Thixotropic silk nanofibril-based hydrogel with extracellular matrix-like structure†
Yingxin
Liu
,
Shengjie
Ling
,
Suhang
Wang
,
Xin
Chen
and
Zhengzhong
Shao
*
State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, Laboratory of Advanced Materials, Fudan University, Shanghai, 200433, People's Republic of China. E-mail: zzshao@fudan.edu.cn; Tel: +86 21 6564 2866
First published on 31st July 2014
Abstract
Silk fibroin (SF) based materials have been widely studied and applied in bio-related areas due to their excellent structural and biological properties. Here, we present an injectable hydrogel formed by SF nanofibrils via simple fibrillation and centrifugation approach. The hydrogels with extracellular matrix-like structure not only perform the sufficient mechanical properties, but also show outstanding thixotropic character, whose storage modulus (G′) can recover to 93% within 40 seconds after a large shearing strain (5000 %). More importantly, the injectable hydrogel exhibits significant biocompatibility for L929 cells cultured in hydrogel after injection, illustrated by cell viability and cytotoxicity assays. All of these results indicate that such SF nanofibril-based hydrogel has promise in application such as a cell therapy carrier.
Injectable hydrogels have been extensively studied as ideal implanted biomaterials for cell therapies in tissue engineering and regenerative medicine.1,2 The reasons for this are (a) injectable matrix can appropriately fill the irregularly shaped tissue defects with minimal surgical wounds and patient discomfort;3 (b) drugs or cells can be incorporated into the hydrogel simply by mixing before injection;4,5 (c) these water-holding, three-dimensional networks, resembling extracellular cell matrix (ECM), possess high permeability for oxygen, nutrients, waste metabolites and intercellular chemical signaling.6,7 However, a comprehensive and systematic approach to prepare such hydrogels with balanced processing–structure–function relationships remains challenging.8–10
From the aspects of processing, an environmentally friendly and facile approach for obtaining low-cost injectable hydrogel is still to be pursued in the areas of both chemical and physical gelation.11,12 Compared to chemical gelation, which normally requires elaborate methods, harsh conditions and cytotoxic solvents in the processing, physical gelation is more reliable to address these issues due to the moderate and cytocompatible preparation conditions such as temperature and pH. Moreover, the strategy to construct biomacromolecular nanofibrillar hydrogels with ECM-like structure are still in their infancy. For example, cellulose nanofibril-based hydrogel has started to emerge only recently.13 The critical roadblock is that ECM is universally hierarchical at a multitude of diverse scales like other biological tissues and materials.14–16
According to the flowing state before injection, injectable hydrogels may be divided into two categories. The common one is that it is a solution before injection and becomes gel at the defective tissue in situ.4 Another is a preformed solid-hydrogel holding shearing-thinning and self-healing characters (so-called thixotropy), and it can be delivered by a syringe.17–19 Obviously, the latter may have better way to prevent unwanted leakage due to insufficient gelation rate.20
Silk fibroin (SF) is an ideal candidate for achieving injectable hydrogels, not only because of its excellent biocompatibility and tunable degradability,21–23 but also due to its strong self-assembly tendency as well as facile processibility.24–26 Zhang et al. reported that they could obtain the aqueous dispersion of SF nanofibrils directly from natural silkworm cocoon silks.27 However, well-dispersed SF nanofibril solution made from SF aqueous solutions has seldom been reported.28,29 This is probably because of the high instability of SF solutions, which show a strong tendency to form hydrogels or aggregates at environmental conditions with low pH,30,31 high temperature,32 shear forces,33 and alcohol treatment.34 Recently, we found that SF molecules could assemble into elongated nanofibrils comparable to amyloid nanofibrils.29 In this study, we show that such a SF nanofibril solution can be further converted to an injectable hydrogel with thixotropic property through a simple one-step centrifugation process. The mechanical strength of such a SF nanofibril-based hydrogel can be tuned easily by adjusting the sodium chloride content in the system. Compared with previous silk injectable hydrogels, SF nanofibril-based hydrogels show higher thixotropy, lower solid content and more homogeneously nanofibrillar network (ECM-like structure). Therefore, such hydrogels can be used as cell carriers with remarkable cell encapsulation and delivery ability.
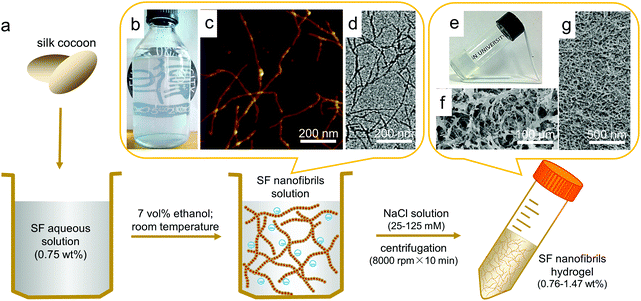
The approach followed to generate the SF nanofibril-based hydrogel is shown in Fig. 1a. First, Bombyx mori silkworm cocoon silk fibers were degummed, dissolved and dialyzed in accordance with a typical protocol described in available literature and ESI,†26 yielding a solution of protein concentration of ca. 5 wt%. The solution was further adjusted to 0.75 wt% and pH 9.5 with 0.5 mol L−1 NaOH aqueous solution, and then mixed with ethanol (the final concentration of ethanol 7 vol%). When this solution was incubated for one day at room temperature, the SF molecules self-assembled into elongated nanofibrils due to appropriate conformation transition of SF induced by ethanol. The obtained SF nanofibril solution (0.14 wt%) is opalescent (Fig. 1b), and those SF nanofibrils are up to 1 μm long in contour length, as observed by AFM and TEM (Fig. 1c and d), which are identical to our previous findings.29 CD curve of SF nanofibril solution, as well as the FTIR spectrum (sharp absorption at 1623 cm−1) of these dried SF nanofibrils prove that β-sheet is the dominant conformation in such nanofibrils (Fig. S1†).35 On account of the existence of electrostatic interactions (the isoelectric point of SF is 4.53,36 and SF nanofibrils were negatively charged at basic pH, confirmed by the zeta-potential test, e.g., the value is −18 mV at pH = 6.8) and bactericidal effect (ethanol) in solution, this SF nanofibril solution can remain stable for more than 6 months without precipitation and mildew, as shown in Fig. 1b.
ECM is an intricate network composed of nanofibrillar proteins (mostly collagen).16 Compared with the diameter and length of constituent nanofibrils, the resultant nanofibrillar 3D network is more important for encapsulated cells.13,16 Considering the structural, mechanical and biocompatible similarities between SF nanofibrils and collagen fibirls,37,38 as well as inherent shear thinning properties of nanofibrillar network,39 we set out to prepare ECM-like injectable hydrogel via simple one-step centrifugation process. To prevent the negative effect of ethanol on cells, we have removed it by dialyzing SF nanofibril solution against deionized water for 3 days before fabricating hydrogels. Initially, we found that electrolyte could induce SF nanofibrils to aggregate, as shown in Fig. S2.† Therefore, SF nanofibril solutions (0.14 wt%) with or without sodium chloride were used by us for the attempts to produce hydrogels. In the case containing NaCl, 0.6 mL NaCl solution with calculated concentration was mixed with 5.4 mL SF nanofibril solutions to obtain final solutions of 25, 50, 75, 100, 125 mmol L−1 NaCl separately, and these ion concentrations have no significantly adverse effects on cells growth and proliferation.20 No hydrogel could form from the SF nanofibril solution without NaCl; nevertheless, SF nanofibril solution with NaCl (for all cases) formed a highly transparent hydrogel after only 10 min centrifugation with 8000 r min−1, as shown in Fig. 1e, whose transmittance is up to 72% at 700 nm (Fig. S3†), close to that of the cornea.40 The corresponding SEM images (Fig. 1f and g) reveal that the resultant hydrogel is closely similar to ECM, displaying uniform porous and fibrous scaffold structure, which could offer a structural support for cells and potentially enhance cell adhesion, proliferation and absorb proteins and growth factors.41,42
As for the difference caused by NaCl in forming hydrogels, it could be a consequence of the Debye length variation. Due to the presence of electrostatic interactions, SF nanofibril solution is stable under the centrifugation or other shearing actions (same as the reason why SF nanofibril solution can stabilize for more than 6 months at room temperature, as mentioned before). In contrast, by adding NaCl, the Debye length of the surface charge on SF nanofibrils becomes shorter, therefore leading to reduced repulsive force between the SF nanofibrils (Fig. S2†). Finally, SF nanofibrils are easily entangled to form three-dimensional networks under centrifugation.
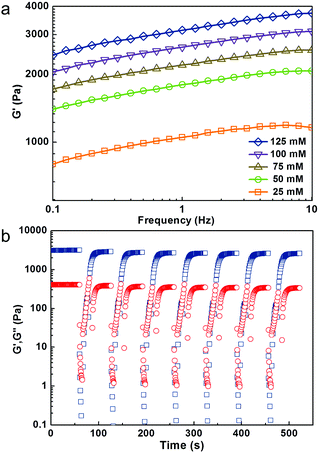
According to our hypothesis, the repulsive forces among SF nanofibrils (which can be easily manipulated by applying different NaCl concentration before gelation) has a critical influence on solid content of the hydrogels (Table 1), and thus will be directly reflected in their mechanical strength. Rheological experiments were performed to evaluate the mechanical properties (viscoelastic behavior) of the SF nanofibril-based hydrogels, as shown in Fig. 2. The relative strain sweep and shear sweep curves are shown in Fig. S4.† Indeed, by progressively increasing the concentration of NaCl from 25 to 125 mmol L−1, the storage modulus (G′) of SF nanofibril-based hydrogels can be increased substantially from 1100 to 3700 Pa. 480 Pa already meets the mechanical requirement for an implanted hydrogel,43 and thus the hydrogels in our case have tunable mechanical properties for different clinical requirements. In view of the physiological salt concentration (150 mM), we studied the variation of solid content and G′ after SF nanofibril-based hydrogels were soaked in such NaCl concentration PBS. Fig. S5† illustrates that solid content and G′ of all hydrogels increased, which could further prevent the possible leakage of hydrogel in the defective tissue.
| NaCl concentration (mmol L−1) | 25 | 50 | 75 | 100 | 125 |
| Solid content (wt%) | 0.76 ± 0.07 | 1.01 ± 0.05 | 1.19 ± 0.01 | 1.32 ± 0.07 | 1.47 ± 0.15 |
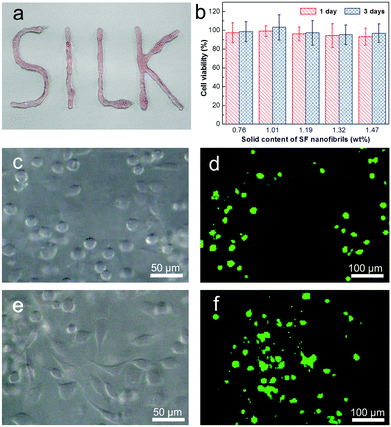
Recovery rate and ratio are crucial parameters to evaluate the injectable properties of a thixotropic hydrogel. Remarkably, SF nanofibril-based hydrogel withstanding a great strain treatment (5000%) and G′ could recover to 90% within 40 seconds, even after continuous repeats for 7 times. To the best of our knowledge, no such ultrahigh recoverability of pristine SF hydrogel has been reported previously. This also indicates that SF nanofibril-based hydrogel can effectively prevent the possible leak due to the inadequate gelation rate. This was also confirmed by our tentative test. The hydrogel can be easily injected into desired styles, as shown in Fig. 3a; moreover, the shape of the injected hydrogel can be fixed perfectly (see ESI video†).
In addition to the structure and mechanical properties, biocompatibility is the most important respect for injectable hydrogels. Here, L929 cells were employed to evaluate the viability and cytotoxicity of the SF nanofibril-based hydrogels via CCK-8 assay. Fig. 3b presents the results of cytotoxicity test for SF nanofibril-based hydrogels at 1 and 3 days of incubation. It can be found that in all samples, the relative cell growth is more than 94%, clearly proving that there is no cytotoxicity. In order to demonstrate the biocompatibility and explore the potential applications in the area of cell-carriers, L929 cells were encapsulated in the SF nanofibril-based hydrogel for a scheduled time (1, 3 and 7 days in our cases), and a corresponding live/dead staining was followed. It should be mentioned at this stage that SF nanofibril-based hydrogels show no collapse in all of incubation times, and there is no detectable degradation in 7 days (Fig. S6†). The confocal images (Fig. 3d, f and S7†) reveal that cells showed uniform dispersion in the SF nanofibril-based hydrogels and demonstrate a similar cytotoxicity result to that of CCK-8 assay shown in Fig. 3b. More importantly, L929 cells showed spreading trend in the hydrogel (Fig. 3c and e), which may be due to the low hydrogel solid content and homogeneous nanofibrillar network. This also indicates that cells behave analogously with their native state in ECM. Given the excellent injectable property, we are confident that these SF nanofibril-based hydrogels will offer benefits for cell therapy.
In summary, we prepared a new injectable SF nanofibril-based hydrogel via a facile centrifugation approach. Such a hydrogel shows a hierarchical structure similar to that of ECM. More importantly, this material has super resistance to ultrahigh shear; moreover, it can maintain outstanding recovering ability under iteratively ultrahigh shears. Finally, the cytotoxicity and biocompatibility results prove that SF nanofibril-based hydrogel is a superb hydrogel for implanted biomaterials. These injectable SF nanofibril-based hydrogels, which combined the advantages of processing, structure, and properties, have promise to be used in cell carriers for different kinds of clinical therapy.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (no. 21034003 and 21274028), the National High Technology Research and Development Program of China (863 Program) (no. 2012AA030309), and Program of Shanghai Subject Chief Scientist (12XD1401000). Shengjie Ling gratefully acknowledges the provision of a scholarship by the China Scholarship Council. The authors also thank Dr Jinrong Yao, Dr Yuhong Yang and Han Cao at Fudan University for their valuable suggestions and discussions.Notes and references
- E. Ruel-Gariépy and J. Leroux, Eur. J. Pharm. Biopharm., 2004, 58, 409–426 CrossRef PubMed.
- Y. Li, J. Rodrigues and H. Tomás, Chem. Soc. Rev., 2012, 41, 2193–2221 RSC.
- J. D. Kretlow, L. Klouda and A. G. Mikos, Adv. Drug Delivery Rev., 2007, 59, 263–273 CrossRef CAS PubMed.
- L. Yu and J. Ding, Chem. Soc. Rev., 2008, 37, 1473–1481 RSC.
- C. Wang, R. R. Varshney and D. Wang, Adv. Drug Delivery Rev., 2010, 62, 699–710 CrossRef CAS PubMed.
- J. L. Drury and D. J. Mooney, Biomaterials, 2003, 24, 4337–4351 CrossRef CAS.
- N. A. Peppas, J. Z. Hilt, A. Khademhosseini and R. Langer, Adv. Mater., 2006, 18, 1345–1360 CrossRef CAS.
- N. Huebsch and D. J. Mooney, Nature, 2009, 462, 426–432 CrossRef CAS PubMed.
- E. S. Place, N. D. Evans and M. M. Stevens, Nat. Mater., 2009, 8, 457–470 CrossRef CAS PubMed.
- K. M. Woo, J. Jun, V. J. Chen, J. Seo, J. Baek, H. Ryoo, G. Kim, M. J. Somerman and P. X. Ma, Biomaterials, 2007, 28, 335–343 CrossRef CAS PubMed.
- W. E. Hennink and C. F. van Nostrum, Adv. Drug Delivery Rev., 2012, 64, 223–236 CrossRef PubMed.
- D. Macaya and M. Spector, Biomed. Mater., 2012, 7, 012001 CrossRef CAS PubMed.
- M. Bhattacharya, M. M. Malinen, P. Lauren, Y. Lou, S. W. Kuisma, L. Kanninen, M. Lille, A. Corlu, C. GuGuen-Guillouzo, O. Ikkala, A. Laukkanen, A. Urtti and M. Yliperttula, J. Controlled Release, 2012, 164, 291–298 CrossRef CAS PubMed.
- H. Fernandes, L. Moroni, C. van Blitterswijk and J. de Boer, J. Mater. Chem., 2009, 19, 5474–5484 RSC.
- W. P. Daley, S. B. Peters and M. Larsen, J. Cell Sci., 2008, 121, 255–264 CrossRef CAS PubMed.
- M. P. Lutolf and J. A. Hubbell, Nat. Biotechnol., 2005, 23, 47–55 CrossRef CAS PubMed.
- J. P. Schneider, D. J. Pochan, B. Ozbas, K. Rajagopal, L. Pakstis and J. Kretsinger, J. Am. Chem. Soc., 2002, 124, 15030–15037 CrossRef CAS PubMed.
- R. V. Rughani, M. C. Branco, D. J. Pochan and J. P. Schneider, Macromolecules, 2010, 43, 7924–7930 CrossRef CAS.
- M. C. Branco, D. J. Pochan, N. J. Wagner and J. P. Schneider, Biomaterials, 2010, 31, 9527–9534 CrossRef CAS PubMed.
- I. M. Geisler and J. P. Schneider, Adv. Funct. Mater., 2012, 22, 529–537 CrossRef CAS.
- U. Kim, J. Park, H. J. Kim, M. Wada and D. L. Kaplan, Biomaterials, 2005, 26, 2775–2785 CrossRef CAS PubMed.
- N. E. Davis, L. N. Beenken-Rothkopf, A. Mirsoian, N. Kojic, D. L. Kaplan, A. E. Barron and M. J. Fontaine, Biomaterials, 2012, 33, 6691–6697 CrossRef CAS PubMed.
- J. G. Hardy and T. R. Scheibel, Prog. Polym. Sci., 2010, 35, 1093–1115 CrossRef CAS PubMed.
- I. Greving, M. Cai, F. Vollrath and H. C. Schniepp, Biomacromolecules, 2012, 13, 676–682 CrossRef CAS PubMed.
- Q. Lu, H. Zhu, C. Zhang, F. Zhang, B. Zhang and D. L. Kaplan, Biomacromolecules, 2012, 13, 826–832 CrossRef CAS PubMed.
- G. Zhou, Z. Shao, D. P. Knight and X. Chen, Adv. Mater., 2009, 21, 366–370 CrossRef CAS.
- F. Zhang, Q. Lu, J. Ming, H. Dou, Z. Liu, B. Zuo, M. Qin, F. Li, D. L. Kaplan and X. Zhang, J. Mater. Chem. B, 2014, 2, 3879–3885 RSC.
- S. Bai, S. Liu, C. Zhang, W. Xu, Q. Lu, H. Han, D. L. Kaplan and H. Zhu, Acta Biomater., 2013, 9, 7806–7813 CrossRef CAS PubMed.
- S. Ling, C. Li, J. Adamcik, Z. Shao, X. Chen and R. Mezzenga, Adv. Mater., 2014, 26, 4569–4574 CrossRef CAS PubMed.
- M. Fini, A. Motta, P. Torricelli, G. Giavaresi, N. N. Aldini, M. Tschon, R. Giardino and C. Migliaresi, Biomaterials, 2005, 26, 3527–3536 CrossRef CAS PubMed.
- U. Kim, J. Park, C. Li, H. Jin, R. Valluzzi and D. L. Kaplan, Biomacromolecules, 2004, 5, 786–792 CrossRef CAS PubMed.
- A. Matsumoto, J. Chen, A. L. Collette, U. Kim, G. H. Altman, P. Cebe and D. L. Kaplan, J. Phys. Chem. B, 2006, 110, 21630–21638 CrossRef CAS PubMed.
- T. Yucel, P. Cebe and D. L. Kaplan, Biophys. J., 2009, 97, 2044–2050 CrossRef CAS PubMed.
- Z. Gong, Y. Yang, L. Huang, X. Chen and Z. Shao, Soft Matter, 2010, 6, 1217–1223 RSC.
- S. Ling, Z. Qi, D. P. Knight, Z. Shao and X. Chen, Biomacromolecules, 2011, 12, 3344–3349 CrossRef CAS PubMed.
- C. W. P. Foo, E. Bini, J. Hensman, D. P. Knight, R. V. Lewis and D. L. Kaplan, Appl. Phys. A: Mater. Sci. Process., 2006, 82, 223–233 CrossRef CAS PubMed.
- M. Miron-Mendoza, J. Seemann and F. Grinnell, Biomaterials, 2010, 31, 6425–6435 CrossRef CAS PubMed.
- S. Ling, C. Li, J. Adamcik, S. Wang, Z. Shao, X. Chen and R. Mezzenga, ACS Macro Lett., 2014, 3, 146–152 CrossRef CAS.
- C. Yan, A. Altunbas, T. Yucel, R. P. Nagarkar, J. P. Schneider and D. J. Pochan, Soft Matter, 2010, 6, 5143–5156 RSC.
- M. Rafat, F. Li, P. Fagerholm, N. S. Lagali, M. A. Watsky, R. Munger, T. Matsuura and M. Griffith, Biomaterials, 2008, 29, 3960–3972 CrossRef CAS PubMed.
- M. Mastrogiacomo, S. Scaglione, R. Martinetti, L. Dolcini, F. Beltrame, R. Cancedda and R. Quarto, Biomaterials, 2006, 27, 3230–3237 CrossRef CAS PubMed.
- Y. Wang, Q. Zhao, H. Zhang, S. Yang and X. Jia, Adv. Mater., 2014, 26, 4163–4167 CrossRef CAS PubMed.
- E. L. Bakota, Y. Wang, F. R. Danesh and J. D. Hartgerink, Biomacromolecules, 2011, 12, 1651–1657 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4bm00214h |
| This journal is © The Royal Society of Chemistry 2014 |