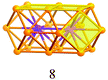
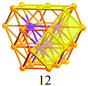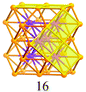Synthesis of a Au44(SR)28 nanocluster: structure prediction and evolution from Au28(SR)20, Au36(SR)24 to Au44(SR)28†
Chenjie
Zeng
,
Yuxiang
Chen
,
Gao
Li
and
Rongchao
Jin
*
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213, USA. E-mail: rongchao@andrew.cmu.edu
First published on 5th November 2013
Abstract
We report the synthesis of a Au44(SR)28 nanocluster (SR = 4-tert-butylbenzenethiolate). Based on the structural rules learned from the known Au28(SR)20 and Au36(SR)24 structures, we propose a plausible structure for Au44(SR)28, which is predicted to comprise a six-interpenetrating cuboctahedral Au36 kernel protected by four dimeric staples and sixteen bridging thiolates, i.e. Au36[Au2(SR)3]4(SR)16.
Atomically precise metal nanoclusters have drawn significant research attention in recent years.1–10 The structural evolution as a function of size constitutes an important issue in the nanocluster research,1,11 which is the basis for obtaining deep insight into the size-dependent catalytic,12,13 optical and electronic properties14 of metal nanoclusters. A number of thiolate-protected Aun(SR)m nanoclusters ranging from ca. a dozen to hundreds of gold atoms have been reported.15–20 The reported Aun(SR)m structures have provided valuable information on the basic rules of structural construction, e.g. a highly symmetric Au kernel protected by staple-like Au-SR oligomers.21 But much effort is still needed in order to gain in-depth understanding of the structural evolution rules.1,15,21–23 Recently, we reported the Au28(SR)20 and Au36(SR)24 nanoclusters protected by 4-tert-butylbenzenethiolate (SPh-t-Bu, TBBT for short) and revealed their face centered cubic (fcc) structures.24,25 These two nanoclusters share some common structural features (Fig. S1, ESI†): a gold kernel composed of interpenetrating cuboctahedra and a ligand shell composed of plain bridging thiolates and dimeric oligomers.
Herein, we report the synthesis of a third gold nanocluster in the TBBT ligand series, formulated as Au44(SR)28, and its predicted structure is based upon the structural rules learned from the Au28(SR)20 and Au36(SR)24 structures.24,25 A clear trend can be seen from the three formulae (28, 20), (36, 24) and (44, 28), i.e. the number of Au atoms uniformly increases by 8 and the number of thiolate ligands by 4 in the series. This clear trend, together with the insight into the structures of Au28(SR)20 and Au36(SR)24, led us to propose a plausible structure for Au44(SR)28 and reveal the evolution in this series of medium-sized gold nanoclusters.
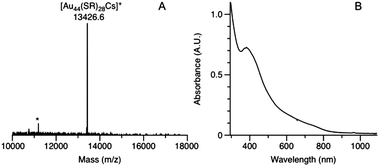
The synthesis of Au44(SR)28 nanoclusters was based on a one-phase method using ethyl acetate as the solvent (see the ESI† for details).26 Briefly, HAuCl4 was first reacted with 4 equivalents of HSPh-t-Bu to form colorless Au(I)SR complexes. Then, NaBH4 (10 equivalents per Au) was added to reduce Au(I)SR to Aun(SR)m nanoclusters. The initially obtained polydispersed nanoclusters were subject to solvent-induced fractionation by diffusion of methanol into a toluene solution of the nanoclusters. After one month, a dark precipitate was formed; the precipitate was then separated from the supernatant, followed by electrospray ionization mass spectroscopy (ESI-MS) to determine the molecular weight of the nanocluster (Fig. 1A). In order to impart charge to the cluster, CsOAc was added to the cluster solution to form cluster–Cs+ adducts. A distinct peak at m/z = 13426.6 was detected. After careful comparison of possible (n, m) values for the formula of [Aun(SR)mCs]+, the peak was assigned to [Au44(SPh-t-Bu)28Cs]+ (theoretical FW: 13426.8, deviation: −0.2). Other combinations of (n, m) are ruled out (Table S1, ESI†) due to large discrepancies between the calculated formula weight and the experimental value. The Au44(SR)28 nanocluster is charge neutral since the addition of one Cs+ atom to the cluster forms the singly charged adduct. A small amount of Au36(SR)24 impurity was found to coexis with Au44(SR)28 (Fig. 1A, the asterisk-labeled peak). Note that in the MALDI-MS spectrum, no intact molecular peak corresponding to Au44(SR)28 was observed (Fig. S2 and inset, Table S2, ESI†); instead, a peak corresponding to one-ligand-lost Au44(SR)27 was observed together with other fragment peaks, similar to the case of Au36(SR)24.27 The Au44(SR)28 formula is further confirmed by using thiophenol as the ligand. A peak at 11856.8 was observed in the ESI-MS spectrum (Fig. S3, ESI†), corresponding to [Au44(SPh)28Cs]+ (theoretical FW: 11855.7).
The optical spectrum of Au44(SR)28 exhibits an absorption band at 380 nm and a broad platform from 600 to 800 nm (Fig. 1B). The energy gap derived from the optical spectrum is ∼1.45 eV. It is worth noting that Price et al.28a previously observed an ∼8.7 kDa species etched from ∼22 kDa clusters and suggested a formula of [Au44(SPh)28]2− but with no precise mass determined. Recently Jiang et al.28b predicted two structures protected by monomeric and dimeric staples.
The most interesting aspect about this Au44(SR)28 nanocluster is its relationship with the recently reported Au28(SR)20 and Au36(SR)24.24,25 In this series, the number of gold atoms increases by eight from Au28 to Au36 to Au44, and the number of thiolate ligands increases by four from Au28(SR)20 to Au36(SR)24 to Au44(SR)28. The Au8(SR)4 increment in the series inspired us to consider the inherent structural connection among these clusters.
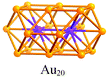
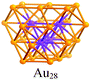
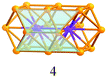
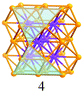
A careful examination of the crystal structures of Au28(SR)20 and Au36(SR)24 reveals some interesting structural rules shared by the two nanoclusters (Table 1). (i) With respect to the kernel structure, both nanoclusters have fcc-type kernels composed of interpenetrating cuboctahedra. Au28(SR)20 has a rod-like Au20 kernel composed of two interpenetrating cuboctahedra with two centres (i.e. twelve-coordinate gold atoms resembling the bulk fcc case), whereas Au36(SR)24 has a Au28 kernel composed of four interpenetrating cuboctahedra with four centres (Au4) arranged into a tetrahedron. (ii) Both the Au20 rod kernel and the Au28 tetrahedral kernel are enclosed by well-defined crystal faces: the Au20 kernel has four trapezoid-shaped {111} facets on the front and back sides of the rod, while the Au28 kernel also has four triangular {111} facets on the four surfaces of the truncated tetrahedron (Table 1, blue shadowed). Besides, the Au20 rod kernel exposes eight square facets corresponding to {100}, and the Au28 tetrahedral kernel possesses twelve squares (Table 1, yellow shadowed). (iii) Regarding the thiolate protecting modes, both Au28(SR)20 and Au36(SR)24 nanoclusters have dimeric staples (–SR-Au-SR-Au-SR–) protecting the {111} facets in a one-on-one fashion (Fig. S4, ESI†), and bridging thiolates (–SR–) residing on the edges of square {100} facets with one –SR– ligand per square (Fig. S5, ESI†). Hence the formulae of Au28(SR)20 and Au36(SR)24 can be deduced as Au20[Au2(SR)3]4(SR)8 and Au28[Au2(SR)3]4(SR)12, respectively (Table 1). It is thus obvious that the eight-gold-atom difference between Au28(SR)20 and Au36(SR)24 is added into the kernel (Fig. 2A), and the four-thiolate difference is due to the difference in the number of bridging thiolates. The number of dimeric staples is kept the same in the structural evolution from Au28(SR)20 to Au36(SR)24.
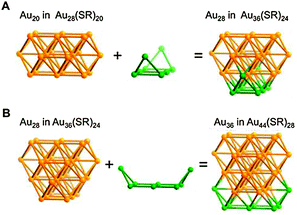 | ||
| Fig. 2 Kernel evolution. (A) Au20 in Au28(SR)20 to Au28 in Au36(SR)24; (B) Au28 in Au36(SR)24 to Au36 in Au44(SR)28. | ||
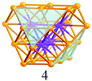
Based on the structural rules learned from Au28(SR)20 and Au36(SR)24, we propose a plausible structural model for Au44(SR)28 which possesses a Au36 kernel composed of six interpenetrating cuboctahedra, in which the six centres (Au6) are arranged into an edge-sharing bi-tetrahedron (Table 1, right). The Au36 kernel can be evolved from the Au28 kernel of Au36(SR)24 by adding eight more gold atoms to the bottom of the Au28 tetrahedron (Fig. 2B). The Au36 kernel also exhibits four triangular {111} facets (Table 1, blue shadowed), as in Au28(SR)20 and Au36(SR)24. Besides, 16 squares can be found on the surface of the proposed Au36 kernel, with 4 on the top and bottom, 6 on the left, and another 6 on the right side of the kernel (Table 1, yellow shadowed). The surface protecting motifs of the Au36 kernel can be deduced from the above rule (iii), that is, one dimeric staple per {111} face and one bridging thiolate per {100} square face; hence, overall four dimeric staples and sixteen bridging thiolates protect the four {111} faces and sixteen {100} faces of the Au36 kernel. The formula of Au44(SR)28 can thus be given as Au36[Au2(SR)3]4(SR)16.
Positioning of the dimeric staples and bridging thiolates in Au44(SR)28 can also be obtained through comparison with Au28(SR)20 and Au36(SR)24 (Fig. S4 and S5, ESI†). Fig. 3A illustrates the positions of the dimeric staples: on the top of the blue diamond unit on each {111} facet is a dimeric staple, with its two sulfur ends bonded to the two farthest vertices of the diamond unit. The positions of the 16 bridging thiolates are shown in Fig. 3B, with the green bond in each square spanned by a bridging thiolate. In this way, all the 30 gold atoms on the surface of the Au36 kernel are bonded to at least one thiolate group, hence the kernel is well protected. The Au44(SR)28 structure is predicted to be chiral as in the case of Au28(SR)20 due to the rotary arrangement of the dimeric staples and bridging thiolates. The Au44S28 framework exhibits a D2 symmetry. Crystallization trials have not been successful, thus the proposed structure remains to be verified in future work. Of note, the [Ag44(SR)30]4− cluster29 has recently been structurally characterized,30,31 and the structure is quite different from the predicted structure of [Au44(SR)28]0 due to different bonding mode of Ag-SR compared to Au-SR.
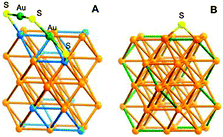 | ||
| Fig. 3 Proposed positioning of 4 dimeric staples (A) and 16 bridging thiolates (B) on the surface of the Au36 kernel. | ||
In summary, we have synthesized Au44(SR)28 nanoclusters. Based upon the structural features learned from the crystal structures of Au28(SR)20 and Au36(SR)24, we have proposed a plausible structural model for Au44(SR)28. The three nanoclusters constitute an intriguing series that exhibits some remarkable structural trends in terms of the kernel and surface motifs.
R.J. is grateful for the financial support by the Air Force Office of Scientific Research under AFOSR Award No. FA9550-11-1-9999 (FA9550-11-1-0147) and the Camille Dreyfus Teacher-Scholar Awards Program.
Notes and references
- H. Qian, M. Zhu, Z. Wu and R. Jin, Acc. Chem. Res., 2012, 45, 1470 CrossRef CAS PubMed.
- P. Maity, T. Wakabayashi, N. Ichikuni, H. Tsunoyama, S. Xie, M. Yamauchia and T. Tsukuda, Chem. Commun., 2012, 48, 6085 RSC.
- C. Kumaraa and A. Dass, Nanoscale, 2012, 4, 4084 RSC.
- A. Dass, K. Holt, J. F. Parker, S. W. Feldberg and R. W. Murray, J. Phys. Chem. C, 2008, 112, 20276 CAS.
- K. Chaudhari, P. L. Xavier and T. Pradeep, ACS Nano, 2011, 5, 8816 CrossRef CAS PubMed.
- S. Knoppe, R. Azoulay, A. Dass and T. Bürgi, J. Am. Chem. Soc., 2012, 134, 20302 CrossRef CAS PubMed.
- Y. Negishi, K. Igarashi, K. Munakata, W. Ohgakea and K. Nobusada, Chem. Commun., 2012, 48, 660 RSC.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662 CrossRef CAS PubMed.
- R. S. McCoy, S. Choi, G. Collins, B. J. Ackerson and C. J. Ackerson, ACS Nano, 2013, 7, 2610 CrossRef CAS PubMed.
- H. Yang, J. Lei, B. Wu, Y. Wang, M. Zhou, A. Xia, L. Zheng and N. Zheng, Chem. Commun., 2013, 49, 300 RSC.
- Y. Pei and X. C. Zeng, Nanoscale, 2012, 4, 4054 RSC.
- G. Li and R. Jin, Acc. Chem. Res., 2013, 46, 1749 CrossRef CAS PubMed.
- (a) Y. Liu, H. Tsunoyama, T. Akita, S. Xie and T. Tsukuda, ACS Catal., 2010, 1, 2 CrossRef; (b) G. Li, D.-e. Jiang, C. Liu, C. Yu and R. Jin, J. Catal., 2013, 306, 177 CrossRef CAS PubMed; (c) X. Nie, C. Zeng, X. Ma, H. Qian, Q. Ge, H. Xu and R. Jin, Nanoscale, 2013, 5, 5912 RSC.
- (a) M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2008, 130, 5883 CrossRef CAS PubMed; (b) S. H. Yau, O. Varnavski and T. Goodson, Acc. Chem. Res., 2013, 46, 1506 CrossRef CAS PubMed.
- (a) P. Maity, S. Xie, M. Yamauchi and T. Tsukuda, Nanoscale, 2012, 4, 4027 RSC; (b) Y. Negishi, C. Sakamoto, T. Ohyama and T. Tsukuda, J. Phys. Chem. Lett., 2012, 3, 1624 CrossRef CAS.
- R. Jin, H. Qian, Z. Wu, Y. Zhu, M. Zhu, A. Mohanty and N. Garg, J. Phys. Chem. Lett., 2010, 1, 2903 CrossRef CAS.
- X. Meng, Q. Xu, S. Wang and M. Zhu, Nanoscale, 2012, 4, 4161 RSC.
- P. R. Nimmala, B. Yoon, R. L. Whetten, U. Landman and A. Dass, J. Phys. Chem. A, 2013, 117, 504 CrossRef CAS PubMed.
- Y. Levi-Kalisman, P. D. Jadzinsky, N. Kalisman, H. Tsunoyama, T. Tsukuda, D. A. Bushnell and R. D. Kornberg, J. Am. Chem. Soc., 2011, 133, 2976 CrossRef CAS PubMed.
- H. Qian, Y. Zhu and R. Jin, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 696 CrossRef CAS PubMed.
- R. Jin, Y. Zhu and H. Qian, Chem.–Eur. J., 2011, 17, 6584 CrossRef CAS PubMed.
- (a) D.-e. Jiang, S. H. Overbury and S. Dai, J. Am. Chem. Soc., 2013, 135, 8786 CrossRef CAS PubMed; (b) A. Tlahuice and I. L. Garzon, Phys. Chem. Chem. Phys., 2012, 14, 3737 RSC; (c) Y. Pei, R. Pal, C. Liu, Y. Gao, Z. Zhang and X. C. Zeng, J. Am. Chem. Soc., 2012, 134, 3015 CrossRef CAS PubMed.
- S. Malola, L. Lehtovaara, S. Knoppe, K.-J. Hu, R. E. Palmer, T. Bürgi and H. Häkkinen, J. Am. Chem. Soc., 2012, 134, 19560 CrossRef CAS PubMed.
- C. Zeng, H. Qian, T. Li, G. Li, N. L. Rosi, B. Yoon, R. N. Barnett, R. L. Whetten, U. Landman and R. Jin, Angew. Chem., Int. Ed., 2012, 51, 13114–13118 CrossRef CAS PubMed.
- C. Zeng, T. Li, A. Das, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2013, 135, 10011–10013 CrossRef CAS PubMed.
- Z. Wu, J. Suhan and R. Jin, J. Mater. Chem., 2009, 19, 622 RSC.
- C. Zeng, C. Liu, Y. Pei and R. Jin, ACS Nano, 2013, 7, 6138 CrossRef CAS PubMed.
- (a) R. C. Price and R. L. Whetten, J. Am. Chem. Soc., 2005, 127, 13750–13751 CrossRef CAS PubMed; (b) D.-e. Jiang, M. Walter and J. Akola, J. Phys. Chem. C, 2010, 114, 15883 CrossRef CAS.
- O. Bakr, V. Amendola, C. M. Aikens, W. Wenseleers, R. Lee, L. Dal Negro, G. C. Schatz and F. Stellacci, Angew. Chem., Int. Ed., 2009, 48, 5921 CrossRef CAS PubMed.
- A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature DOI:10.1038/nature12523.
- H. Yang, Y. Wang, H. Huang, L. Gell, L. Lehtovaara, S. Malola, H. Häkkinen and N. Zheng, Nat. Commun. DOI:10.1038/ncomms3422.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc47089j |
| This journal is © The Royal Society of Chemistry 2014 |