Advanced nanocomposites for bone regeneration
Kevin
Baler
ab,
Jordan P.
Ball
c,
Zdravka
Cankova
a,
Ryan A.
Hoshi
a,
Guillermo A.
Ameer
*ab and
Josephine B.
Allen
*c
aBiomedical Engineering Department, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208, USA. E-mail: g-ameer@northwestern.edu; Tel: +1 (847) 467-2992
bChemistry of Life Processes Institute, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208, USA
cDepartment of Materials Science and Engineering, University of Florida, Rhines Hall, Gainesville, FL 32611, USA. E-mail: jallen@mse.ufl.edu; Tel: +1 (352) 846-3328
First published on 23rd June 2014
Abstract
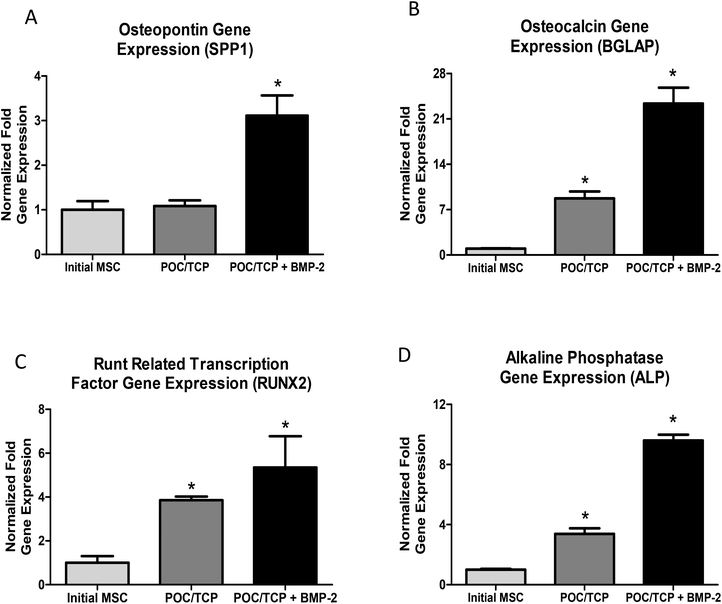
The field of orthopedic tissue engineering is quickly expanding with the development of novel materials and strategies designed for rapid bone regeneration. While autologous bone grafts continue to be the standard of care, drawbacks include donor-site morbidity and short tissue supplies. Herein we report a novel nanocomposite sponge composed of poly(1,8-octanediol-co-citrate) (POC) and the bioactive ceramic β-tricalcium phosphate (TCP). We show that these nanocomposite sponges can be used as a depot for bone-producing (a.k.a. osteogenic) growth factors. In vitro bioactivity is demonstrated by significant upregulation of osteogenic genes, osteopontin (∼3 fold increase), osteocalcin (∼22 fold increase), alkaline phosphatase (∼10 fold increase), and transcription factor, RUNX2 (∼5 fold increase) over basal expression levels in mesenchymal stem cells. In vivo osteogenicity and biocompatibility is demonstrated in a standard subcutaneous implant model in rat. Results show that the nanocomposite sponge supports complete cell infiltration, minimal adverse foreign body response, positive cellular proliferation, and cellular expression of osteogenic markers in subcutaneous tissue. The results shown herein are encouraging and support the use of this sponge for future bone tissue engineering efforts.
1. Introduction
Autologous bone grafts continue to be the standard of care to repair bone defects due to their intrinsic osteoconductivity, osteogenicity and osteoinductivity. However, their use is limited because of donor-site morbidity from the additional surgery and short supply of host tissue for grafting.1–3 Consequently, the need to develop new orthobiologic materials to aid in the management of bone defects remains. As such, the field of orthopedic tissue engineering has been flooded in recent years with a variety of biomaterials proposed as bone grafts. The challenge is to ensure that there is a balance between the appropriate structural and mechanical requirements for the graft material and the necessary biological components to promote the growth of vascularized bone.A major goal is to develop bone tissue alternatives for the treatment of bone defects from non-union fractures, traumatic bone injury, bone tumor resection, and spinal fusion cases where spontaneous bone restoration does not occur.2 The diversity and complexity of these clinical problems requires the development of graft materials that are versatile, facilitating the customization of properties for the intended application. Bone grafts can be made from synthetic or naturally occurring materials.4,5 Typical synthetic bone grafting materials come in a variety of physical forms such as liquids, powders, granules, putties, and porous rigid blocks to facilitate handling and delivery to the surgical site. Synthetic bone grafts or substitutes also come in a variety of compositions including bioactive ceramics, glasses, polymers, and polymer–ceramic composites.6,7 There is increasing interest in developing synthetic grafts that are biologically active and will support bone regeneration but such devices are not commercially available to date.6 Grafts derived from naturally occurring materials may consist of collagen-based scaffolds or demineralized bone tissue. These grafts represent a majority of off-the-shelf bone grafts on the market. More recently, there has been a significant clinical interest in the use of sponge-like orthobiologic grafts or matrices due to their ability to compress and readily expand into bone defects and the option to absorb marrow or cell suspensions into the sponge pore structure. All of the sponge-like bone grafts currently on the market are derived from demineralized allogeneic or xenogeneic bone that has been specially processed to maintain some degree of elasticity.8,9 These materials have unpredictable performance and resorption characteristics due to the heterogenous tissue source and their long-term performance has yet to be evaluated. There are also reports in the literature on composite scaffolds that combine the properties of both synthetic elastomers and brittle materials.10–12 While some of the results look promising, most approaches have limitations associated with biocompatibility, lack of tunability, lack of bioactivity, and toxicity.13
This report describes the characterization of a novel nanocomposite sponge that consists of a citric acid-based elastomer and bioceramic nanoparticles for orthopedic tissue engineering applications. The sponge consists of the elastomeric polymer, poly(1,8-octanediol-co-citrate) (POC) and β-tricalcium phosphate (β-TCP, Ca3(PO4)2) nanoparticles. POC is a well-described biocompatible, elastomeric biomaterial that has been investigated by our group and others for a variety of tissue engineering applications.14–19 The bioactive β-TCP is a ceramic material similar to the mineral found in native bone, hydroxyapatite (Ca10(PO4)6(OH)2). Both β-TCP and hydroxyapatite are calcium phosphate based minerals commonly found in nature with the hydroxyapatite mineral containing an additional hydroxide ion beyond the basic calcium phosphate block. While hydroxyapatite is found in human bone, β-TCP is more soluble than hydroxyapatite, thus making it more available for uptake by cells producing extracellular matrix during tissue regeneration.20,21 However, the direct downside to this increased solubility, is that the accelerated release of Ca2+ and PO43− ions will reduce the available surface area for bone cell proliferation.22 While previous work explored the use of a POC/hydroxyapatite system, here we explored the inclusion of β-TCP into the POC elastomeric polymer as a strategy to enhance bone regeneration and limit the loss in available osteogenic surface area. This combination has been investigated due to the reported osteogenic properties of the constituents as well as an ideal balance between β-TCP solubility and the rate of new bone formation.23–27 Overall, the POC/TCP sponge is degradable and shows great promise as a biologically active tissue engineering scaffold.
2. Experimental
2.1. Nanocomposite fabrication
Synthesis of poly(1,8-octanediol-co-citrate) (POC) was carried out as previously described.28 All chemicals used in the synthesis were purchased from Sigma Aldrich (St. Louis, MO). Medical grade Beta Tricalcium phosphate nanocrystals (β-TCP) were purchased from Berkeley Advanced Biomaterials (San Leandro, CA). Briefly, equimolar amounts of citric acid and 1,8-octanediol were melted together at 160 °C while stirring. Once melted, the temperature was decreased to 140 °C and the mixture was stirred for approximately 1 hour to obtain the POC pre-polymer (pre-POC). The POC pre-polymer was purified to remove any unreacted monomers by precipitation in water and then freeze-dried. The purified pre-POC was mixed with sodium chloride (∼250–300 μm diameter) and β-tricalcium phosphate (β-TCP) nanocrystals (100 nm diameter, specific surface area 50–70 m2 g−1). The resulting composite was 85% sodium chloride and 15% POC/β-TCP. Of the 15% POC/β-TCP, we fabricated scaffolds with 40% TCP nanocrystal concentration. The composite was post polymerized at 80 °C for 3 days. The polymerized composites were then submerged in water to remove the 85% salt component which resulted in nanocomposite “sponge” with a theoretical overall porosity of 85%.2.2. Nanocomposite characterization
The compressive properties of the nanocomposite scaffolds were obtained using an Instron 5500 mechanical tester with a 500N load cell. Nanocomposite scaffolds were fabricated with 0%, 20%, and 40% TCP content and polymerized as previously described. Following the leaching out of the salt phase to create the porous construct, the scaffolds were cut into cubes (∼125 mm3) and subjected to compressive tests to obtain the compressive modulus (Ec). Six samples of each composition were evaluated.
In addition, following previously described methods; the compressive recovery ratio of the scaffolds was measured.29,30 Porous scaffolds were cut into rectangular pieces 6 mm by 5 mm with a height of 4.5 mm. After 4 hours of soaking in PBS, samples were compressed to one fifth their height. The heights of the pieces were measured using electronic calipers before and after 2 min following 1 and 15 compressions using a TA.XT Plus Texture Analyzer (Texture Technologies, Scarsdale, NY) with a crosshead speed of 2 mm min−1. Calculations were performed using eqn (1). Compressive moduli were also obtained from stress measurements while obtaining recovery ratio information with the Texture Analyzer.
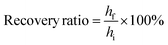 | (1) |
Recovery ratio is calculated by dividing the final height (hf) of a sample by its initial height (hi), multiplied by 100%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per scaffold in a volume of ∼25 μl per scaffold. The cells were allowed to attach to the scaffolds for 1 hour at 37 °C. After 1 hour, the culture wells were filled and the cell seeded scaffolds submerged in complete media for each cell type. The cells were cultured under static culture conditions for up to seven days. At various time points 1, 3, 5, and 7 days (n = 6) scaffolds were removed from culture, rinsed, then incubated for 20 minutes in 0.1% Triton-X solution to lyse the cells contained within the scaffold. The scaffolds were then subjected to sonication for 10 minutes to ensure complete lysis of the cells. The DNA was collected and quantified via Pico-green DNA assay kit (Life Technologies, Grand Island, NY).
000 cells per scaffold in a volume of ∼25 μl per scaffold. The cells were allowed to attach to the scaffolds for 1 hour at 37 °C. After 1 hour, the culture wells were filled and the cell seeded scaffolds submerged in complete media for each cell type. The cells were cultured under static culture conditions for up to seven days. At various time points 1, 3, 5, and 7 days (n = 6) scaffolds were removed from culture, rinsed, then incubated for 20 minutes in 0.1% Triton-X solution to lyse the cells contained within the scaffold. The scaffolds were then subjected to sonication for 10 minutes to ensure complete lysis of the cells. The DNA was collected and quantified via Pico-green DNA assay kit (Life Technologies, Grand Island, NY).
| Gene | Symbol | NCBI ref. seq. | 5′-Forward primer-3′ |
|---|---|---|---|
| 5′-Reverse primer-3′ | |||
| Alkaline phosphatase | ALPL | NM_001177520 | GCTTCTTGTCTGTGTCACTCA |
| ACCATTCCCACGTCTTCAC | |||
| Osteocalcin | BGLAP | NM_199173 | GGTCTCTTCACTACCTCGCT |
| CTCACACTCCTCGCCCTAT | |||
| Osteopontin | SPP1 | NM_001040060 | GTGATGTCCTCGTCTGTAGC |
| CCCCACAGTAGACACATATGATG | |||
| Runt-related transcription factor 2 | RUNX2 | NM_004348 | AGGCGGTCAGAGAACAAAC |
| CTTCACAAATCCTCCCCAAGT | |||
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH | NM_002046.3 | TCCACCACCCTGTTGCTGTA |
| ACCACAGTCCATGCCATCAC |
RT-qPCR consisting of 40 cycles of 95 °C for 10 s and 60 °C for 30 s, was carried out in a CFX Connect thermocycler (Bio-Rad Laboratories, Hercules, CA). 20 μL reactions were performed, in triplicate, using 1 μL (10 μM) each of forward and reverse primers, 6 μL nuclease-free water, 2 μL template, and 10 μL Advanced SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). Primers were obtained from IDT DNA Technologies (Coralville, IA). RT-qPCR quality was assessed using gel electrophoresis to ensure amplicon length and melt curve analysis was performed to confirm appropriate amplification of the target gene without genomic DNA contamination.
2.3. In vivo biocompatibility evaluation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), cell proliferation marker, Ki67 (Abcam, Cambridge, MA diluted 1
500), cell proliferation marker, Ki67 (Abcam, Cambridge, MA diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), osteoblast marker, osteopontin (Abcam, Cambridge, MA diluted 1
500), osteoblast marker, osteopontin (Abcam, Cambridge, MA diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250), and osteocalcin (Abcam, Cambridge, MA diluted 1
250), and osteocalcin (Abcam, Cambridge, MA diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100), endothelial cell marker, CD31 (Abcam, Cambridge, MA dilution 1
100), endothelial cell marker, CD31 (Abcam, Cambridge, MA dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200), and endothelial nitric oxide synthase (eNOS, Abcam, Cambridge, MA dilution 1
200), and endothelial nitric oxide synthase (eNOS, Abcam, Cambridge, MA dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200). All antibodies were prepared in diluent solution composed of 5% FBS and 0.1% Triton X-100 in 1× PBS. Following overnight incubation, the sections were incubated with appropriate secondary antibodies for 1 hour at room temperature. Finally, the sections were rinsed with 1× PBS and mounted with DAPI containing mounting media for nucleus visualization. Sections were evaluated via standard light microcopy or fluorescence microscopy.
200). All antibodies were prepared in diluent solution composed of 5% FBS and 0.1% Triton X-100 in 1× PBS. Following overnight incubation, the sections were incubated with appropriate secondary antibodies for 1 hour at room temperature. Finally, the sections were rinsed with 1× PBS and mounted with DAPI containing mounting media for nucleus visualization. Sections were evaluated via standard light microcopy or fluorescence microscopy.
3. Results and discussion
3.1. Nanocomposite fabrication
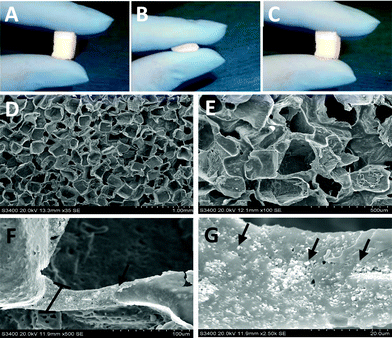
Structure and composition are important characteristics to consider in the development of any composite biomaterial system for use in tissue engineering. Specifically, scaffold architecture, porosity, pore size, as well as the distribution of the phases contained within the scaffold can affect the implant's performance. These scaffold characteristics are critical to promote the cellular processes at work during tissue formation and regeneration. Porous compressible composite scaffolds with up to 40% TCP nanocrystal concentration were successfully fabricated. The scaffolds were sponge-like and compressible with full recovery from deformation immediately upon release with no visible signs of rips, tears, or permanent deformation (Fig. 1a–c).3.2. Nanocomposite characterization
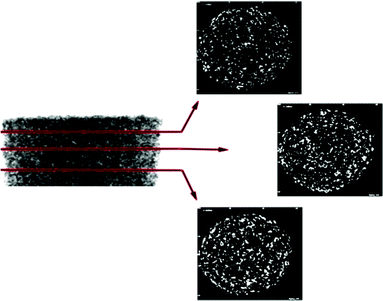
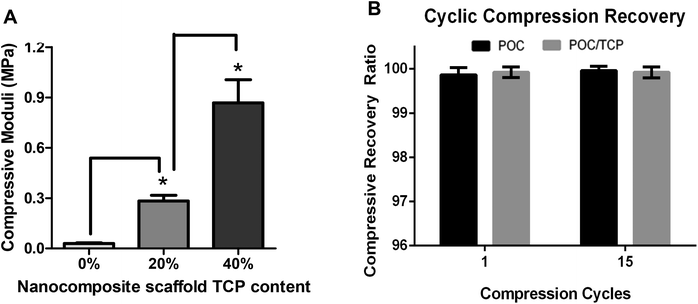
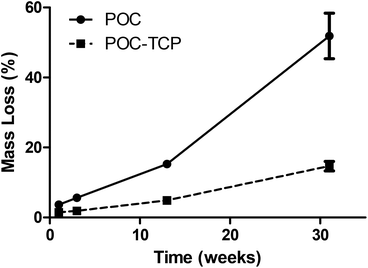
Characterization of the nanocomposites included; morphological assessment, computer tomography, mercury intrusion porosimetry, mechanical testing for compressive moduli and recovery from deformation, degradation studies, as well as quantitative analysis of microarchitecture. The micro architecture of the fabricated nanocomposites was observed visually via scanning electron microscopy (SEM). Representative images of the scaffolds are shown in Fig. 1d–g. The fabrication method used resulted in a homogenous pore size distribution (∼250–300 μm) with interconnected pore structure. At high magnification, the distribution of the TCP nanocrystals becomes evident. The images show that the TCP nanocrystals are embedded in the POC polymer yet still exposed on the cell-contacting surface while some agglomeration between nanocrystals is apparent. Despite the agglomeration, the bioceramic TCP phase provides the attached cells with an osteogenic niche within the POC phase of the composite. Micro-CT scan images of the scaffolds confirm that the distribution of TCP nanocrystals is homogenous (Fig. 2). These data suggest that the incorporated bioceramic will be present throughout the degradation process, providing for the maintenance of osteogenic stimuli throughout the bone forming process, an important requirement of a bone graft.Mechanical testing for compressive moduli reveals that the presence of the ceramic TCP nanocrystals within the walls of the POC increased the compressive moduli and thus the stiffness (Fig. 3a). With increasing TCP content (0–40%), the compressive modulus increased (p < 0.05). The POC/TCP composites achieve 100% recovery from compressive deformation following multiple compressive cycles (Fig. 3b). Regarding nanocomposite sponge degradation, over a period of 32 weeks the 40% TCP nanocomposites degraded significantly slower than POC scaffolds (14.7 ± 1.4% vs. 51.9 ± 6.5%, respectively). At a 50 week timepoint (not shown), the 40% TCP nanocomposite showed a 25% mass loss while the POC samples were completely degraded (Fig. 4). Collectively, the incorporation of β-TCP nanocrystals into the construct contributes to an increase in the overall mechanical properties of the scaffold as well as significantly reduces the rate of degradation, as compared to a scaffold composed of purely POC. We postulate that the inclusion of β-TCP within the composite acts to buffer the acidic degradation product of POC, thus reducing the degradation rate. This mechanism was shown to be evident in a study with polylactic glycolic acid (PLGA) as the composite polymer phase.32
Using mercury intrusion porosimetry, the actual sponge porosity was quantified and found to be 86.5%, which is in agreement with calculated values, validating our fabrication method to produce sponges of a desired porosity (data not shown). The characterization of the nanocomposites presented is very positive, and it should be noted that the structure and composition are quite tunable, due to the inherent flexibility of the polymeric POC phase, and the ability to control the nanocrystal ceramic phase. For example, the polymeric phase is a cross-linked network that may be modified by the selection of diol as well as increasing or decreasing the degree of crosslinking during polymerization. The modifications described have been shown to significantly affect the mechanical properties and the rate of degradation of the polymer phase, while still maintaining the scaffold biocompatibility.28,33 Finally, the composite scaffolds can be embedded with increased or decreased concentration of the bioactive ceramic, β-TCP, thus further affecting the overall scaffold properties. The high compressibility and elastomeric nature of the POC/TCP sponges is also beneficial when it comes to the surgical handling of this material. More specifically, the inherent challenges of handling the brittle ceramic β-TCP are avoided by the addition of the elastomeric polymer. This along with recovery ratio testing demonstrates that even if compression of the scaffold is necessary for implantation, it is expected to regain its original shape. Also advantageous from a clinical standpoint is that this material can be easily cut into any desired size or shape during surgery to match a specific bone defect.
3.3. In vitro evaluation of cell compatibility
Cell compatibility studies were conducted with the POC/TCP nanocomposite scaffolds. Cell proliferation over 7 days in culture was measured for both primary human osteoblast cells (HOst) and human umbilical vein endothelial cells (HUVEC) (Fig. 5). There was an increase in the DNA content isolated from the scaffolds at each time point. The results show that the POC/TCP nanocomposite sponge supports the attachment and proliferation of bone osteoblast and vascular endothelial cells over 7 days, processes that are important to the generation of vascularized bone. In addition, the overall rate of proliferation of both cell types was consistent over the course of the experiment. To allow for complete restitution of a bone defect, a bone graft should not only be resorbable but support the replacement of the cellular constituents of newly formed bone.34,35 Therefore, of great importance for this study is the ability of this nanocomposite construct to support bone and vascular cell infiltration and proliferation, which ultimately leads to bone formation and the assembly of a vascular network. These results are not surprising since POC is known to be very conducive to the attachment and growth of several different cell phenotypes including osteoblasts, chondrocytes, and bone marrow derived mesenchymal stem cells.18,36,37 Finally, in contrast to many other polymeric and ceramic scaffold designs, the POC/TCP nanocomposites were not coated or modified in any way to enhance cell attachment and proliferation, such as through the use of adhesion peptides, or proteins such as fibronectin, simplifying manufacture and regulatory path.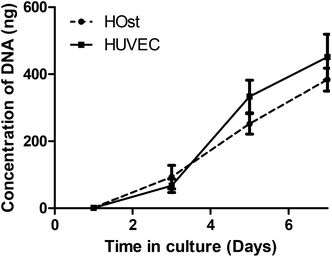 | ||
| Fig. 5 POC/TCP nanocomposite scaffold biocompatibility as measured by proliferation of primary cells types, HUVEC and HOst over 7 days. Data shown are Mean ± SD, with n = 6 for each time point. | ||
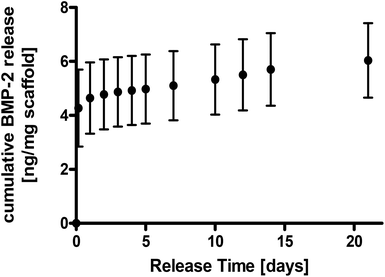
3.4. BMP-2 release from nanocomposites and bioactivity assessments
3.5. In vivo biocompatibility
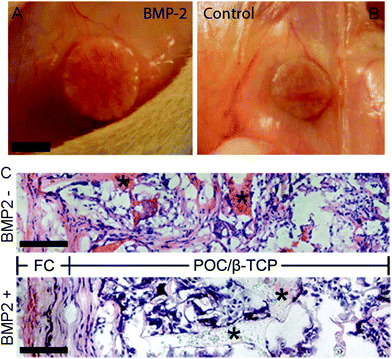
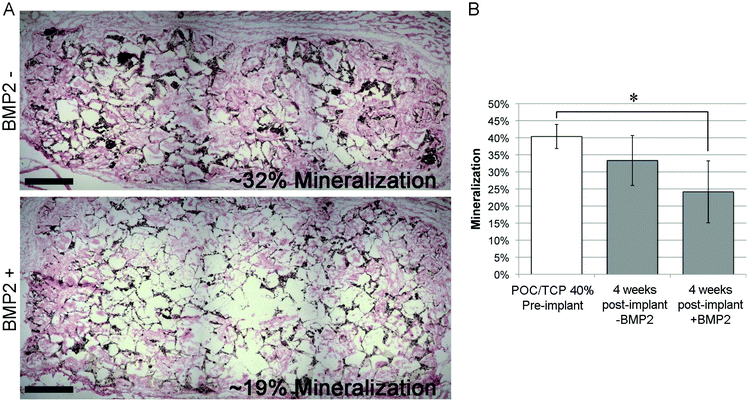
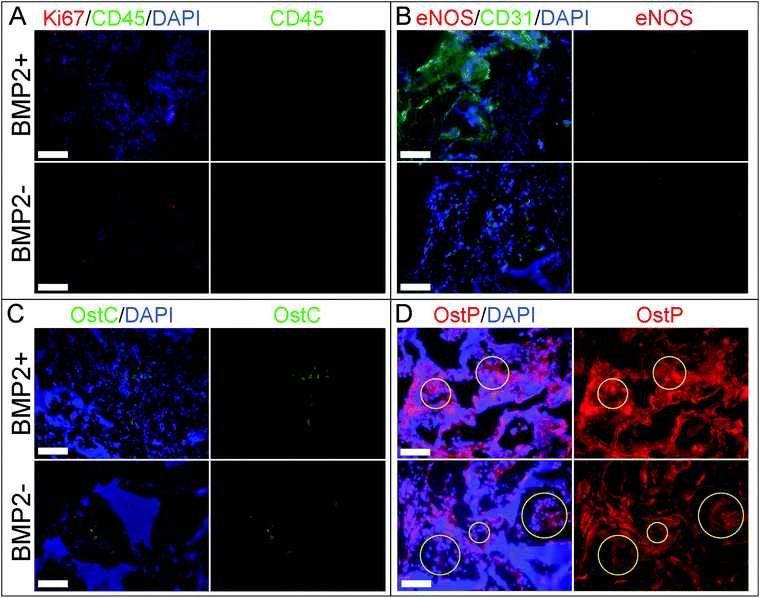
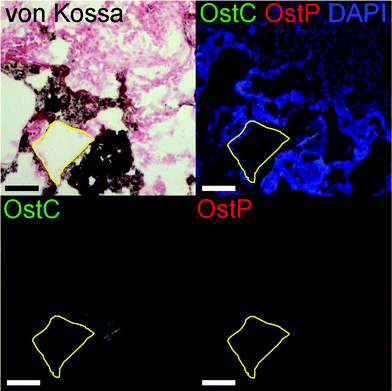
The osteogenic potential of the scaffold on the migrating and differentiating cells was evaluated by staining for osteocalcin and osteopontin. Osteocalcin and osteopontin are both markers associated with osteoblast cells. While osteopontin can stain positively for fibroblasts and other osteogenic cell types, osteocalcin is a very specific marker for osteoblasts.50,51 Overall we observed highly specific osteocalcin staining and diffuse osteopontin staining in POC/β-TCP scaffolds with and without BMP-2 (Fig. 10c and d). Interestingly, while we expected osteoblasts or osteoclasts to be localized near the scaffold itself, we found that osteocalcin-positive cells tended to cluster together towards the center of scaffold pores. Osteopontin staining was more difficult to interpret due to diffuse red background staining and autofluorescence of POC which was consistent throughout the scaffolds and across samples. However, in the regions of interest (yellow circles) where there are cells and no polymer scaffold, elevated osteopontin staining is observed. To link these osteogenicity markers together, we attempted to perform serial sections of alternating von Kossa staining and immunofluorescent osteocalcin and osteopontin staining to provide qualitative co-localization for the cell markers and calcium deposits in a BMP-2 containing POC/β-TCP scaffold (Fig. 11). The yellow trapezoidal shape outlines a pore in the POC/β-TCP scaffold and is used as a distinctive feature near a calcium deposit to help the reader align the serial sections. While co-localization cannot be confirmed with the current data set, the presence of all these markers in the same pore near a large calcium deposit is encouraging. Adjacent to the longest side of the trapezoid, a dark calcium deposit was present with cell nuclei discernible at the top corner of the deposit. The cells in this area also stained positively for osteocalcin while osteopontin staining remained diffuse throughout the area.
The positive and highly specific osteocalcin staining is very promising due to the fact that these scaffolds were implanted without cells, therefore the observed osteoblasts are either locally differentiated cells or migratory osteogenic cells from the surrounding tissues. It is interesting to note that most of the osteoblasts are localized towards the center of the scaffold pores where there would be reduced contact with the embedded β-TCP in the scaffold walls. This suggests that a more ideal niche for osteoblasts is not directly on the scaffold surface but rather in the center of pores where there may be different mechanical forces, concentrations of BMP-2 or other factors that are not on the pore wall surface. Surprisingly, we found the presence of osteoblasts in the POC/β-TCP scaffolds without BMP-2 suggesting that the scaffold itself is sufficient to recruit the proper cell type in the absence of growth factors indicating its small but intrinsic osteogenic potential. Because of the subcutaneous model we selected, we know that the levels of endogenous BMP-2 due to injury from the surgery are minimal (in comparison to BMP-2 levels reported in the muscle pouch model) and not likely to affect the total concentration in the scaffold.46 These in vivo results are consistent with our in vitro studies which show that mesenchymal stem cells had significant upregulation of osteopontin and osteocalcin after culturing them for 6 weeks on POC/β-TCP scaffolds. However, contrary to our in vitro results, our in vivo data do not show an enhancement in osteogenesis in scaffolds with immobilized, BMP-2. This may be due to either insufficient BMP-2 on the scaffold to see an effect in vivo where unlike in vitro, many additional factors can impact osteogenesis.
4. Conclusion
The field of orthopedic tissue engineering is growing at a rapid pace with the introduction of new materials that promote bone healing. Of particular interest within the field is the introduction of novel biocompatible materials with unique mechanical properties for easy delivery and to enhance bone regeneration. Herein we report a novel nanocomposite sponge that is compressible allowing for minimally invasive delivery and supports the immobilization and release of BMP-2. In addition, the in vivo data shows that the nanocomposite sponge supports complete cell infiltration, minimal adverse foreign body response, positive cellular proliferation and cellular expression of osteogenic markers when evaluated in subcutaneous tissue. The results support the further development and study of this sponge for use in bone tissue engineering.Disclosures
None.Acknowledgements
The authors would like to acknowledge Steven Strathmann for his work in obtaining the micro-CT data. The authors would also like to acknowledge Baxter-Northwestern Alliance for funding support for this project.References
- A. J. Salgado, O. P. Coutinho and R. L. Reis, Macromol. Biosci., 2004, 4(8), 743–765 CrossRef CAS PubMed.
- U. Kneser, D. J. Schaefer, E. Polykandriotis and R. E. Horch, J. Cell. Mol. Med., 2006, 10(1), 7–19 CrossRef CAS PubMed.
- C. Laurencin, Y. Khan and S. F. El-Amin, Expert Rev. Med. Devices, 2006, 3(1), 49–57 CrossRef CAS PubMed.
- S. Bose, M. Roy and A. Bandyopadhyay, Trends Biotechnol., 2012, 30(10), 546–554 CrossRef CAS PubMed.
- T. Cordonnier, J. Sohier, P. Rosset and P. Layrolle, Adv. Eng. Mater., 2011, 13(5), B135–B150 CrossRef.
- M. M. Stevens, Mater. Today, 2008, 11(5), 18–25 CrossRef CAS.
- C. Ohtsuki, M. Kamitakahara and T. Miyazaki, J. R. Soc. Interface, 2009, 6(Suppl 3), S349–S360 CrossRef CAS PubMed.
- T. T. Roberts and A. J. Rosenbaum, Organogenesis, 2012, 8(4), 114–124 CrossRef PubMed.
- A. S. Greenwald, S. D. Boden, V. M. Goldberg, Y. Khan, C. T. Laurencin and R. N. Rosier, J. Bone Joint Surg. Am., 2001, 83-A(Suppl 2 Pt 2), 98–103 Search PubMed.
- S. S. Kim, M. Sun Park, O. Jeon, C. Yong Choi and B. S. Kim, Biomaterials, 2006, 27(8), 1399–1409 CrossRef CAS PubMed.
- S. Liao, W. Wang, M. Uo, S. Ohkawa, T. Akasaka, K. Tamura, F. Cui and F. Watari, Biomaterials, 2005, 26(36), 7564–7571 CrossRef CAS PubMed.
- R. Zhang and P. X. Ma, J. Biomed. Mater. Res., 1999, 44(4), 446–455 CrossRef CAS.
- D. Sengupta and S. C. Heilshorn, Tissue Eng., Part B, 2010, 16(3), 285–293 CrossRef CAS PubMed.
- H. Qiu, J. Yang, P. Kodali, J. Koh and G. A. Ameer, Biomaterials, 2006, 27(34), 5845–5854 CrossRef CAS PubMed.
- D. Motlagh, J. Allen, R. Hoshi, J. Yang, K. Lui and G. Ameer, J. Biomed. Mater. Res., Part A, 2007, 82(4), 907–916 CrossRef PubMed.
- J. Yang, D. Motlagh, A. R. Webb and G. A. Ameer, Tissue Eng., 2005, 11(11–12), 1876–1886 CrossRef CAS PubMed.
- E. J. Chung, P. Kodali, W. Laskin, J. L. Koh and G. A. Ameer, J. Mater. Sci. Mater. Med., 2011, 22(9), 2131–2138 CrossRef CAS PubMed.
- C. G. Jeong, H. Zhang and S. J. Hollister, Acta Biomater., 2011, 7(2), 505–514 CrossRef CAS PubMed.
- M. P. Prabhakaran, A. S. Nair, D. Kai and S. Ramakrishna, Biopolymers, 2012, 97(7), 529–538 CrossRef CAS PubMed.
- C. Knabe, F. C. Driessens, J. A. Planell, R. Gildenhaar, G. Berger, D. Reif, R. Fitzner, R. J. Radlanski and U. Gross, J. Biomed. Mater. Res., 2000, 52(3), 498–508 CrossRef CAS.
- S. Yamada, D. Heymann, J. M. Bouler and G. Daculsi, Biomaterials, 1997, 18(15), 1037–1041 CrossRef CAS.
- T. W. Kim, Y. M. Park, D. H. Kim, H. H. Jin, K. K. Shin, J. S. Jung, H. C. Park and S. Y. Yoon, Ceram. Int., 2012, 38(3), 1965–1974 CrossRef CAS PubMed.
- H. Rojbani, M. Nyan, K. Ohya and S. Kasugai, J. Biomed. Mater. Res., Part A, 2011, 98(4), 488–498 CrossRef PubMed.
- E. R. Luvizuto, T. P. Queiroz, R. Margonar, S. R. Panzarini, E. Hochuli-Vieira, T. Okamoto and R. Okamoto, J. Craniofac. Surg., 2012, 23(5), e430–e433 CrossRef PubMed.
- Y. Kang, S. Kim, M. Fahrenholtz, A. Khademhosseini and Y. Yang, Acta Biomater., 2013, 9(1), 4906–4915 CrossRef CAS PubMed.
- E. Kato, J. Lemler, K. Sakurai and M. Yamada, Clin. Implant Dent. Relat. Res., 2014, 16(2), 202–211 CrossRef PubMed.
- B. Ozdemir and E. Okte, J. Biomed. Mater. Res., Part B, 2012, 100(4), 976–983 CrossRef PubMed.
- J. Yang, A. R. Webb, S. J. Pickerill, G. Hageman and G. A. Ameer, Biomaterials, 2006, 27(9), 1889–1898 CrossRef CAS PubMed.
- Y. Kang, J. Yang, S. Khan, L. Anissian and G. A. Ameer, J. Biomed. Mater. Res., Part A, 2006, 77(2), 331–339 CrossRef PubMed.
- H. Kweon, M. K. Yoo, I. K. Park, T. H. Kim, H. C. Lee, H. S. Lee, J. S. Oh, T. Akaike and C. S. Cho, Biomaterials, 2003, 24(5), 801–808 CrossRef CAS.
- G. C. Oliver Jr., B. M. Parker, D. L. Brasfield and C. W. Parker, J. Clin. Invest., 1968, 47(5), 1035–1042 CrossRef CAS PubMed.
- F. Yang, W. J. Cui, Z. Xiong, L. Liu, J. Z. Bei and S. G. Wang, Polym. Degrad. Stab., 2006, 91(12), 3065–3073 CrossRef CAS PubMed.
- A. R. Webb, B. D. Macrie, A. S. Ray, J. E. Russo, A. M. Siegel, M. R. Glucksberg and G. A. Ameer, Ann. Biomed. Eng., 2007, 35(8), 1357–1367 CrossRef PubMed.
- U. Maus, S. Andereya, S. Gravius, J. A. Ohnsorge, C. Niedhart and C. H. Siebert, J. Biomater. Appl., 2008, 22(6), 559–576 CrossRef CAS PubMed.
- A. R. Vaccaro, Orthopedics, 2002, 25(5 Suppl), s571–s578 Search PubMed.
- F. S. Shirazi, E. Moghaddam, M. Mehrali, A. A. Oshkour, H. S. Metselaar, N. A. Kadri, K. Zandi and N. A. Abu, J. Biomed. Mater. Res., Part A, 2013 DOI:10.1002/jbm.a.35074.
- A. K. Sharma, P. V. Hota, D. J. Matoka, N. J. Fuller, D. Jandali, H. Thaker, G. A. Ameer and E. Y. Cheng, Biomaterials, 2010, 31(24), 6207–6217 CrossRef CAS PubMed.
- S. Dohzono, Y. Imai, H. Nakamura, S. Wakitani and K. Takaoka, Clin. Orthop. Relat. Res., 2009, 467(12), 3206–3212 CrossRef PubMed.
- H. Schliephake, H. A. Weich, C. Dullin, R. Gruber and S. Frahse, Biomaterials, 2008, 29(1), 103–110 CrossRef CAS PubMed.
- Z. Gamie, G. T. Tran, G. Vyzas, N. Korres, M. Heliotis, A. Mantalaris and E. Tsiridis, Expert Opin. Biol. Ther., 2012, 12(6), 713–729 CrossRef CAS PubMed.
- T. J. Heino and T. A. Hentunen, Curr. Stem. Cell Res. Ther., 2008, 3(2), 131–145 CrossRef CAS.
- J. E. Aubin, J. Cell. Biochem., 1998, 72(Issue Supplement 30–31), 73–82 CrossRef.
- J. E. Aubin, Rev. Endocr. Metab. Disord., 2001, 2(1), 81–94 CrossRef CAS.
- I. Satokata, L. Ma, H. Ohshima, M. Bei, I. Woo, K. Nishizawa, T. Maeda, Y. Takano, M. Uchiyama, S. Heaney, H. Peters, Z. Q. Tang, R. Maxson and R. Maas, Nat. Genet., 2000, 24(4), 391–395 CrossRef CAS PubMed.
- R. S. Siffert, J. Exp. Med., 1951, 93(5), 415–426 CrossRef CAS.
- M. A. Scott, B. Levi, A. Askarinam, A. Nguyen, T. Rackohn, K. Ting, C. Soo and A. W. James, Stem Cells Dev., 2012, 21(5), 655–667 CrossRef CAS PubMed.
- J. Handschel, H. P. Wiesmann, U. Stratmann, J. Kleinheinz, U. Meyer and U. Joos, Biomaterials, 2002, 23(7), 1689–1695 CrossRef CAS.
- H. Kaneko, T. Arakawa, H. Mano, T. Kaneda, A. Ogasawara, M. Nakagawa, Y. Toyama, Y. Yabe, M. Kumegawa and Y. Hakeda, Bone, 2000, 27(4), 479–486 CrossRef CAS.
- E. C. Shors, Orthop. Clin. North Am., 1999, 30(4), 599–613 CrossRef CAS.
- N. Ashizawa, K. Graf, Y. S. Do, T. Nunohiro, C. M. Giachelli, W. P. Meehan, T. L. Tuan and W. A. Hsueh, J. Clin. Invest., 1996, 98(10), 2218–2227 CrossRef CAS PubMed.
- N. K. Lee, H. Sowa, E. Hinoi, M. Ferron, J. D. Ahn, C. Confavreux, R. Dacquin, P. J. Mee, M. D. Mckee, D. Y. Jung, Z. Zhang, J. K. Kim, F. Mauvais-Jarvis, P. Ducy and G. Karsenty, Cell, 2007, 130(3), 456–469 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |