Open Access Article
Open Access ArticleBiferrocenium salts with magnetite-like mixed-valence iron: coexistence of Fe3+ and Fe2.5+ in the crystal†
Tomoyuki
Mochida
*ab,
Eri
Nagabuchi
b,
Masashi
Takahashi
b and
Hatsumi
Mori
c
aDepartment of Chemistry, Graduate School of Science, Kobe University, Rokkodai, Nada, Hyogo 657-8501, Japan. E-mail: tmochida@platinum.kobe-u.ac.jp; Fax: +81 78 803 5679; Tel: +81 78 803 5679
bDepartment of Chemistry, Faculty of Science, Toho University, Miyama, Funabashi, Chiba 274-8510, Japan
cInstitute for Solid State Physics, The University of Tokyo, Kashiwanoha, Kashiwa, Chiba 277-8581, Japan
First published on 13th January 2014
Abstract
The biferrocene-based salt [Bifc]2[Ni(mnt)2]3 (Bifc = bis(isopropylthio)biferrocene; mnt = maleonitriledithiolate) contains a biferrocenium monocation and dication within the same crystal. The coexistence of Fe3+ and mixed-valence Fe2.5+, which resembles the valence state of magnetite, was confirmed by Mössbauer spectroscopy.
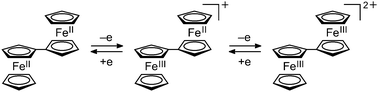
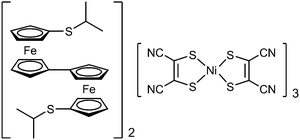
Magnetite (Fe3O4) is a basic mixed-valence compound that has attracted significant attention.1 In the inverse spinel structure of magnetite, the tetrahedral sites (A sites) and octahedral sites (B sites) are occupied by Fe3+ ions and Fe2.5+ ions, respectively, and the latter ions comprise Fe2+ and Fe3+ ions that undergo rapid electron exchange, as demonstrated by 57Fe Mössbauer spectroscopy and other methods.1,2 Mixed valency in molecular compounds and metal complexes has attracted considerable interest.3,4 Biferrocenium salts are well-known organometallic mixed-valence compounds.5 Biferrocenes exhibit three redox states, i.e., neutral, monocation, and dication (Fig. 1), and produce salts with mixed-valence monocations. We previously reported a biferrocenium salt that undergoes a phase transition from a monocationic to dicationic salt at low temperatures, which demonstrates that the dicationic species can exist when stabilized by electrostatic interactions.6 Herein, we report the intriguing valence state of a biferrocene-based salt, i.e., [Bifc]2[M(mnt)2]3 (1, Bifc = 1′,1′′′-bis(isopropylthio)-1,1′′-biferrocene, Fig. 2) that exhibits a 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 cation/anion ratio and contains both a mixed-valence monocation and dication in the same crystal.‡ [Ni(mnt)2]− is a planar paramagnetic anion, which has produced intriguing ferrocenium-based salts.7
3 cation/anion ratio and contains both a mixed-valence monocation and dication in the same crystal.‡ [Ni(mnt)2]− is a planar paramagnetic anion, which has produced intriguing ferrocenium-based salts.7
The crystal structure of 1 was determined at 173 K (space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ).8 The packing diagram of 1 viewed along the a-axis is shown in Fig. 3. There are two crystallographically independent cations, i.e., A and B, which are both located on the inversion center. The [Ni(mnt)2]−1 anions form centrosymmetric trimers, which are further stacked along the a-axis to form a columnar structure (ESI†).9 The cations are located between the columns of anions. Examination of the intramolecular geometry revealed that cations A and B have different valence states and are dicationic and monocationic, respectively. The average Fe–C(Cp) distance in cation A (2.085 Å) is similar to that of a ferrocenium cation (2.08 Å), whereas that in cation B (2.063 Å) is intermediate of that for a ferrocenium cation and neutral ferrocene (2.04 Å) and thus corresponds to a mixed-valence monocation.10 The valence state of 1 is hence represented as [BifcA]2+[BifcB]+[{Ni(mnt)2}3]3−. Because the two Fe atoms in each cation are crystallographically equivalent, cations A and B contain Fe3+ and averaged-valence Fe2.5+, respectively. The corresponding Pt-containing salt, i.e., [Bifc]2[Pt(mnt)2]32, has a similar structure11 and the same valence state characteristics.
).8 The packing diagram of 1 viewed along the a-axis is shown in Fig. 3. There are two crystallographically independent cations, i.e., A and B, which are both located on the inversion center. The [Ni(mnt)2]−1 anions form centrosymmetric trimers, which are further stacked along the a-axis to form a columnar structure (ESI†).9 The cations are located between the columns of anions. Examination of the intramolecular geometry revealed that cations A and B have different valence states and are dicationic and monocationic, respectively. The average Fe–C(Cp) distance in cation A (2.085 Å) is similar to that of a ferrocenium cation (2.08 Å), whereas that in cation B (2.063 Å) is intermediate of that for a ferrocenium cation and neutral ferrocene (2.04 Å) and thus corresponds to a mixed-valence monocation.10 The valence state of 1 is hence represented as [BifcA]2+[BifcB]+[{Ni(mnt)2}3]3−. Because the two Fe atoms in each cation are crystallographically equivalent, cations A and B contain Fe3+ and averaged-valence Fe2.5+, respectively. The corresponding Pt-containing salt, i.e., [Bifc]2[Pt(mnt)2]32, has a similar structure11 and the same valence state characteristics.
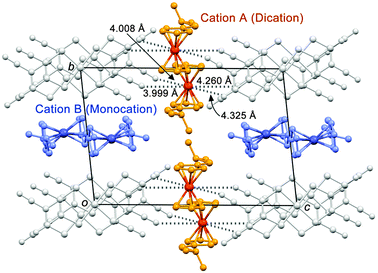 | ||
| Fig. 3 Packing diagram of 1 viewed along the a-axis. Hydrogen atoms are omitted for clarity. Dotted lines indicate the close Fe⋯NC distances between the cation and the anion. | ||
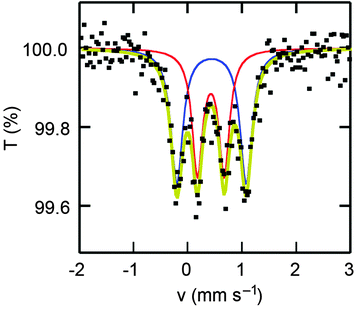
The valence states of the iron atoms in the cations were unambiguously determined using 57Fe Mössbauer spectroscopy. Fig. 4 shows the spectrum recorded at 300 K, which features two equal-intensity components. The inner doublet, which has an isomer shift of 0.43 mm s−1 (relative to α-iron) and a quadrupole splitting of 0.49 mm s−1, corresponds to Fe3+ in the biferrocenium dication (cation A). The outer doublet, which has an isomer shift of 0.44 mm s−1 and a quadrupole splitting of 1.28 mm s−1, corresponds to Fe2.5+ in a mixed-valence biferrocenium monocation that undergoes rapid valence exchange (>108 s−1, cation B).5 The spectral features remain unchanged down to 6 K (Fig. S1, ESI†).
This salt, which contains Fe3+ and Fe2.5+, is isoelectronic to magnetite in terms of the valence state of the iron, while the electron transfer between Fe2+ and Fe3+ is intramolecular. Although magnetite undergoes a charge-ordering transition at around 119 K,11 exhibits no valence localization of the Fe2.5+ site, at least down to 6 K; thus, it is highly likely that valence tautomerization of 1 occurs via electron tunneling.12 The origin of the coexistence of a dication and monocation in 1 is reasonably attributed to differences in the local electrostatic interactions. The ferrocenium sites in biferrocenium-[Ni(mnt)2] salts are stabilized by neighboring cyano groups.9 In 1, the Fe atom in cation A is surrounded by four electronegative cyano groups from the anion (Fe⋯NC distances: 3.999(4)–4.325(4) Å, indicated by dotted lines in Fig. 3), which strongly stabilizes the cationic state; in contrast, cation B has no such interactions.
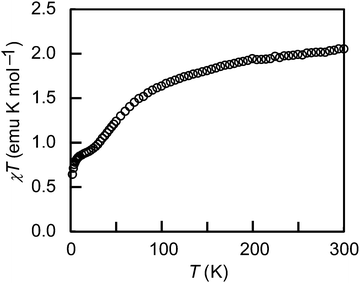
This salt exhibits paramagnetic behavior. The temperature dependence of the χT value is shown in Fig. 5. 1 has a magnetic susceptibility of 2.06 emu K mol−1 at room temperature and contains three spins of the ferrocenium cation: two from cation A and one from cation B. The magnetic moment of the ferrocenium cation is 0.6–0.7 emu K mol−1, which is larger than the spin-only value because of orbital contributions.6 Hence, the total magnetic moment of the cations in 1 is expected to be 1.8–2.1 emu K mol−1; this corresponds with the observed value. The contributions of the anion spins are small because of strong antiferromagnetic interactions in the column.9,10a The gradual decrease of the magnetic moment down to about 30 K is ascribed to loss of the orbital contributions; the magnetic moment at this temperature is close to the spin-only value of 3 × 0.375 = 1.13 emu K mol−1. This temperature-dependent loss of the orbital contribution in biferrocenium salts is exceptional5d,6 and likely results from the electronic effects of sulfur.13 The susceptibility suddenly decreases at 5 K, which is probably ascribed to singlet formation in the dication (cation A). These magnetic behaviors are consistent with the valence state of the salt.
In summary, we discovered an organometallic compound that contains Fe3+ and Fe2.5+ and exhibits a magnetite-like mixed-valence state of the iron ions. Intramolecular charge separation to Fe3+ and Fe2.5+ has been observed in a few trinuclear mixed-valence complexes at low temperatures;14 however, the present example involves intermolecular charge separation, which originates from the different local electronic interactions around each cation. This phenomenon is interesting from the perspective of the recent interest in charge separation in molecular charge-transfer salts.15 A somewhat related phenomenon is the inclusion of neutral ferrocene in [Fe(C5H5)2]2[Ni(mnt)2]2·[Fe(C5H5)2].7a
This work was supported financially by KAKENHI grant number 23110719. We thank Y. Funasako (Kobe University) for his help with X-ray crystallography. This work was performed using facilities at the Institute for Solid State Physics, University of Tokyo.
Notes and references
- (a) J. Garcia and G. Subias, J. Phys.: Condens. Matter, 2004, 16, R145–R178 CrossRef CAS; (b) F. Walz, J. Phys.: Condens. Matter, 2002, 14, R285–R340 CrossRef CAS; (c) E. J. W. Verwey and P. W. Haayman, Physica, 1941, 8, 979–987 CrossRef CAS.
- (a) R. S. Hargrove and W. Kündig, Solid State Commun., 1970, 8, 303–308 CrossRef CAS; (b) N. N. Greenwood and T. C. Gibb, Mössbauer Spectroscopy, Chapman and Hall, London, 1971 Search PubMed.
- (a) Mixed Valency Systems: Applications in Chemistry, Physics, and Biology, Series C, ed. K. Prassides, NATO ASI Series, Kluwer, Dordrecht, 1991, vol. 343 Search PubMed; (b) A. Heckmann and C. Lambert, Angew. Chem., Int. Ed., 2012, 51, 326–392 CrossRef CAS PubMed; (c) K. D. Demadis, C. M. Hartshorn and T. J. Meyer, Chem. Rev., 2001, 101, 2655–2685 CrossRef CAS PubMed.
- (a) S. Barlow and D. O'Hare, Chem. Rev., 1997, 97, 637–670 CrossRef CAS PubMed; (b) H. Nishihara, Adv. Inorg. Chem., 2002, 53, 41–86 CrossRef CAS.
- (a) T.-Y. Dong, L.-S. Chang, G.-H. Lee and S.-M. Peng, Organometallics, 2002, 21, 4192–4200 CrossRef CAS; (b) H. Sano, Hyperfine Interact., 1990, 53, 97–112 CrossRef CAS; (c) D. N. Hendrickson, S. M. Oh, T.-Y. Dong, T. Kambara, M. J. Cohn and M. F. Moore, Comments Inorg. Chem., 1985, 4, 329–349 CrossRef CAS; (d) T. Mochida, S. Yamazaki, S. Suzuki, S. Shimizu and H. Mori, Bull. Chem. Soc. Jpn., 2003, 76, 2321–2328 CrossRef CAS.
- T. Mochida, K. Takazawa, M. Takahashi, M. Takeda, Y. Nishio, M. Sato, K. Kajita, H. Mori, M. M. Matsushita and T. Sugawara, J. Phys. Soc. Jpn., 2005, 74, 2214–2216 CrossRef CAS.
- (a) M. W. Day, J. Qin and C. Yang, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1998, 54, 1413–1416 Search PubMed; (b) S. Zürcher, V. Gramlich, D. von Arx and A. Togni, Inorg. Chem., 1998, 37, 4015–4021 CrossRef; (c) A. E. Pullen, C. Faulmann, K. I. Pokhodnya, P. Cassoux and M. Tokumoto, Inorg. Chem., 1998, 37, 6714–6720 CrossRef CAS PubMed; (d) O. Jeannin, R. Clérac and M. Fourmigué, J. Am. Chem. Soc., 2006, 128, 14649–14656 CrossRef CAS PubMed; (e) T. Mochida, T. Koinuma, T. Akasaka, M. Sato, Y. Nishio, K. Kajita and H. Mori, Chem.–Eur. J., 2007, 13, 1872–1881 CrossRef CAS PubMed.
- Crystallographic parameters for 1 at 173 K: triclinic P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.4988(14) Å, b = 12.0318(16) Å, c = 17.227(2) Å, α = 95.436(3)°, β = 92.971(3)°, γ = 103.641(3)°, V = 2099.0(5) Å3, Z = 1, R1 = 0.0536, and wR2 = 0.1317. See ESI† for details. The crystal structure at 295 K was unchanged from that at 173 K.
, a = 10.4988(14) Å, b = 12.0318(16) Å, c = 17.227(2) Å, α = 95.436(3)°, β = 92.971(3)°, γ = 103.641(3)°, V = 2099.0(5) Å3, Z = 1, R1 = 0.0536, and wR2 = 0.1317. See ESI† for details. The crystal structure at 295 K was unchanged from that at 173 K. - [Ni(mnt)] in 1 is monoanionic, as evidenced by the infrared CN stretching band (νCN = 2207 cm−1) and intramolecular geometry (Ni–S bond lengths: 2.138(1)–2.153(1) Å).9 The intratrimer and intertrimer Ni⋯Ni distances are 3.94 Å and 4.35 Å, respectively. The intratrimer and intertrimer overlap integrals between the SOMOs of the anions calculated using the extended Hückel method are 4.8 × 10−3 and 11.4 × 10−3, respectively.
- (a) T. Mochida, K. Takazawa, H. Matsui, M. Takahashi, M. Takeda, M. Sato, Y. Nishio, K. Kajita and H. Mori, Inorg. Chem., 2005, 44, 8628–8641 CrossRef CAS PubMed; (b) T. Mochida, T. Kobayashi and T. Akasaka, J. Organomet. Chem., 2013, 741–742, 72–77 CrossRef CAS.
- Crystallographic parameters for 2 at 173 K: triclinic P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 11.084(2) Å, b = 12.270(3) Å, c = 17.696(3) Å, α = 100.459(4)°, β = 99.486(4)°, γ = 112.620(4)°, V = 2110.4(7) Å3, Z = 1, R1 = 0.0625, and wR2 = 0.1284. The crystal structure at 295 K was unchanged from that at 173 K. The intratrimer and intertrimer Pt⋯Pt distances are 3.77 Å and 5.68 Å, respectively. See ESI† for details.
, a = 11.084(2) Å, b = 12.270(3) Å, c = 17.696(3) Å, α = 100.459(4)°, β = 99.486(4)°, γ = 112.620(4)°, V = 2110.4(7) Å3, Z = 1, R1 = 0.0625, and wR2 = 0.1284. The crystal structure at 295 K was unchanged from that at 173 K. The intratrimer and intertrimer Pt⋯Pt distances are 3.77 Å and 5.68 Å, respectively. See ESI† for details. - M. Nakano, M. Sorai, P. M. Hagen and D. N. Hendrickson, Chem. Phys. Lett., 1992, 196, 486–490 CrossRef CAS.
- T. Mochida, Y. Funasako, E. Nagabuchi and H. Mori, unpublished.
- (a) M. Manago, S. Hayami, Y. Yano, K. Inoue, R. Nakata, A. Ishida and Y. Maeda, Bull. Chem. Soc. Jpn., 1999, 72, 2229–2234 CrossRef CAS; (b) T. Sato, F. Ambe, K. Endo, M. Katada, H. Maeda, T. Nakamoto and H. Sano, J. Am. Chem. Soc., 1996, 118, 3450–3458 CrossRef CAS.
- H. Seo, C. Hotta and H. Fukuyama, Chem. Rev., 2004, 104, 5005–5036 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, molecular structures and crystal structures, temperature dependence of 57Fe Mössbauer spectra, and crystallographic data. CCDC 263333 (1) and 263334 (2). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc49568j |
| ‡ 1′,1′′′-Bis(isopropylthio)-1,1′′-biferrocene was prepared via the reaction of dilithioferrocene and diisopropyl disulfide in hexane (ESI†). The cyclic voltammogram of this molecule showed two reversible redox waves at E1/2(1) = −0.07 V and E1/2(2) = 0.22 V (vs. [Fe(Cp)2]0/+), which are similar to those of biferrocene, i.e., E1/2(1) = −0.10 V, E1/2(2) = 0.24 V. Single crystals of 1 were obtained as black plates in an ∼40% yield by vapor diffusion of pentane into a dichloromethane solution of equimolar amounts of biferrocene and [Fe(C5H5)2][Ni(mnt)2]. Anal. calcd for C76H60S16N12Fe4Ni3: C, 44.44; H, 2.94; N, 8.18%. Found: C, 44.15; H, 3.03; N, 8.04%. |
| This journal is © The Royal Society of Chemistry 2014 |