From left to right: structures of TKX-50,2 MAD-X13 and 5,5′-bis(trinitromethyl)-3,3′-bi-(1,2,4-triazole).5 | ||
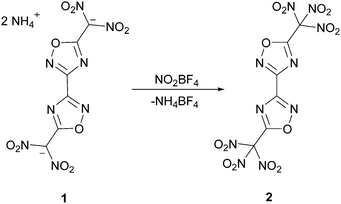
The nitration of previously reported diammonium 5,5′-bis-(dinitromethanide)-3,3′-bi-(1,2,4-oxadiazole) 17 with nitronium tetrafluoroborate was performed in anhydrous acetonitrile at 0 °C for one hour (Scheme 2). The solvent was evaporated and the resulting crude product was extracted with anhydrous diethylether. TNM2-BOD 2 was obtained as colourless crystals suitable for X-ray diffraction. The optimization of the synthesis (yield 27%) is under investigation. It was characterized using 13C and 14N NMR, IR and Raman spectroscopy, mass spectrometry and elemental analysis. Additionally X-ray diffraction measurements were performed.
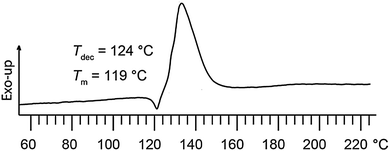
The vibrational analysis shows the characteristic asymmetric NO2 stretching vibrations νas at 1608 cm−1 in the IR, and at 1621 cm−1 in the Raman spectrum. The symmetric NO2 stretching vibrations νs are observed at 1271 cm−1 in the IR, and at 1275 cm−1 in the Raman spectrum.8,9 The 13C NMR spectrum shows the chemical shifts of the carbon atoms in the ring and the trinitromethyl moiety in the expected range: δ = 163.8 (C5), 160.2 (C3) and 117.8 (C(NO2)3) ppm.10 The nitro groups in the 14N NMR spectrum are shifted to δ = −35 ppm.11 Compound 2 has a high oxygen balance with respect to the formation of CO during combustion with 29.3%, and a moderate oxygen balance with respect to CO2 with 7.3%.12 According to DSC measurements compound 2 melts at Tm = 119 °C with subsequent decomposition at Tdec = 124 °C (Tonset, Fig. 1). This has been checked using a melting apparatus (Büchi). Furthermore 2 burns without residues in the open flame as required for energetic oxidizers. The sensitivities towards shock and friction were determined using a drop hammer tester and a friction tester according to BAM standard methods.13 According to the UN recommendations on the transport of dangerous goods14 TNM2-BOD 2 shall be classified as sensitive against both impact (10 J) and friction (80 N) at a grain size of <100 μm. For a practical use as an energetic oxidizer the benchmark sensitivities are defined not to be higher than that of pentaerythrityl tetranitrate (PETN: impact sensitivity = 4 J, friction sensitivity = 80 N). Thus, the sensitivities of compound 2 are in an acceptable range.
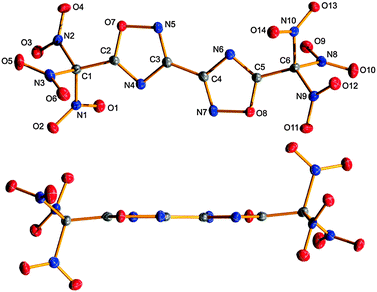
TNM2-BOD crystallizes from diethyl ether or dichloromethane as very thin colourless plates in the monoclinic space group P21/c with four molecules per unit cell and a remarkable high density of 2.02 g cm−3 at 100 K. A measurement at ambient temperature showed a density of 1.94 g cm−3. Fig. 2 presents the molecular structure of TNM2-BOD with perpendicular and lateral views of the rings demonstrating the planarity of the bi-(1,2,4-oxadiazole) unit.
Compared to other solid trinitromethyl containing materials, which tend to have X-ray densities between 1.47 (2,4,6-trimethyl-1-(2,2,2-trinitroethyl)benzene)15 and 2.05 g cm−3 (hexanitroethane),9 TNM2-BOD 2 belongs to the compounds showing the highest densities.16 Among the hydrogen free and neutral CNO-compounds TNM2-BOD 2 displays a remarkably high density, which lies in the range of that observed for various furazane derivatives,17 although not reaching the values of the pernitroalkanes hexanitroethane (2.05)9 and octanitrocubane (1.98),18 or pernitroarenes as hexanitrobenzene (1.99).19 In contrast to the volatile hexanitroethane,9 TNM2-BOD shows practically no tendency to sublime at ambient temperature, however.
The high density might be explained by the presence of weak attractive electrostatic interactions (short contacts) between the nitro groups within the trinitromethyl moieties. In the crystal, intramolecular as well as intermolecular short N⋯O contacts are observed. Such interactions have been widely observed for the solid state in polynitro compounds.21 Within the molecules of 2 a series of N⋯O distances are found, which are clearly shorter than the sum of van der Waals radii (N,O = 3.07 Å),20 as shown in Fig. 3. The distances N1⋯O3, N2⋯O5 and N3⋯O2 are about 2.55 Å (Fig. 2, black dashed), while for N2⋯O1 and N1⋯O6, a distance around 2.94 Å (Fig. 3, gray dashed) is observed. Overall the TNM group arranges to the typical propeller-like orientation of the nitro groups with torsion angles C2–C1–N–O between −36.6(3)° and −50.9(2)° (Fig. 3).22 There are also weak attractions of N2 and O1 from the TNM group to O7 and N4, respectively, of the oxadiazole ring (Fig. 3, light blue dashed). Very similar interactions are observed in the second trinitromethyl group at the opposite side of the molecule.
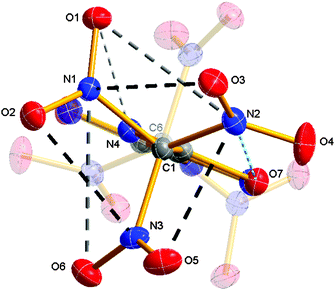 | ||
| Fig. 3 View along C1–C6 displaying the intramolecular interactions within the trinitromethyl moiety; atom distances [Å] and torsion angles [°]: N1–O3 2.543(2), N1–O6 2.967(2), N2–O1 2.924(3), N2–O5 2.568(3), N2–O7 2.981(2), N3–O2 2.566(2), N4–O1 2.896(2); C2–C1–N1–O1 −50.9(2), C2–C1–N2–O4 −36.6(3), C2–C1–N3–O6 −42.9(2) and ∑vdW radii (N,O) = 3.07 Å.20 | ||
There is also a series of short intermolecular N⋯O contacts observed in the crystal, indicating the presence of weak interactions between the single molecules in the solid state. The respective atom distances are listed in Table 1. The interactions occur mostly between the oxygen atoms of the nitro groups and all nitrogen atoms of the rings (N4, N5, N6 and N7). No intermolecular contacts between the bi-(1,2,4-oxadiazole) rings shorter than the sum of van der Waals radii are observed (Fig. 4).
| ∑vdW radii (N,O) = 3.07 Å.8 | |
|---|---|
| N1⋯O12 | 2.927(3) |
| N3⋯O1 | 2.945(3) |
| N6⋯O3 | 2.946(2) |
| N10⋯O3 | 3.010(3) |
| N4⋯O12 | 3.011(2) |
| N5⋯O11 | 3.040(3) |
| N7⋯O4 | 3.055(3) |
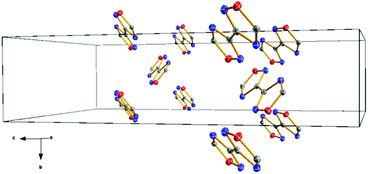 | ||
| Fig. 4 Unit cell content with omitted trinitromethyl groups for demonstrating the orientation of the bi-(1,2,4-oxadiazole) units in one direction and their wave-like arrangement. | ||
The hitherto unknown CNO-compound TNM2-BOD has been synthesized by nitration of diammonium 5,5′-bis(dinitromethanide)-3,3′-bi-(1,2,4-oxadiazole) using nitronium tetrafluoroborate. The density, as measured from single crystal X-ray studies at 100 K (2.02 g cm−3) and at ambient temperature (1.94 g cm−3), is remarkably high. This can be rationalized by various intra- and intermolecular electrostatic N⋯O interactions involving the nitro groups. The new compound TNM2-BOD represents a rare example of a ternary CNO-species. As compared to the volatile hexanitroethane, it combines its properties with those of the bi-(1,2,4-oxadiazole) ring system and represents an interesting non volatile high-energetic alternative.
Financial support of this work by the Ludwig-Maximilian University of Munich (LMU), the U.S. Army Research Laboratory (ARL) under grant no. W911NF-09-2-0018, the Armament Research, Development and Engineering Center (ARDEC) under grant no. W911NF-12-1-0467 & W911NF-12-1-0468, and the Office of Naval Research (ONR) under grant no. ONR.N00014-10-1-0535 and ONR.N00014-12-1-0538 is gratefully acknowledged. The authors acknowledge collaborations with Dr Mila Krupka (OZM Research, Czech Republic), in the development of new testing and evaluation methods for energetic materials and with Dr Muhamed Suceska (Brodarski Institute, Croatia), in the development of new computational codes to predict the detonation and propulsion parameters of novel explosives. We are indebted to and thank Drs Betsy M. Rice and Brad Forch (ARL, Aberdeen, Proving Ground, MD) for many inspired discussions. The X-ray team of Prof. Dr K. Karaghiosoff is gratefully thanked for various measurements.
Notes and references
- (a) T. M. Klapötke, Chemie der hochenergetischen Materialien, de Gruyter GmbH & Co., KG, Berlin, New York, 2009 Search PubMed; (b) T. M. Klapötke, Chemistry of High-Energy Materials, de Gruyter GmbH & Co., KG, Berlin, New York, 2011 Search PubMed.
- T. M. Klapötke, N. Fischer, D. Fischer, D. Piercey, J. Stierstorfer and M. Reymann, WO Pat. 026768 A1, 2013.
- A. Dippold, T. M. Klapötke and F. A. Martin, Z. Anorg. Allg. Chem., 2011, 637, 1181–1193 CrossRef CAS.
- (a) NASA, Space Shuttle News Reference, 2–20–22–21, http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19810022734_1981022734.pdf; (b) NASA, press release: STS-122 The Voyage of Columbus, 2008, 82–84, http://www.nasa.gov/pdf/203212main_sts 122_presskit2.pdf.
- (a) M. Göbel and T. M. Klapötke, Z. Anorg. Allg. Chem., 2007, 633, 1006–1017 CrossRef; (b) Q. J. Axthammer, M. A. Kettner, T. M. Klapötke, R. Moll and S. F. Rest, Proceedings of Seminar on New Trends in Research of Energetic Materials, NTREM, Pardubice, Czech Republic, April 10–12th, 2013, Part 1, 29–39; (c) Q. J. Axthammer, M. A. Kettner, T. M. Klapötke, R. Moll and S. F. Rest, Development of High Energy Dense Oxidizers based on CHNO(F)-materials, EUCASS Conference, Munich, July 3rd 2013.
- M. A. Petrie, G. Koolpe, R. Malhotra and P. Penwell, ONR final report, SRI international, SRI Project No. PI8608, March 2012.
- (a) M. Weyrauther, T. M. Klapötke and J. Stierstorfer, Proceedings of Seminar on New Trends in Research of Energetic Materials, NTREM, Pardubice, Czech Republic, April 10–12th, 2013, Part 1, 982–995; (b) T. M. Klapötke, N. Mayr, J. Stierstorfer and M. Weyrauther, Chem.–Eur. J., 2013 DOI:10.1002/chem.201303825.
- G. Socrates, Infrared and Raman Characteristic Group Frequencies: Tables and Charts, John Wiley & Sons, Chichester, 3rd edn, 2004 Search PubMed.
- D. Bougeard, R. Boese, M. Polk, B. Woost and B. Schrader, J. Phys. Chem. Solids, 1986, 47(12), 1129–1137 CrossRef CAS.
- H.-O. Kalinowski, S. Berger and S. Braun, 13C-NMR Spektroskopie, Georg Thieme Verlag KG, Stuttgart, New York, 1984 Search PubMed.
- M. Witanowski, L. Stefaniak and G. A. Webb, Annual Reports on NMR Spectroscopy, 25, Academic Press Inc., London, 1993 Search PubMed.
- B. Aas, M. A. Kettner, T. M. Klapötke and C. Zoller, Eur. J. Inorg. Chem., 2013, 2013(35), 6028–6036 CrossRef CAS.
- (a) Bundesanstalt für Materalforschung (BAM), http://www.bam.de; laying down test methods pursuant to Regulation (EC) No. 1907/2006 of the European Parliament and of the Council on the Evaluation, Authorisation and Restriction of Chemicals (REACH), ABl. L 142, 2008; (b) T. M. Klapötke, B. Krumm, N. Mayr, F. X. Steemann and G. Steinhauser, Safety Science, 2010, 48, 28–34 CrossRef PubMed.
- (a) Test methods according to the UN Manual of Tests and Criteria, Recommendations on the Transport of Dangerous Goods, United Nations Publication, New York, Geneva, 4th revised edn, 2003 Search PubMed; (b) www.reichel-partner.de .
- C. P. Butts, L. Eberson, K. L. Fulton, M. P. Hartshorn, W. T. Robinson and D. J. Timmerman-Vaughan, Acta Chem. Scand., 1996, 50, 991–1008 CrossRef CAS PubMed.
- M. Göbel and T. M. Klapötke, Adv. Funct. Mater., 2009, 19, 347–365 CrossRef.
- (a) N. I. Golovina, A. N. Titkov, A. V. Raevskii and L. O. Atovmyan, J. Solid State Chem., 1994, 113, 229–238 CrossRef CAS; (b) A. B. Sheremetev, E. A. Ivanova, N. P. Spiridonova, S. F. Melnikova, I. V. Tselinsky, K. Y. Suponitsky and M. Y. Antipin, J. Heterocycl. Chem., 2005, 42, 1237–1242 CrossRef CAS; (c) P. W. Leonard, C. J. Pollard, D. E. Chavez, B. M. Rice and D. A. Parrish, Synlett, 2011, 2097–2099 CrossRef CAS PubMed.
- M.-X. Zhang, P. E. Eaton and R. Gilardi, Angew. Chem., Int. Ed., 2000, 39(2), 401–404 CrossRef CAS.
- Z. A. Akopyan, Y. T. Struchkov and V. G. Dashevii, Zh. Strukt. Khim., 1966, 7(3), 408–416 CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- A. Sikorski and D. Trzybinski, J. Mol. Struct., 2013, 1049, 90–98 CrossRef CAS PubMed.
- Y. Oyumi, T. B. Brill and A. L. Rheingold, J. Phys. Chem., 1985, 89, 4824–4828 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General methods, experimental section, and crystallographic data sheet of TNM2-BOD. CCDC 963150. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc49879d |
| This journal is © The Royal Society of Chemistry 2014 |