Self-assembled solvato-morphologically controlled photochromic crystals†
Larisa
Florea
a,
Silvia
Scarmagnani
a,
Fernando
Benito-Lopez
*ab and
Dermot
Diamond
*a
aThe INSIGHT Centre for Data Analytics, Dublin City University, Ireland. E-mail: fernando.lopez@dcu.ie; dermot.diamond@dcu.ie
bCIC microGUNE, Arrasate-Mondragón, Spain. Fax: +34 943739805
First published on 24th October 2013
Abstract
Here we describe, for the first time, intriguing solvato-morphological control of spiropyran-based microcrystalline structures. These microstructures exhibit reversible photoisomerization upon light irradiation (UV/Vis) in the solid-state. Finally, light-guided aggregation of these microstructures at the liquid/air interface is also demonstrated.
Self-assembly is omnipresent in nature as components of any size (from molecules to galaxies) may self-assemble under favorable conditions.1,2 From the assembly of an ant colony to the folding of a polypeptide chain into a protein, self-assembly is inspiring scientists (chemists, physicists, biologists, and materials scientists) to employ this approach as a “bottom-up” tool to create intriguing structures for different applications.3–5 Until now, most research in self-assembly has been focused on molecular components, but more recently, the development of supramolecular chemistry and the direction of technology toward micrometer- and nanometer-scale structures have broadened this focus to include assembly of larger structures.2–4 In principle, self-assembly can provide a highly innovative solution to the challenges brought by the continued drive to reduce device feature scale6 while also providing great potential for building complex 3D microstructures in a practical way.3
Besides molecular aggregation in solution, interfacial self-assembly has attracted great interest in recent years mainly because of the creative and unique principles of this method for forming complex structures, such as self-assembled monolayers, Langmuir–Blodgett films, nanocrystals, clusters, and capsules.7 Among the self-assembly architectures, a nano- or microstructures with photo-responsive properties is of particular interest due to important potential applications in optical memories, optical switches and displays.8,9 It is becoming increasingly obvious that the next generation of these materials will incorporate intelligent design of externally addressable photo-responsive units with self-assembly capabilities, as these can produce highly pre-organized nano- or microstructures in a spontaneous manner under the right conditions.
Among the various classes of photochromic compounds, spirobenzopyrans are one of the most studied, and have been successfully used in applications such as non-linear optics, data recording, optical and electrical switching, and chemical sensing, among others.10 The photochromic behaviour of spiropyrans is due to the reversible heterolytic cleavage and rebinding of the Cspiro–O bond upon irradiation with UV light and white light, respectively (Scheme 1). These characteristics offer the possibility to modulate or switch various functions at the molecular or supramolecular level using light as the trigger.
Several assemblies of spiropyran (SP) derivatives and related photomodulation have been previously reported, indicating the great potential of merocyanine (MC) to be used as a self-assembly unit. Krongauz and coworkers have described the formation of MC aggregates composed of Tiny globules (0.1–0.4 μm) referred to as “quasicrystals” obtained after UV irradiation of SP-long chain derivatives in aliphatic hydrocarbons, or when exposed to a constant external electric field (V = 25 kV cm−1).11,12 Larger MC crystals (10–100 μm) were obtained from aliphatic hydrocarbons and non-polar solvents upon prolonged UV irradiation (30 min)13 or when MC functionalized polymers were used.14 Other assemblies reported include Langmuir–Blogget films15 and aggregates of spiropyran-functionalized dendrons.16
Here we report for the first time the spontaneous formation of photochromic spirobenzopyran aggregates of 1′-(3-carboxypropyl)-3,3′-dimethyl-6-nitrospiro-1-benzopyran-2,2′-indoline (SP-COOH, Scheme 1) at an liquid–air interface when the liquid phase is an ethanol–water mixture. This process leads to the spontaneous production of highly organized assemblies composed of three-dimensional daisy-like microstructures. By changing the alcohol/water ratio of the liquid phase (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water), selective control of the formation of these 3D molecular assemblies, and their morphology (flat crystals to ribbon-like structures) and dimensions (∼10–80 μm) is possible. Moreover, these microstructures exhibit reversible photoisomerization upon light irradiation in the solid-state. To our knowledge, such structures and behaviour have not been reported previously, and they are distinctly different from “quasicrystals” and larger-sized aggregates because of their high degree of organization at the microscopic level, morphological complexity, facile solvato-morphological control, and lack of residual amorphous material. This study provides the first direct measurement of the structural, spectroscopic, and photochromic properties of such SP-COOH microstructures.
water), selective control of the formation of these 3D molecular assemblies, and their morphology (flat crystals to ribbon-like structures) and dimensions (∼10–80 μm) is possible. Moreover, these microstructures exhibit reversible photoisomerization upon light irradiation in the solid-state. To our knowledge, such structures and behaviour have not been reported previously, and they are distinctly different from “quasicrystals” and larger-sized aggregates because of their high degree of organization at the microscopic level, morphological complexity, facile solvato-morphological control, and lack of residual amorphous material. This study provides the first direct measurement of the structural, spectroscopic, and photochromic properties of such SP-COOH microstructures.
The self-assembled microstructures are prepared as described in the ESI.† Depending on the final EtOH volume percent (V%) in the water–EtOH mixture (15%, 20%, 25%), three different types of microstructures are obtained, namely SP-COOH 15%, SP-COOH 20% and SP-COOH 25%, respectively. These microscopic structures are produced predominantly in the MC and MC-H+ form (indicated by their reddish colour) and remain stable within the aqueous solution most likely due to the stabilization of individual structures in such a highly polar environment. Previously, aggregation of MC derivatives was always observed due to photo-induced formation of MC units surrounded by a non-polar environment. This caused spontaneous association of MC in order to minimize the overall energy of the system.13,16,17 In the case presented here however, these conditions are not satisfied, no spontaneous association is observed, and the turbidity of the solution exhibits good homogeneity (ESI,† Fig. S2). Following this, 10 mL of the SP-COOH EtOH–water dispersion (15%, 20% and 25% EtOH, respectively, prepared as described in the ESI,† Section S3) was poured in a Petri dish (5.5 cm OD) in order to ensure high surface area for the liquid/air interface and exposed to daylight for 2 min.
This caused rapid switching of the EtOH–water bulk phase from a reddish to a light yellow colour, due to the switching of the remaining dissolved MC/MCH+ to SP/MCH+ forms in response to day light (ESI,† Fig. S3B and Scheme S1). This process was accompanied by spontaneous assembly of aggregates at the air/liquid interface. The proposed mechanism for this process relies on the fact that, as the polarity of the bulk liquid phase decreases (due to the switching of the polar MC unit to the less polar SP form), MC solid structures start to aggregate, due to their polar nature. Another important observation is that evaporation of EtOH from the surface facilitates the movement of these structures towards the liquid/air interface. This process was very rapid, and a continuous aggregation film forms at the interface within minutes. A video showing this spontaneous assembly can be seen in ESI,† Video S1 and Fig. S5, for the case of SP-COOH 25%. Very similar behaviour was found to occur with all the other solutions studied (Fig. S6 and S7, ESI†). However, the morphology of the obtained structures and their dimensions were different and very dependent on the percentage of water used. Scanning electron microscopy images of these structures (Fig. 1) show that the morphology of the assembled structures vary in form from ribbon-like shapes in the case of SP-COOH 15% (Fig. 1, left) to daisy-shaped structures composed of flat crystals in the case when SP-COOH 25% is used (Fig. 1, right). The structures obtained for SP-COOH 20% resemble the daisy-like shape of SP-COOH 25%, but the terminations of the units are hyper branched and twisted (Fig. 1, centre). These structures appear to be reasonably homogeneous across the entire aggregated film obtained at the liquid–air interface and closely maintain their morphology with great reproducibility from structure to structure as well as from solution to solution (Fig. S8–S10, ESI†). Analysis of the diameter of the individual self-assembled structures shows that by varying the water content of the liquid phase, structures with average diameters from ∼24 μm (SP-COOH 15%) to ∼71 μm (SP-COOH 25%) can be easily achieved (Fig. S8–S10, ESI†). For SP-COOH 15% and 20%, the width of the fiber-like structures is about 300 nm while for the SP-COOH 25% the width of the flat crystal-like structures is about 5 μm (at the central point), thickness about 200 nm or less, and they are partially transparent (Fig. 1, right). Moreover, it was found that the morphology of the photochromic structures is not overly dependent on the concentration of the SP-COOH in the final solution, but is greatly related to the water/EtOH ratio. In fact, identical morphologies were obtained for structures formed using different SP-COOH concentrations and after different times (12 h to several months), as long as the water/EtOH ratio was kept constant. It should be appreciated however, that there are limits to the water/EtOH ratios in which such complex, highly symmetrical structures are obtained. For example, at %EtOH > 40% such structures were hardly observed due to the high content of EtOH, ensuring total solubility of the spiropyran derivative at the concentrations used. Similarly, when %EtOH < 10%, the low overall concentration of spiropyran (i.e. ≥10-fold dilution) renders it completely soluble in the highly aqueous liquid phase, through the spontaneous formation of MCH+–COO− form. Under these conditions, aggregation at the air/liquid interface was minimal, and the material obtained after evaporation of the solution is a rather amorphous solid. It is most likely that the self-assembly of individual structures (“petals”) in the “daisy flower-shaped structure” is governed by intermolecular forces between MC-COOH units, including π–π stacking and hydrogen-bonding between individual MC-COOH units (e.g. through the carboxylic acid group) and between MC-COOH molecules and water.9,16,18
Our studies suggest that the microstructures obtained present photochromic properties not only at the liquid/air interface (ESI,† Fig. S11), but also in the solid state, see Fig. 2 (SP-COOH 25%). After irradiation with UV light for 5 minutes, these crystals change colour from yellow to red, due to an absorbance band centred around 550 nm. Until recently it was believed that spiropyrans do not typically exhibit such photochromism in the solid state. However, recent results have shown that in some cases, this behaviour can be found in spiropyran or spirooxazine crystals even at room temperature. The authors suggest that this can occur as there is enough free volume within the structures to facilitate the transformation between the two isomers.19 Photo-stability over time has to be considered when dealing with spiropyran photochromic dyes, as a well-documented photo-bleaching process20 occurs when SP is exposed to UV-white light radiation for extended periods of time. We have found that after four switching cycles, the efficiency remains constant in the case of SP-COOH 25% microstructures (Fig. 2, inset), indicating that these cycles are repeatable, with no detectable hysteresis, at least for the durations of exposure we have studied. Moreover, microscopy imaging between irradiation cycles showed that the microstructures do not appear to suffer any obvious physical degradation arising from this degree of photo-exposure.
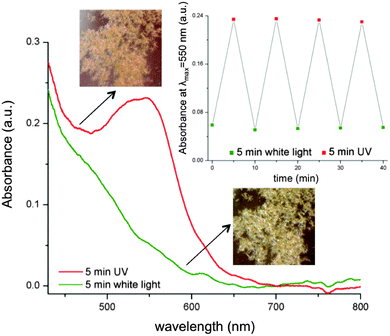 | ||
| Fig. 2 Absorbance spectra and photos of SP-COOH 25% self-assembled microstructures under different illumination conditions. Inset shows absorbance at 550 nm over four switching cycles. | ||
Further investigation including determination of the structures of the self-assembling units for both species (SP, MC) using X-ray crystallography is in progress.
Moreover, as it was experimentally found that the interfacial aggregation is driven by the evaporation of EtOH, guided assembly was realized at the liquid–air interface by using a Halogen lamp, 150 W DDL Polytec GmbH Waldbronn (Video S2, ESI†). The heating effect produced by this lamp (∼4 °C) caused the assembly of the SP-COOH 25% microstructures largely in the vicinity of the irradiated area (where there is predominant EtOH evaporation). The “crystal moths” (in that the crystals appear to spontaneously migrate towards the light source) behavior can be very useful for fast harvesting of the self-assembled microstructures.
In conclusion, we demonstrated a convenient protocol to fabricate dimensionally controlled micrometer structures of carboxylic acid functionalized spiropyran (SP-COOH) and their thermal guided aggregation at the liquid–air interface. The material has been characterized by structural (see ESI†), spectroscopic and imaging methods in order to derive structural and photochromic information on the aggregates. A mechanism for the association was proposed. The process described here permits efficient production of highly organized aggregates that present reversible photochromic properties. The good optical properties and the ease of formation suggest that the self-assembled aggregates can be used for optical memory, non-linear optics and Langmuir–Blodgett films.
The project has been carried out with the support of Science Foundation Ireland under INSIGHT award SFI/12/RC/2289.
Notes and references
- C. M. Sanchez, H. Arribart and M. M. G. Guille, Nat. Mater., 2005, 4, 277–288 CrossRef CAS.
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418–2421 CrossRef CAS PubMed.
- B. A. Grzybowski, C. E. Wilmer, J. Kim, K. P. Browne and K. J. Bishop, Soft Matter, 2009, 5, 1110–1128 RSC.
- M. R. Jones and C. A. Mirkin, Nature, 2012, 491, 42–43 CrossRef CAS PubMed.
- A. Piermattei, et al. , Angew. Chem., Int. Ed., 2006, 45, 7543–7546 CrossRef CAS PubMed.
- M. Ieong, B. Doris, J. Kedzierski, K. Rim and M. Yang, Science, 2004, 306, 2057–2060 CrossRef CAS PubMed.
- H. Ma and J. Hao, Chem. Soc. Rev., 2011, 40, 5457–5471 RSC.
- A. Patra, R. Métivier, F. o. Brisset and K. Nakatani, Chem. Commun., 2012, 48, 2489–2491 RSC.
- X. Zhou, Y. Duan, S. Yan, Z. Liu, C. Zhang, L. Yao and G. Cui, Chem. Commun., 2011, 47, 6876–6878 RSC.
- L. Florea, D. Diamond and F. Benito-Lopez, Macromol. Mater. Eng., 2012, 297, 1148–1159 CrossRef CAS.
- V. Krongauz, S. Fishman and E. Goldburt, J. Phys. Chem., 1978, 82, 2469–2474 CrossRef CAS.
- F. P. Shvartsman, I. R. Cabrera, A. L. Weis, E. J. Wachtel and V. A. Krongauz, J. Phys. Chem., 1985, 89, 3941–3946 CrossRef CAS.
- Y. Onai, M. Mamiya, T. Kiyokawa, K. Okuwa, M. Kobayashi, H. Shinohara and H. Sato, J. Phys. Chem., 1993, 97, 9499–9505 CrossRef CAS.
- V. K. Kotharangannagari, A. Sánchez-Ferrer, J. Ruokolainen and R. Mezzenga, Macromolecules, 2011, 44, 4569 CrossRef CAS.
- H. Tachibana, Y. Yamanaka, H. Sakai, M. Abe and M. Matsumoto, J. Lumin., 2000, 87, 800–802 CrossRef.
- Q. Chen, Y. Feng, D. Zhang, G. Zhang, Q. Fan, S. Sun and D. Zhu, Adv. Funct. Mater., 2010, 20, 36–42 CrossRef CAS.
- P. Uznanski, Langmuir, 2003, 19, 1919–1922 CrossRef CAS.
- Q. Liao, H. Fu, C. Wang and J. Yao, Angew. Chem., Int. Ed., 2011, 50, 4942–4946 CrossRef CAS PubMed.
- J. Harada, Y. Kawazoe and K. Ogawa, Chem. Commun., 2010, 46, 2593–2595 RSC.
- G. Baillet, G. Giusti and R. Guglielmetti, J. Photochem. Photobiol., A, 1993, 70, 157–161 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc46699j |
| This journal is © The Royal Society of Chemistry 2014 |


