Tunable synthesis of poly(ethylene imine)–gold nanoparticle clusters†
Florian
Kretschmer
ab,
Ulrich
Mansfeld
ab,
Stephanie
Hoeppener
ab,
Martin D.
Hager
abc and
Ulrich S.
Schubert
*abc
aLaboratory of Organic and Macromolecular Chemistry (IOMC), Friedrich Schiller University Jena, Humboldtstr. 10, 07743 Jena, Germany. E-mail: ulrich.schubert@uni-jena.de; Fax: +49 3641 948202
bJena Center for Soft Matter (JCSM), Friedrich Schiller University Jena, Philosophenweg 7, 07743 Jena, Germany
cDutch Polymer Institute (DPI), P.O. Box 902, 5600 AX Eindhoven, The Netherlands
First published on 7th August 2013
Abstract
The reaction of tetrachloroauric acid in DMF with poly(ethylene imine) (PEI) as a reducing agent yields spherical nanoparticles. Depending on the reaction conditions single gold nanoparticles or gold–PEI clusters with tunable size up to 200 nm in diameter were obtained which could serve as potential building blocks for metamaterials.
Directing the assembly of noble metal nanoparticles has attracted considerable interest in recent years as a source for potential analytical, medical and optoelectronic applications.1,2 Among the most intriguing materials that can be obtained in this way are metamaterials due to their unique properties not found in nature.3 Being able to manipulate electromagnetic waves in a defined way enables the creation of novel devices like superlenses4 or cloaking devices.5 Meta-atoms are the building blocks of such materials. Structures, which can serve as building blocks, can be obtained via spherical cluster formation or satellite assemblies of plasmonic nanoparticles.6 One strategy to synthesize these structures is to employ spherical templates usually consisting of silica,7 polymers8 or micelles9 and the subsequent synthesis of nanoparticles in their interior. Moreover, already formed nanoparticles can be assembled on their outer surface via electrostatic or strong sulfur–metal particle interactions.10 A second approach is the synthesis of particles, followed by their encapsulation in a spherical shell.11 The approach described herein involves the simultaneous synthesis of the nanoparticles and cluster formation.12 Our strategy involves cheap commercial reagents and requires only one reaction step to create size-tunable gold nanoparticle clusters.
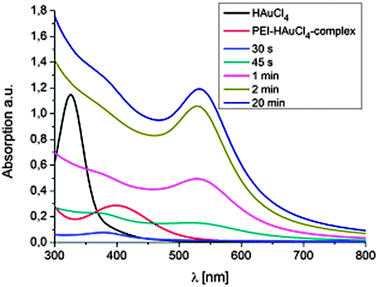
The proposed mechanism for the formation of gold nanoparticle–polymer clusters is depicted in Scheme 1. In general a solution of branched poly(ethylene imine) (bPEI) (25 kDa) and HAuCl4 in DMF is heated, which results in the formation of nanoparticles encapsulated by the polymer. Mixing the solutions leads immediately to a large bathochromic shift (Fig. 1, Fig. S1 and S2, ESI†). The first step in this process is the protonation of the primary, secondary and tertiary amine groups of the polymer. The Au3+ ions are colored yellow in solution and the color change indicates the complex formation between the gold ions and bPEI. It is also possible for one gold ion to complex multiple polymer chains which can facilitate crosslinking. Upon heating, Au3+ ions are first reduced to colorless Au+ ions and then to elemental gold. This can also be followed by UV-vis spectroscopy which shows at first a decrease in absorption due to the formation of Au+ but over time the plasmon band of the nanoparticles evolves and increases in intensity. Metal centers are frequently used in dehydrogenation reactions,13 and organic amines are commonly used for the synthesis of gold nanoparticles.14 Dehydrogenative oxidation of primary and secondary amine groups leads first to imine formation and, depending on the reaction time and temperature, in a second step the formation of nitrile groups in the case of primary amines is possible. For the tertiary amines, transformation to an imine can take place, in addition, formation of radical species can occur which is also a known mechanism in photocatalytic processes in which, e.g., triethylamine acts as an electron donor.15
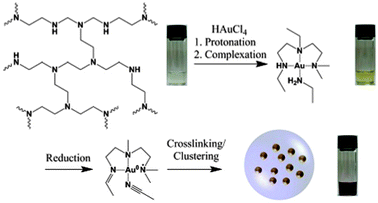 | ||
| Scheme 1 Schematic representation of the cluster formation. Photographs depict the solutions at the different stages of the synthesis. | ||
To gather further insights into the cluster formation process we first started investigating the effect of the PEI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HAuCl4 mass ratio on the formation of nanoparticles (Fig. S3 and S5, ESI†). At a 1
HAuCl4 mass ratio on the formation of nanoparticles (Fig. S3 and S5, ESI†). At a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio a blue solution with an absorption maximum at 622 nm could be obtained which is an indication of the formation of nanoparticles. In this case the amount of reducing agent might be too low to reduce all gold ions to elemental gold. Besides this, the stabilization of the formed particles by the polymer shell could be insufficient, resulting in larger aggregates of nanoparticles. Increasing the ratio to 1
4 ratio a blue solution with an absorption maximum at 622 nm could be obtained which is an indication of the formation of nanoparticles. In this case the amount of reducing agent might be too low to reduce all gold ions to elemental gold. Besides this, the stabilization of the formed particles by the polymer shell could be insufficient, resulting in larger aggregates of nanoparticles. Increasing the ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 results in a stable nanoparticle solution with an absorption maximum at 544 nm. TEM imaging showed spherical nanoparticles with a rather large size distribution and the formation of a polymer film around the particles. However, there is no distinct segregation between the clusters, and most of them appear to be fused together. Increasing the ratio to 1
2 results in a stable nanoparticle solution with an absorption maximum at 544 nm. TEM imaging showed spherical nanoparticles with a rather large size distribution and the formation of a polymer film around the particles. However, there is no distinct segregation between the clusters, and most of them appear to be fused together. Increasing the ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 led to the formation of clearly separated gold–polymer clusters without aggregated gold particles as evidenced by a plasmon band at 532 nm. It is worth noting that the polymer shell shows considerable stability against water. It is possible to perform three times a centrifugation–redispersion cycle without dissolution of the polymer shell or release of gold nanoparticles (Fig. S6, ESI†). This supports the hypothesis of a crosslinking mechanism as pure bPEI is readily soluble in water. The nanoparticles can be etched by addition of cyanide which, however, also results in aggregation of the empty polymer shells.
1 led to the formation of clearly separated gold–polymer clusters without aggregated gold particles as evidenced by a plasmon band at 532 nm. It is worth noting that the polymer shell shows considerable stability against water. It is possible to perform three times a centrifugation–redispersion cycle without dissolution of the polymer shell or release of gold nanoparticles (Fig. S6, ESI†). This supports the hypothesis of a crosslinking mechanism as pure bPEI is readily soluble in water. The nanoparticles can be etched by addition of cyanide which, however, also results in aggregation of the empty polymer shells.
Motivated by these initial results, we sought to explore the effect of changing concentrations of bPEI and HAuCl4 at a fixed ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Surprisingly, increasing or decreasing the amount of the compounds in solution does not result in more or less clusters in solution but, in fact, causes a significant change in the cluster size (Fig. 2 and 3). Decreasing the concentration of each of the reactants to 1 mg mL−1 leads to the formation of single nanoparticles with an average size of 33 nm. There is no obvious cluster formation and only occasionally a thin polymer shell is visible. UV-vis spectroscopy indicates a bathochromic shift of the absorption to 561 nm. Increasing the concentrations to 10, 20, 50 and 100 mg mL−1 yielded polymer shells with sizes of 40, 60, 120 and 230 nm, respectively, with small encapsulated gold nanoparticles (∼3 to 8 nm). The maximum of the absorption changes only insignificantly except for the highest concentration. To address the effect of the polymer size and shape on the formation of nanoparticles we investigated branched PEIs with different molar masses and one linear PEI (lPEI, 25 kDa) (Fig. S4 and S5, ESI†). Application of low molar mass bPEI (0.6 kDa) resulted in the formation of nanoparticles; however, precipitation with simultaneous color change to blue occurred during the reaction. Hence, it was concluded that the polymer might simply be too short to stabilize the particles against aggregation. After increasing the molar mass to 1.8 kDa TEM imaging showed the formation of clusters. However, it should be noted that still some precipitation was observed due to incomplete stabilization, which can also be visualized by the UV-vis spectrum that showed a small broadening of the surface plasmon resonance. Increasing the molar mass to 10 kDa (bPEI) resulted in a stable solution and cluster formation without any evidence of aggregation. Synthesis of clusters with lPEI gave similar results to the ones with the low molar mass bPEI; nanoparticles were formed, but aggregation occurred during the synthesis. Even though the size of the lPEI should be sufficient to stabilize the nanoparticles, the linear structure could compromise the crosslinking of the PEI chains, resulting only in a small or no polymer layer around the nanoparticles. This is, however, not corroborated by experiments conducted with polyallylamine (PAAm). The main difference is the nature of the amine unit, which is primary in the case of PAAm. While the overall polymer structure still corresponds to a linear chain, PAAm was also able to generate particle clusters.
1. Surprisingly, increasing or decreasing the amount of the compounds in solution does not result in more or less clusters in solution but, in fact, causes a significant change in the cluster size (Fig. 2 and 3). Decreasing the concentration of each of the reactants to 1 mg mL−1 leads to the formation of single nanoparticles with an average size of 33 nm. There is no obvious cluster formation and only occasionally a thin polymer shell is visible. UV-vis spectroscopy indicates a bathochromic shift of the absorption to 561 nm. Increasing the concentrations to 10, 20, 50 and 100 mg mL−1 yielded polymer shells with sizes of 40, 60, 120 and 230 nm, respectively, with small encapsulated gold nanoparticles (∼3 to 8 nm). The maximum of the absorption changes only insignificantly except for the highest concentration. To address the effect of the polymer size and shape on the formation of nanoparticles we investigated branched PEIs with different molar masses and one linear PEI (lPEI, 25 kDa) (Fig. S4 and S5, ESI†). Application of low molar mass bPEI (0.6 kDa) resulted in the formation of nanoparticles; however, precipitation with simultaneous color change to blue occurred during the reaction. Hence, it was concluded that the polymer might simply be too short to stabilize the particles against aggregation. After increasing the molar mass to 1.8 kDa TEM imaging showed the formation of clusters. However, it should be noted that still some precipitation was observed due to incomplete stabilization, which can also be visualized by the UV-vis spectrum that showed a small broadening of the surface plasmon resonance. Increasing the molar mass to 10 kDa (bPEI) resulted in a stable solution and cluster formation without any evidence of aggregation. Synthesis of clusters with lPEI gave similar results to the ones with the low molar mass bPEI; nanoparticles were formed, but aggregation occurred during the synthesis. Even though the size of the lPEI should be sufficient to stabilize the nanoparticles, the linear structure could compromise the crosslinking of the PEI chains, resulting only in a small or no polymer layer around the nanoparticles. This is, however, not corroborated by experiments conducted with polyallylamine (PAAm). The main difference is the nature of the amine unit, which is primary in the case of PAAm. While the overall polymer structure still corresponds to a linear chain, PAAm was also able to generate particle clusters.
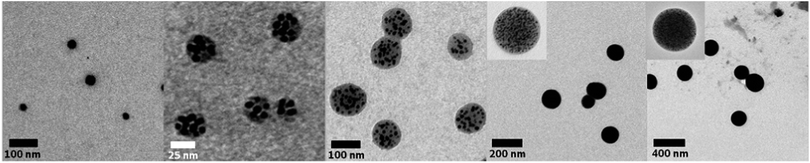 | ||
Fig. 2 TEM images of single particles and clusters synthesized from bPEI 25 kDa and HAuCl4 at a fixed ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Concentrations of the reactants: 1, 10, 20, 50, 100 mg mL−1 (from left to right). 1. Concentrations of the reactants: 1, 10, 20, 50, 100 mg mL−1 (from left to right). | ||
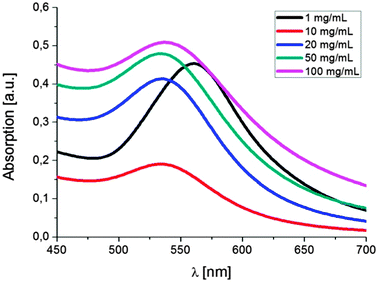 | ||
| Fig. 3 UV-vis spectra of single particles and clusters synthesized at different concentrations of the reactants (in DMF). | ||
As the formation of metal nanoparticles in water via PEI is well known,16,17 though, without formation of a polymer shell, we suggest that the solvent might also contribute to the shell formation. To elucidate this issue we investigated the impact of the solvent on the cluster formation (Fig. S7 and S8, ESI†). It turned out to be difficult to identify a solvent in which bPEI and HAuCl4 are soluble at the same time. At first we performed similar experiments reported in the literature with water as the solvent and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the compounds which resulted in single spherical particles. Due to the aforementioned difficulties ethanol was used as the solvent yielding also no clusters. As ethanol and many other organic solvents are also capable of reducing gold ions to elemental gold, the formation of nanoparticles might merely be attributed to the reducing properties of the organic solvent while the polymer acts only as a capping agent. DMF is also reported to be a reducing agent for gold salts;18 however, the reduction proceeds only at high temperature or over a prolonged period of time. To exclude this effect we conducted the reaction in DMF at room temperature. In contrast to the reactions performed at 130 °C the reaction was complete only after several days. Cluster formation takes place, but as is evident from the TEM image, the clusters are less defined and the polymer shell is considerably thinner (Fig. S8, ESI†). In addition, occasionally rather large nanoparticles are formed and the particles appear more aggregated. This is in agreement with the UV-vis spectrum of the solution which shows a slight bathochromic shift in conjunction with an overall broadening of the plasmon resonance compared to the samples prepared at elevated temperature.
1 ratio of the compounds which resulted in single spherical particles. Due to the aforementioned difficulties ethanol was used as the solvent yielding also no clusters. As ethanol and many other organic solvents are also capable of reducing gold ions to elemental gold, the formation of nanoparticles might merely be attributed to the reducing properties of the organic solvent while the polymer acts only as a capping agent. DMF is also reported to be a reducing agent for gold salts;18 however, the reduction proceeds only at high temperature or over a prolonged period of time. To exclude this effect we conducted the reaction in DMF at room temperature. In contrast to the reactions performed at 130 °C the reaction was complete only after several days. Cluster formation takes place, but as is evident from the TEM image, the clusters are less defined and the polymer shell is considerably thinner (Fig. S8, ESI†). In addition, occasionally rather large nanoparticles are formed and the particles appear more aggregated. This is in agreement with the UV-vis spectrum of the solution which shows a slight bathochromic shift in conjunction with an overall broadening of the plasmon resonance compared to the samples prepared at elevated temperature.
Next to gold, silver is the most interesting material for plasmonic applications due to its more favorable optical properties and lower costs.19 In case the reaction is conducted with an equivalent amount of silver nitrate instead of HAuCl4 a yellow solution is formed. TEM imaging and UV-vis spectroscopy showed the formation of silver nanoparticles with a broad size distribution, however, no cluster formation could be observed. Two factors could contribute to this result: on the one side, in contrast to gold, DMF is a good reducing agent for silver ions which results in a similar combination to the aforementioned ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HAuCl4
HAuCl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEI system. On the other hand, the counter ion might contribute to the formation of the polymer shell. While bPEI is soluble in water and some polar organic solvents, bPEI hydrochloride is soluble only in water but not in DMF. No formation of clusters is evident directly after mixing the reactants, however, as hydrochloric acid evolves during complexation and reduction of HAuCl4, more protonation of the polymer takes place, resulting in the formation of clusters. In order to confirm this hypothesis we conducted a control experiment with NH4Cl. Upon heating NH3 is driven off and HCl is formed. This resulted in the formation of polymer nanoparticles as evidenced by TEM imaging, however, devoid of gold particles (Fig. S2, ESI†).
PEI system. On the other hand, the counter ion might contribute to the formation of the polymer shell. While bPEI is soluble in water and some polar organic solvents, bPEI hydrochloride is soluble only in water but not in DMF. No formation of clusters is evident directly after mixing the reactants, however, as hydrochloric acid evolves during complexation and reduction of HAuCl4, more protonation of the polymer takes place, resulting in the formation of clusters. In order to confirm this hypothesis we conducted a control experiment with NH4Cl. Upon heating NH3 is driven off and HCl is formed. This resulted in the formation of polymer nanoparticles as evidenced by TEM imaging, however, devoid of gold particles (Fig. S2, ESI†).
The optical properties of nanoparticle assemblies are also dependent on the size of the gold particles in the cluster and the filling fraction of gold. While the size of the gold particles cannot be increased by increasing the gold concentration it turned out to be possible to utilize pre-synthesized gold particles which are encapsulated during the synthesis. Citrate-stabilized Au nanoparticles were transferred into DMF, and, in a similar way as introduced before, small or large clusters can be synthesized. TEM imaging showed for a low concentration of initial particles concomitantly present small and large particles (Fig. S9 and S10, ESI†). However, the fraction of small particles is significantly lower compared to the synthesis without initial particles. In addition, empty clusters can also be observed. In the case of a high initial particle concentration no secondary nucleation is found. This observation indicates that the formation mechanism of these clusters remains essentially the same as previously described, except that in addition the seeded growth of the initial particles takes place. In accordance with theoretical predictions6 an increased filling fraction leads to a more pronounced broadening of the localized surface plasmon resonance as indicated by UV-vis spectroscopy.
In conclusion, we synthesized tuneable gold nanoparticle–PEI clusters and investigated the effects of the polymer structure, solvent and concentration of the reactants. The mechanism relies on the reducing properties of the amine moieties with concomitant insolubilization of the polymer via HCl and crosslinking of the polymer chains. The filling fraction and size can be increased via encapsulation of pre-synthesized gold nanoparticles. The introduced approach presents a versatile route to synthesize cluster systems with good control and potentially promising properties for further research, e.g., for application in metamaterials or the design of functional polyamine–plasmonic particle composites.
Financial support by the Federal Ministry of Education and Research (Spitzencluster PHONA) is acknowledged.
Notes and references
- N. J. Halas, S. Lal, W. S. Chang, S. Link and P. Nordlander, Chem. Rev., 2011, 111, 3913–3961 CrossRef CAS PubMed.
- Y. Jin, Adv. Mater., 2012, 24, 5153–5165 CrossRef CAS PubMed.
- Y. Liu and X. Zhang, Chem. Soc. Rev., 2011, 40, 2494–2507 RSC.
- N. Fang, H. Lee, C. Sun and X. Zhang, Science, 2005, 308, 534–537 CrossRef CAS PubMed.
- L. H. Gabrielli, J. Cardenas, C. B. Poitras and M. Lipson, Nat. Photonics, 2009, 3, 461–463 CrossRef CAS.
- J. Dintinger, S. Mühlig, C. Rockstuhl and T. Scharf, Opt. Mater. Express, 2012, 2, 269–278 CrossRef CAS.
- M. Xiao, C. Zhao, H. Chen, B. Yang and J. Wang, Adv. Funct. Mater., 2012, 22, 4526–4532 CrossRef CAS.
- J. Cho and F. Caruso, Chem. Mater., 2005, 17, 4547–4553 CrossRef CAS.
- X. Chen, Y. An, D. Zhao, Z. He, Y. Zhang, J. Cheng and L. Shi, Langmuir, 2008, 24, 8198–8204 CrossRef CAS PubMed.
- S. Mühlig, A. Cunningham, S. Scheeler, C. Pacholski, T. Burgi, C. Rockstuhl and F. Lederer, ACS Nano, 2011, 5, 6586–6592 CrossRef PubMed.
- M. Grzelczak, A. Sanchez-Iglesias, H. H. Mezerji, S. Bals, J. Perez-Juste and L. M. Liz-Marzán, Nano Lett., 2012, 12, 4380–4384 CrossRef CAS PubMed.
- J. Kim, M. J. Sadowsky and H. G. Hur, Biomacromolecules, 2011, 12, 2518–2523 CrossRef CAS PubMed.
- F. Richard Keene, Coord. Chem. Rev., 1999, 187, 121–149 CrossRef.
- J. D. Newman and G. J. Blanchard, Langmuir, 2006, 22, 5882–5887 CrossRef CAS PubMed.
- S. Rau, B. Schäfer, D. Gleich, E. Anders, M. Rudolph, M. Friedrich, H. Görls, W. Henry and J. G. Vos, Angew. Chem., Int. Ed., 2006, 45, 6215–6218 CrossRef CAS PubMed.
- X. Sun, S. Dong and E. Wang, Langmuir, 2005, 21, 4710–4712 CrossRef CAS.
- K.-S. Shin, J.-H. Kim, I.-H. Kim and K. Kim, Bull. Korean Chem. Soc., 2012, 33, 906–910 CrossRef CAS.
- I. Pastoriza-Santos and L. M. Liz-Marzán, Langmuir, 2002, 18, 2888–2894 CrossRef CAS.
- N.-H. Shen, T. Koschny, M. Kafesaki and C. Soukoulis, Phys. Rev. B, 2012, 85, 075120–075124 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc45090b |
| This journal is © The Royal Society of Chemistry 2014 |