Biomimetic ECM coatings for controlled release of rhBMP-2: construction and biological evaluation†
Ying
Huang‡
a,
Qiaojie
Luo‡
a,
Guangyu
Zha
a,
Jianxiang
Zhang
b,
Xiaohui
Li
b,
Shifang
Zhao
a and
Xiaodong
Li
*a
aDepartment of Oral and Maxillofacial Surgery, the Affiliated Stomatology Hospital, College of Medicine, Zhejiang University, Hangzhou, P. R. China. E-mail: cisarli@zju.edu.cn; Fax: +8657187217433; Tel: +8657188208378
bDepartment of Pharmaceutics, College of Pharmacy, Third Military Medical University, Chongqing 400038, China
First published on 14th February 2014
Abstract
As we all know biochemical surface modification is promising for implantable biomedical device applications due to its ability to directly provide therapeutic molecular cues for tissue repair. However, presenting multiple molecular cues on implant surfaces in the proper way is challenging. In this study, a multi-component polyelectrolyte multilayer (PEM) coating composed of collagen type I, RGD peptide functionalized hyaluronic acid, and recombined human BMP-2 (rhBMP-2) was constructed on Ti via a layer by layer technique. Subsequently, this coating was crosslinked via disulfide bonds to form a surface gel coating with a semi-interpenetrating network. A disulfide-crosslinked RGD-containing biomimetic extracellular matrix coating that could serve as a reservoir for rhBMP-2 was thus obtained. The embedded rhBMP-2 displayed a sustained release profile and a strong resistance to the physiological environment. In vitro biological evaluation revealed that the resultant disulfide crosslinking bioactive coating could effectively modulate cellular behaviors of pre-osteoblasts such as adhesion, proliferation and differentiation. In vivo study further revealed that this coating could enhance the bone-to-implant integration characterized by the increased removal torque values.
1. Introduction
Surface modification of dental implant surfaces with bioactive molecules like collagen type I,1 fibronectin,2,3 vitronectin,3 and growth factors such as bone morphogenetic proteins (BMPs)4 and fibroblast growth factors (FGFs),5 is attracting increasing attention.6 Since such biochemical modifications can endow implants with direct molecular cues for wound healing and tissue regeneration, they are regarded as the most promising approaches to accelerate osteointegration, shorten healing time and increase clinical success rates.There are two key issues involved in the biochemical modification of Ti implants. One is the selection of biomolecules with special functions to regulate osteogenic cell responses. Proteins that present an RGD motif, such as fibronectin and vitronectin, mainly mediate the initial cell recognition of biomaterial surfaces and influence the subsequent cell adhesion.7 Meanwhile, water soluble osteogenic growth factors such as BMPs8 and FGFs,9 can stimulate the proliferation and/or differentiation of osteogenic cells in vitro and accelerate new bone formation in vivo. Actually, biomolecules in a native extracellular matrix (ECM) function as a whole in regulating cell behaviors and only their cooperation allows the spatiotemporal regulation of tissue-specific cell responses, guiding appropriate cell behaviors at the special cell growth stage, including recruitment, adhesion, proliferation and differentiation.6,10 Inspired by these biological events, the introduction of multiple biomolecules onto the implant surfaces to mimic the components of ECM was supposed to provide more tissue-specific molecule signals for rapid and robust osteointegration.
The other core issue of the biochemical modification concerns the development of an appropriate strategy to incorporate biomolecules onto a Ti implant. In general, biomolecules can be immobilized onto the implant surface through physical adsorption, covalent binding or self-organizing organic layers.11 However, almost all the available methods are confronted with the dilemma of either unsatisfactory stability of the resultant coating or decreased activities of the introduced biomolecules.12 Especially, for the construction of multi-component bioactive coatings, the difficulties reside in not only keeping the bioactivities of the employed biomolecules, but also organizing them in an appropriate way for the purpose of exerting their synergistic effects. It is well known that the main components of the native ECM interlock into a network structure, which presents adhesive peptides on collagen matrices to support the cell adhesion, but also serves as a reservoir for growth factors in vivo, protects them from protease,13,14 and delivers them in a localized and controlled way to activate cell functions at different stages. The way in which biomolecules are arranged in the native ECM provides an ideal model to learn how to organize the multi-component coating on Ti implant surfaces.
Collagen type I (collagen) and hyaluronic acid (HA) are two basic components of the native ECM, while RGD and rhBMP-2 are two mostly used regulatory molecules for bone repair. In this study, a biomimetic strategy was developed to organize collagen, HA-GRGDSPC-SH (an RGD grafted derivative of hyaluronic acid, synthesized in our lab15) and rhBMP-2 to construct a disulfide-crosslinked biomimetic ECM coating on the Ti surface via a modified layer by layer (LbL) technique. It is anticipated that the disulfide crosslinking can endow the resultant coating a promoted stability and redox-responsive characteristics without harm to the bioactivities of the RGD sequence and rhBMP-2. Moreover, the stable polyelectrolyte membrane (PEM) coating can further serve as a reservoir to deliver rhBMP-2 in a localized and sustained way. Therefore, this study focused on the release behaviors of the rhBMP-2 from the crosslinked coatings, and the effects of the anchored RGD sequence and released rhBMP-2 on cell functions of pre-osteoblasts in vitro and bone-to-implant integration in vivo.
2. Materials and methods
2.1. Synthesis of HA derivatives
HA-GRGDSPC-(SH): Synthesis of HA-GRGDSPC-(SH) was carried out according to our previous report.15 In brief, HA was reacted with GRGDSPC-(S-S)-CPSDGRG in an aqueous solution containing 1-ethyl-3-3-dimethylaminopropyl carbodiimide (EDC) and N-hydroxysuccinimide (NHS) to obtain HA-GRGDSPC-(S-S)-CPSDGRG-HA. Subsequently, the product was reduced by dithiothreitol (DTT) at room temperature (RT) to obtain HA-GRGDSPC-(SH).
HA-GRGDSP: HA was reacted with GRGDSP (the molar ratio of carboxylic groups in HA to GRGDSP was about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1) in an aqueous solution containing EDC and NHS to obtain HA-GRGDSP.
1.1) in an aqueous solution containing EDC and NHS to obtain HA-GRGDSP.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at RT for 1 h. The acid etched Ti discs (AE-Ti) were divided into four groups according to different treatments.
1) at RT for 1 h. The acid etched Ti discs (AE-Ti) were divided into four groups according to different treatments.
CHC-Ti (Col/HA coating) group: The freshly prepared AE-Ti discs with abundant hydroxyl groups on the surfaces were alternatively deposited into 1.0 mg ml−1 collagen solution (pH 4.0) and 0.5 mg ml−1 HA solution (pH 4.0) to form PEM coatings. Each deposition lasted for 15 min and was followed by three thorough washes with deionized water. A collagen deposition plus an HA deposition procedure was considered as one assembly cycle. The Col/HA PEM coating on Ti was achieved by depositing with 5 assembly cycles.
CHRC-Ti (Col/HA-GRGDSP coating) group: In this group, 0.5 mg ml−1 HA solution was replaced with 0.5 mg ml−1 HA-GRGDSP solution to form the Col/HA-GRGDSP PEM coating on AE-Ti discs. The other experimental conditions are the same as those employed for the CHC-Ti group.
BEC-Ti [(Col + rhBMP-2)/HA-GRGDSP coating] group: In this group, 1 mg ml−1 collagen solution was replaced with 1 mg ml−1 collagen solution containing 20 μg ml−1 rhBMP-2 to form (Col + rhBMP-2)/HA-GRGDSP PEM coating on the AE-Ti discs. The other experimental conditions are the same as those used for the CHRC-Ti group.
BEC-CL-Ti [crosslinked (Col + rhBMP-2)/HA-GRGDSPC-(SH) coating]: In this group, 0.5 mg ml−1 HA-GRGDSP solution was replaced with 0.5 mg ml−1 HA-GRGDSPC-(SH) to form (Col + rhBMP-2)/HA-GRGDSPC-(SH) PEM coating on the AE-Ti discs. The other experimental conditions are the same as those adopted for the BEC-Ti group. Ti discs with [(Col + rhBMP-2)/HA-GRGDSPC-SH]5 PEM coatings were immersed into 10 mM MES (2-(N-morpholino)-ethane-sulfonic acid) buffer solution (pH 6) containing 2 mM chloramine T (CaT) at RT for 60 s. The discs were taken out of the Mes/CaT solution and in turn thoroughly rinsed with 10 mM pH 6.5 PBS solution and then deionized water.
All the samples were dried under the N2 atmosphere and sterilized via ultraviolet irradiation before further experiments.
2.2. Characterization
The topological information of all the Ti discs was characterized by scanning force microscopy (SFM) under the tapping mode with a Nanoscope III multimode scanning force microscope (Digital Instruments, Santa Barbara, CA). The hydrophilicity of different coatings was evaluated with contact angle measurements (GBX Instruments, Valence, France). The thickness of the multilayer coatings was measured on a variable-angle spectroscopic ellipsometer (model VASE; J.A. Woollam Inc., Lincoln, NE). The measurement was performed at incident angles of 65°, 70° and 75° within a wavelength range of 400–1700 nm. The thickness was calculated from the ellipsometric parameters, Δ and ψ, using a Cauchy model. Each thickness value was averaged from 5 parallel measurements on different places of the film.XPS detection was performed on a RBD upgraded PHI-5000C ESCA system (PerkinElmer, USA) with Al Kα radiation (hν = 1486.6 eV). In general, the X-ray anode was run at 250 W and the high voltage was kept at 14.0 kV with a detection angle at 54°. The base pressure of the analyzer chamber was about 5 × 10−8 Pa. A survey scan (0–1000 eV binding energy range, 93.9 eV pass energy) and a high-resolution scan of all the elements (23.5 eV pass energy) were run for each sample. Binding energies were calibrated with the contaminated carbon (C1s = 284.6 eV). The data were analyzed with the RBD AugerScan 3.21 software provided by RBD Enterprises.
2.3. Study of the degradation behaviors of PEM coatings
Collagen was labeled with fluorescein isothiocyanate (FITC, Sigma).15 Glass slides (1 cm × 2 cm) were cleaned and then treated with piranha (a mixture of H2SO4 and H2O2, the volume ratio of H2SO4–H2O2 = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) at 60 °C for 2 h. Four types of coatings were deposited on the clean glass slides according to the assembly methods mentioned above: Col-FITC/HA PEM coating, Col-FITC/HA-GRGDSP PEM coating, (Col-FITC + rhBMP-2)/HA-GRGDSP PEM coating and crosslinked (Col-FITC + rhBMP-2)/HA-GRGDSPC-(SH) PEM coating.
3) at 60 °C for 2 h. Four types of coatings were deposited on the clean glass slides according to the assembly methods mentioned above: Col-FITC/HA PEM coating, Col-FITC/HA-GRGDSP PEM coating, (Col-FITC + rhBMP-2)/HA-GRGDSP PEM coating and crosslinked (Col-FITC + rhBMP-2)/HA-GRGDSPC-(SH) PEM coating.
Five glass slides of each group were immersed into PBS solution (pH 7.2). Each day, the UV absorbance at 490 nm of the glass slides was measured on a UV-visible spectrophotometer (UV-2550, SHIMADZU). In the case of the crosslinked (Col-FITC + rhBMP-2)/HA-GRGDSPC-(SH) PEM coating, five glass slides were immersed into 5 mM glutathione (GSH) solution or 10 μm GSH solution, respectively. The mean values of the five samples of each group at the same day were plotted against the corresponding immersion times.
2.4. rhBMP-2 release behaviors of BEC-Ti and BEC-CL-Ti groups in various media
Six BEC-Ti discs and six BEC-CL-Ti discs were put into 6-well plates, respectively, one disc in one well, into which 1 ml of PBS solution (pH 7.2) was added. At predetermined time intervals (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, and 14 days), the medium in each well was respectively collected and stored at −20 °C, and then 1 ml fresh PBS solution was refilled. The same procedures were employed for the release study either in 5 mM GSH solution or in 10 μM GSH solution. The concentration of enzymatically active rhBMP-2 in either PBS or GSH solution collected from each sample was measured by enzyme-linked immunosorbent assay (ELISA) using a BMP Quantikine kit (Quantikine, R&D Systems, USA) according to the manufacturer's instructions.2.5. Cell culture
The effect of the different coatings on the proliferation and differentiation of newborn mouse calvaria-derived MC3T3-E1 pre-osteoblasts (Cellbank of the Chinese Academy of Science, China) were studied. Cells were cultured in α modified minimal essential medium (α-MEM, Invitrogen Co., Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Invitrogen Co., Carlsbad, CA) and maintained at 37 °C in a 5% CO2 humidified atmosphere. The cell growth medium was replaced every 2–3 days and confluent cells were subcultured through trypsinization. Differentiation was induced by adding 50 μg ml−1 ascorbic acid (Sigma, ST. Louis, MO, USA) and 10 mM β-glycerophosphate (Sigma, ST. Louis, MO, USA) into the growth medium. Cells were seeded on a Ti disc at a density of 1 × 105. The differentiation medium was changed every 3 days, with sample analysis or preparation for proliferation assessment at 1 h and 3 days and differentiation assessment at day 4, 7 and 14. For all in vitro experiments, all measurements were performed in triplicate.2.6. Cell attachment and proliferation
The cellular double-stranded DNA (dsDNA) of each cell in a certain cell line remains constant, thus the amount of cellular DNA reflects the number of cells that survived on the Ti discs. Total cellular dsDNA was determined by using a commercial PicoGreen®dsDNA Kit (Invitrogen). In brief, cells were lysed and cell lysate was incubated with aqueous solution of dsDNA reagent in a microplate for 2–5 min at RT. The fluorescent intensity was measured on a fluorescence spectrometer (Sunrise-Basic TECAN, Austria). Lambda DNA (supplied by the kit) of known concentration was used to generate the standard curve.2.7. Cell differentiation
2.8. Surgery procedures and removal torque tests (RTQ test)
The animal experiment was approved by the Animal Ethic Committee of Zhejiang University, Hangzhou, China. Screw titanium implants were kindly supplied by Zhejiang Guangci Biomedical Instrument Company (Zhejiang, P. R. China) with an external diameter of 3.0 mm and a length of 10 mm. 15 adult male New Zealand white rabbits weighing 2.5–3.0 kg were used. Each rabbit received one BEC-CL-implant and one AE-implant. The surface treatment for these two implant groups are the same as those employed in the case of corresponding AE-Ti group and BEC-CL-Ti group. Briefly, animals were anesthetized and the implant sites 3.0 mm in diameter were prepared on the flat surface on the medial condyle of the femur. The implants were gently screwed into the place by hand until the inferior margin of covering abutment was level with the bone surface. Postoperatively, the animals received antibiotics (penicillin, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U per day) for 3 days. 2, 4 and 8 weeks after the operation, femurs containing implants were retrieved through segmental osteotomy. The implant-to-bone binding strength was measured by removal torque tests in N m using the electronic device (CTT2500, MTS, P. R. China). We recorded the peak value of the resistance to reverse torque to denote the bonding strength of implants with bony tissue. In order to reduce the bias, a single blinded examiner conducted all removal torque tests.
000 U per day) for 3 days. 2, 4 and 8 weeks after the operation, femurs containing implants were retrieved through segmental osteotomy. The implant-to-bone binding strength was measured by removal torque tests in N m using the electronic device (CTT2500, MTS, P. R. China). We recorded the peak value of the resistance to reverse torque to denote the bonding strength of implants with bony tissue. In order to reduce the bias, a single blinded examiner conducted all removal torque tests.
2.9. Statistical analysis
Statistical analysis was carried out using an SPSS statistical software package (V11, SPSS, Inc., Chicago, IL, USA). All the results are expressed as mean ± standard deviation. All the in vitro results were tested for statistical significance with one-way ANOVA test while the values of RTQ were tested for statistical significance with Paired-Samples T test. p ≤ 0.05 was considered to be statistically significance.3. Results and discussion
3.1. Fabrication and characterization of the polyelectrolyte multilayer coatings
In this study, getting inspiration from the stable interlocked-network structure of ECM and its cell-growth modulating functions, a disulfide-crosslinked, RGD and rhBMP-2 containing PEM coating was constructed. Collagen and rhBMP-2 molecules were physically intertwined with the 3-dimensional crosslinked HA-GRGDSPC network to form a surface gel coating possessed semi-interpenetrating network (semi-IPN) (Scheme 1).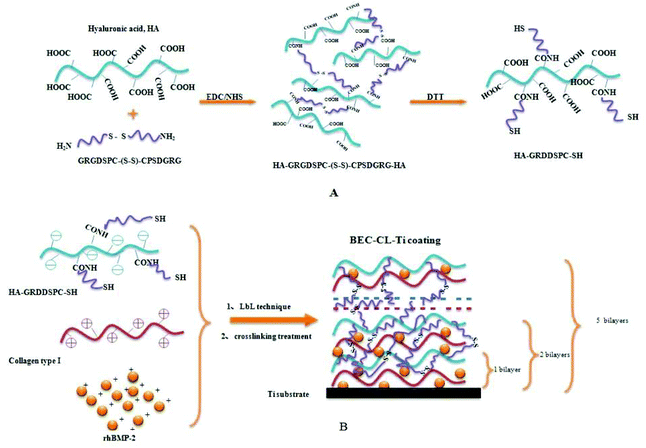 | ||
| Scheme 1 (A) Synthesis of HA-GRGDSPC-SH; (B) formation of the BEC-CL-Ti coating. EDC, 1-ethyl-3-3-dimethylaminopropyl carbodiimide; NHS, N-hydroxysuccinimide; DTT, dithiothreitol. | ||
FT-IR detection showed the successful synthesis of HA-GRGDSP and HA-GRGDSPC-SH (Fig. S1†). SFM was used to observe the surface topography of the Ti discs. For the AE-Ti disc without coating, it displayed a typical honeycomb-like acid-etched surface topography17 (Fig. S2†). In contrast, uniform and fine coatings appeared on the surfaces of the four PEM-coated groups (Fig. 1A–D), which are from the LbL assembly procedure. The micro- or nano-structure of the PEM coatings in the CHC-Ti group, CHRC-Ti group and BEC-Ti group was highly similar. It seemed that many particles of tens of nanometers piled up to produce the uniform coatings (Fig. 1A–C). However, for the crosslinked BEC-CL-Ti group, the coating was fabricated more evenly and smoothly when compared to that in the BEC-Ti group (Fig. 1D). This should be attributed to the formation of disulfide bonds within the PEM coating.
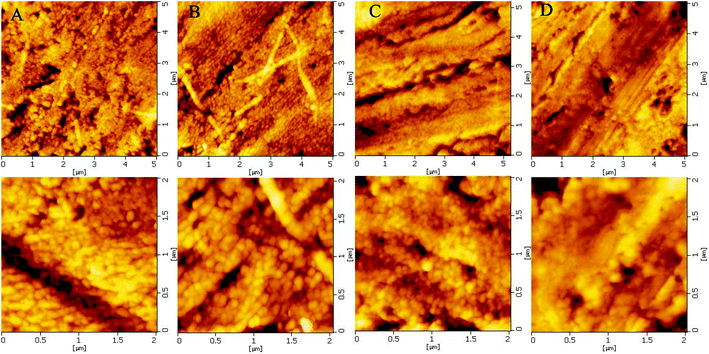 | ||
| Fig. 1 The representative SFM images of (A) CHC-Ti group; (B) CHRC-Ti group; (C) BEC-Ti group; (D) BEC-CL-Ti group. The images on the bottom row are the magnified images of the top row. | ||
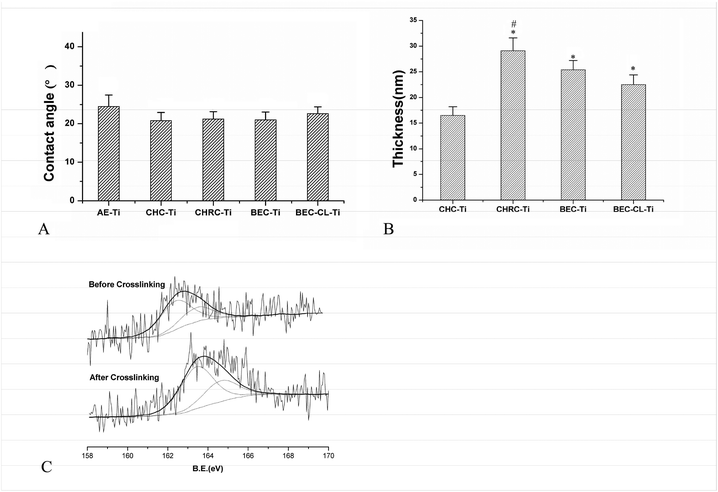
The values of the contact angle represent the surface hydrophilicity of the Ti discs (Fig. 2A). All four groups possessed good hydrophilicity, which is favorable for the cell responses.18 The three uncrosslinked groups almost possessed the same hydrophilicity, and were slightly superior to the crosslinked BEC-CL-Ti group. This may be due to the fact that the crosslinking procedure changed the surface topography and reduced the number of hydrophilic groups in the coating of the BEC-CL group to some extent. Moreover, based on the ellipsometric measurement, the coating thickness of the CHC-Ti group, CHRC-Ti group, BEC-Ti group and BEC-CL-Ti group was 16.2, 29, 24.3, and 21.2 nm, respectively (Fig. 2B). The difference in the coating thickness of the four groups should be attributed to the different assembled polyelectrolyte pairs.
Changes in surface chemical compositions of samples in the BEC-CL-Ti group before and after crosslinking were detected by XPS. The C1s and N1s spectra as well as the C, N content of the uncrosslinked coating were almost the same as those of the crosslinked coating (Fig. S3†). Nevertheless, the S2p spectrum of the uncrosslinked coating was different from that of the crosslinked coating due to the transition of free sulphydryls into disulfides (Fig. 2C). Before crosslinking, the binding energy of S 2p (–SH) was 162.7 ± 0.3(eV), whereas after crosslinking, the binding energy of S 2p (–S–S–) was changed to 163.8 ± 0.3(eV). Here, the results of SFM observation, ellipsometric measurement and XPS detection proved the successful construction of the crosslinked (Col + rhBMP-2)/HA-GRGDSPC-(SH) coating on the Ti substrate (Fig. 1 and 2).
3.2. The degradation behaviors of the four coatings
The degradation profiles of the four coatings in PBS are shown in Fig. 3A. For the uncrosslinked coatings, i.e. the Col-FITC/HA PEM coating, Col-FITC/HA-GRGDSP PEM coating and (Col-FITC + rhBMP-2)/HA-GRGDSP PEM coating, similar degradation profiles were found. All the uncrosslinked coatings thoroughly degraded within 6 days. In the case of the crosslinked (Col-FITC + rhBMP-2)/HA-GRGDSPC-(SH) PEM coating, it displayed a slower degradation profile. About 18% of the total coating degraded within the first day, and the complete degradation needs more than 14 days. The disulfide crosslinking markedly improved the stability of the resultant PEM coating.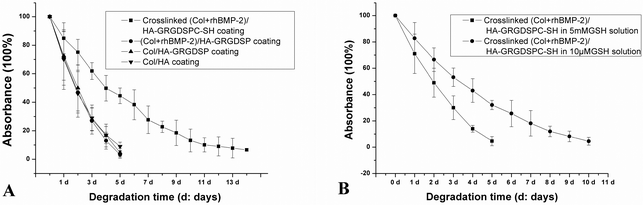 | ||
| Fig. 3 The degradation behaviors of different coatings in PBS solution (A) and the BEC-CL-Ti group in GSH solutions (B). The values represent mean ± standard deviation. | ||
GSH is related to the antioxidant capacity of all kinds of organisms and cells. In the presence of 5 mM GSH (Fig. 3B), the degradation rate of the crosslinked coating was fast. During the first 5 days, more than 90% of the coating degraded. However, in 10 μM GSH solution, its degradation rate was relatively slow, and the total degradation period was about 10 days. 10 μM GSH solution provided a condition mimicking physiological environment to examine the influence of in vivo reducing substances (like GSH) on the degradation behavior of the disulfide crosslinked coating, since the concentration of GSH is 2–10 μM in extracellular surroundings.19 The results here revealed that the degradation behavior of the disulfide crosslinked PEM coating presented in a GSH concentration-dependent degradation manner, which means this coating may respond to the redox environment in physiological conditions.
3.3. Release kinetics of rhBMP-2 from the BEC-Ti and BEC-CL-Ti coatings
Although the employment of rhBMP-2 could render the implant surfaces with osteoinduction capacity,20 expanding the application of rhBMP-2 in the field of oral implantology is restrained by the lack of effective carriers that can deliver rhBMP-2 in a localized and sustained way. Herein, the disulfide crosslinking could endow the coating of the BEC-CL-Ti group with stability and adjustable bio-responsive degradation behaviors,21 which would inevitably influence the release behaviors of rhBMP-2 from the BEC-CL-Ti coating.The release profiles of rhBMP-2 from the BEC-CL-Ti coatings in PBS (Fig. 4A) and 10 μM GSH solutions (Fig. 4B) are depicted in Fig. 4. Unexpectedly, rhBMP-2 could not be detected when the release experiments were carried out in 5 mM GSH solution. For the BEC-Ti group, a burst release and a short release period of rhBMP-2 was observed for rhBMP-2 either in PBS solution or in 10 μM GSH solutions. In PBS solution, about 77% of the total rhBMP-2 was released during the first day, and the release period was 4 days. Similarly, in 10 μM GSH solution, above 80% of the total rhBMP-2 was released within the first day and the release period was 3 days. In the case of the BEC-CL-Ti group, due to the crosslinking procedure, the release of rhBMP-2 was in a prolonged and sustained manner. In PBS solution, 26% of the total rhBMP-2 amount was released during the first day, and rhBMP-2 still could be detected even at day 10, while in 10 μM GSH solution, about 30% of the total rhBMP-2 amount was released during the first day and the total release period was about 7 days. Delivery routes of bioactive molecules in LbL film contain both passive diffusion and degradation of polyelectrolyte pairs.22 Compared with the burst release profiles of rhBMP-2 in the BEC-Ti group, the formation of semi-IPN PEM coating via disulfide crosslinking not only stabilized the polyelectrolyte membrane coating but also slowed the diffusion rate of rhBMP-2 in the coating, resulting in a sustained release of rhBMP-2 both in PBS solution and GSH solution.
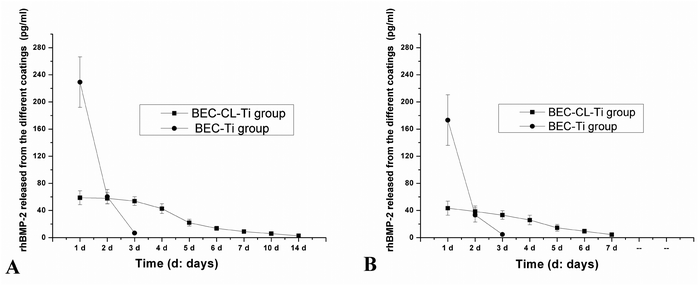 | ||
| Fig. 4 rhBMP-2 release profiles of the BEC-Ti group and the BEC-CL-Ti group in PBS solution (A) and 10 μM GSH solution (B). The values represent mean ± standard deviation. | ||
According to the rhBMP-2 release profiles in PBS solution (Fig. 4A), the total rhBMP-2 amount of each BEC-Ti disc and BEC-CL-Ti disc was 296 and 264 pg, respectively. The loss of a small amount of rhBMP-2 from the BEC-CL-Ti disc should be attributed to the crosslinking procedure. However, according to the release profiles in the 10 μM GSH solution (Fig. 4B), the total rhBMP-2 amount of each BEC-Ti disc and BEC-CL-Ti disc was 201 and 203 pg, less than the corresponding values in PBS solution. This should be attributed to the nature of rhBMP-2. rhBMP-2 is a small, basic protein consisting of two identical polypeptide chains of 114 amino acids covalently linked together via a disulfide bond to form the biologically active protein of 25.8 kDa. In GSH solution, the disulfide bonds could be cleaved. One rhBMP-2 molecule may change into two identical polypeptide chains, and the mono polypeptide chain could not be detected by the ELISA assay. As a result, part of rhBMP-2 was destroyed in 10 μM GSH solution, and the detectable rhBMP-2 was less than that in PBS solution. Because a high concentration of GSH can rapidly cleave all the disulfide bonds, no rhBMP-2 could be detected in the 5 mM GSH solution. Provided that the amounts of the wrapped rhBMP-2 on each BEC-CL-Ti disc and each BEC-Ti disc were constant, it could be estimated that the total detectable rhBMP-2 of the BEC-CL-Ti group and BEC-Ti group in 10 μm GSH solution was 77% and 68% of that in PBS solution, respectively. It was thus clear that the disulfide crosslinking in the BEC-CL-Ti group not only provided a sustained release profile of rhBMP-2, but also rendered rhBMP-2 a stronger resistance to the physiological environment.
3.4. In vitro biological evaluations
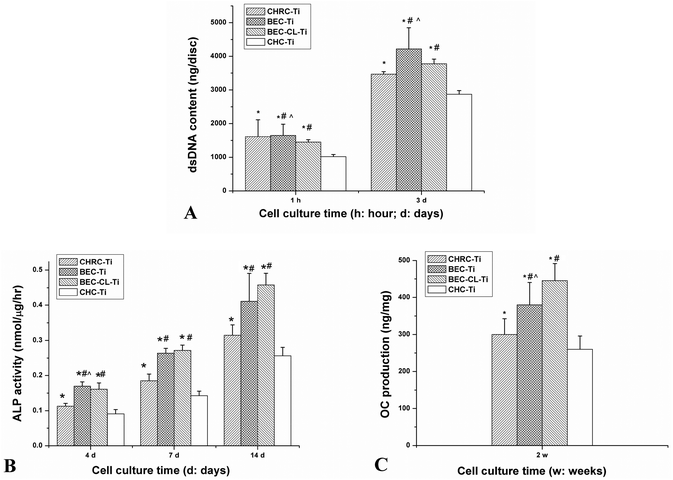
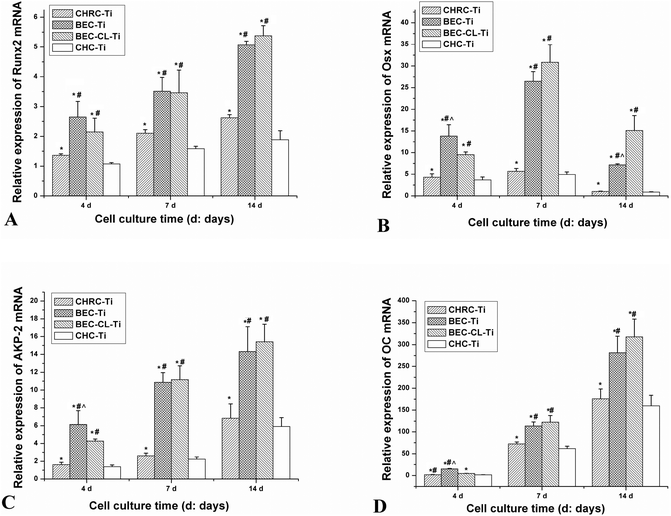
RGD sequences could mediate recognition and improve adhesion to biomaterials for many kinds of cells23,24 and cellular responses to RGD-modified biomaterials including proliferation25 and differentiation26 could also be improved because RGD sequences binding with cellular membrane receptors would trigger the intracellular signal transduction pathways that may further regulate cell functions.27 In this study, three RGD containing groups (CHRC-Ti group, BEC-Ti group and BEC-CL-Ti group) showed a better adhesion, proliferation and differentiation capacity of pre-osteoblasts than the CHC-Ti group (Fig. 5 and 6). Nevertheless, rhBMP-2 molecules had little influence on cell adhesion, which could account for the insignificant difference of cell numbers between the BEC-Ti group and CHRC-Ti group. Moreover, BMP-2 molecules, as a member of the TGF-β family, could promote proliferation and differentiation of osteogenic cells.28 Thus, the two rhBMP-2 containing groups were more favorable to cell proliferation than the CHRC-Ti group (Fig. 5A). ALP and OC are markers of osteogenic cell differentiation and both of them participate in bone matrix formation and mineralization.26 ALP is an early indicator,29,30 while OC appears at the later stage of cell differentiation.31 Runx-2 and Osterix, two key transcription factors that regulate osteogenesis, direct the expression of bone-specific genes such as AKP-2 and OC.31 Due to the tremendous differentiation stimulating effects of rhBMP-2 molecules, the BEC-Ti and BEC-CL-Ti groups showed obviously improved cellular differentiation capacities compared to that in the CRHC-Ti group, with an increased ALP activity and OC production and up-regulated mRNA expression levels (Fig. 5B, D and 6).The above mentioned results revealed that the composition of the PEM coatings has a critical influence on the cell responses. The single-factor RGD treated coating (CHRC-Ti) showed a limited promotion of cell proliferation and differentiation behaviors, while the two-factor RGD and rhBMP-2 containing groups (BEC-Ti and BEC-CL-Ti) showed a significant promotion of cell proliferation and differentiation behaviors. Clearly, a multi-signaling strategy is more benefitial for optimal cell responses. Both collagen and HA are important components of ECM. In a way that is different to the common strategies that use polymers as the matrices of the PEM coating,32,33 the strategy used here takes advantage of the provided component-close-to-ECM microenvironment for osteogenic cells16 on one hand and provides more molecular cues for wound healing on the other hand. However, the increased number of the types of components means that organizing them in a proper way is harder. A lyophilization strategy was used to construct the multi-component organic coating on the implant surfaces.34 The arrangement of the multiple components in this way was disorderly and the resultant coating was unstable. Fast disintegration of the coating and burst release of the regulatory molecules will lead to high local concentrations of bioactive molecules, which often counteracts or even reverses their effects on tissue regeneration.35 For instance, free RGD peptide sequences in culture systems can be an antagonist for cell adhesion to fibronectin-modified biomaterials.36 Even worse, the transiently high local concentration of rhBMP-2 has frequently been related to undesirable side-effects37 such as tissue swelling, inflammatory reactions and heterotopic ossification.38
The strategy used in this study allowed the organization of multiple components in a careful and ordered way. The RGD sequences were fixed into the 3-dimensional skeleton of the semi-IPN gel structure, and rhBMP-2 was reserved in this semi-IPN gel structure (Scheme 1). The BEC-Ti group showed better cell responses (Fig. 5 and 6) than the BEC-CL-Ti group at the early stage, but the differentiation capacity of the BEC-CL-Ti group exceeded that of the BEC-Ti group at the late stage of the cell culture (Fig. 6). This should be attributed to the fact that rhBMP-2 could steadily elicit its biological effects in the BEC-CL-Ti group over more than 10 days, while it was cleared away within 3 days though either proteolysis or fresh medium replacement in the case of the BEC-Ti group. Sustained local delivery of rhBMP-2 is thought to more closely replicate the natural healing process.39 Such results suggested that the sustained release of rhBMP-2 would be more favorable for the cell differentiation, just as the previous studies reported.38
3.5. Biomechanical test
Although encouraging in vitro results (promoting cell adhesion capacity and stimulating cell proliferation and differentiation) were observed, whether the novel coating of the BEC-CL-Ti group could stimulate cell responses in vivo and finally improve the degree of implant integration in bone tissue was the vital issue that we concerned about. It is difficult to track cellular events in the healing process on an implant surface after implantation, but the bone binding behaviors to implants can be detected by biomechanical tests at predetermined time points. The removal torque test is one of the most valuable methods to evaluate the bone anchorage strength of implants.40,41 In this study, compared with the AE-implant group, higher RTQ values of BEC-CL-implant group in each test point indicated the greatly enhanced bone to implant binding strength of BEC-CL-implant group (Fig. 7). These results may be attributed to the synergistic effect of the RGD peptide and BMP-2 molecules in the biomimetic coating. The biomimetic ECM coating could be conductive to implant-bone integration though modulating tissue-implant interactions caused by providing more molecular cues for tissue repairing.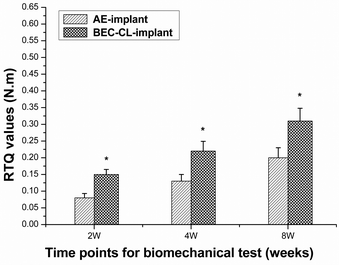 | ||
| Fig. 7 RTQ values of the AE-implant group and the BEC-CL-implant group. The values represent mean ± standard deviation. *p < 0.05, v. BEC-CL-implant. | ||
4. Conclusion
In conclusion, a disulfide-crosslinked collagen/HA-GRGDSPC PEM coating was constructed on a Ti implant, in which rhBMP-2 was incorporated. The resultant biomimetic coating showed improved stability, GSH-responsive biodegradation properties and a sustained release profile of rhBMP-2. The in vitro and in vivo results indicated that the biomimetic ECM coating, which anchored the RGD sequence and delivered rhBMP-2 in a sustained way, effectively promoted the cell responses of pre-osteoblasts and increased bone-to-implant binding strength. This biomimetic coating is a type of novel coating. The strategy employed herein can also be extended for designing other functional coatings on implantable devices.The authors declare no competing financial interest.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (no. 51173163, no. 50803055 and no. 30872902), the National Science-technology Support Plan project of China (2012BAI07B01) and the fundamental Research Funds for the Central Universities (2012QNA7043).References
- S. Rammelt, T. Illert, S. Bierbaum, D. Scharnweber, H. Zwipp and W. Schneiders, Biomaterials, 2006, 27, 5561–5571 CrossRef CAS PubMed.
- Y. Z. Yang, R. Glover and J. L. Ong, Colloids Surf., B, 2003, 30, 291–297 CrossRef CAS.
- I. Degasne, M. F. Basle, V. Demais, G. Hure, M. Lesourd, B. Grolleau, L. Mercier and D. Chappard, Calcif. Tissue Int., 1999, 64, 499–507 CrossRef CAS PubMed.
- H. Schliephake, A. Aref, D. Scharnweber, S. Bierbaum, S. Roessler and A. Sewing, Clin. Oral Implants Res., 2005, 16, 563–569 CrossRef PubMed.
- S. Y. Lee, J. Y. Koak, S. J. Heo, S. K. Kim, S. J. Lee and S. Y. Nam, Int. J. Oral Maxillofac. Implants, 2010, 25, 315–320 Search PubMed.
- M. Morra, Eur. Cell. Mater., 2006, 12, 1–15 CAS.
- W. J. Grzesik and P. G. Robey, J. Bone Miner. Res., 1994, 9, 487–496 CrossRef CAS PubMed.
- Y. Liu, R. O. Huse, K. de Groot, D. Buser and E. B. Hunziker, J. Dent. Res., 2007, 86, 84–89 CrossRef CAS PubMed.
- J. B. Park, J. Craniofac. Surg., 2011, 22, 1880–1882 CrossRef PubMed.
- M. Morra, Expert Rev. Med. Devices, 2007, 4, 361–372 CrossRef CAS PubMed.
- H. Schliephake and D. Scharnweber, J. Mater. Chem., 2008, 18, 2404–2414 RSC.
- M. C. Porte-Durrieu, F. Guillemot, S. Pallu, C. Labrugere, B. Brouillaud, R. Bareille, J. Amedee, N. Barthe, M. Dard and C. Baquey, Biomaterials, 2004, 25, 4837–4846 CrossRef CAS PubMed.
- V. Kumar, A. K. Abbas, N. Fausto and J. C. Aster, in Tissue Repair: Regeration, Healing, and Fibrosis, ed. V. Kumar, A. K. Abbas, N. Fausto and J. C. Aster, Elsevier Saunders, Philadelphia, 8th edn, 2009, pp. 79–110 Search PubMed.
- O. Jeon, C. Powell, L. D. Solorio, M. D. Krebs and E. Alsberg, J. Controlled Release, 2011, 154, 258–266 CrossRef CAS PubMed.
- Y. Huang, Q. J. Luo, X. D. Li, F. Zhang and S. Zhao, Acta Biomater., 2012, 8, 866–877 CrossRef CAS PubMed.
- X. J. Li, Q. J. Luo, Y. Huang, X. D. Li, F. Zhang and S. F. Zhao, Polym. Adv. Technol., 2012, 23, 756–764 CrossRef CAS.
- A. Nanci, J. D. Wuest, L. Peru, P. Brunet, V. Sharma, S. Zalzal and M. D. McKee, J. Biomed. Mater. Res., 1998, 40, 324–335 CrossRef CAS PubMed.
- G. Mendonca, D. B. S. Mendonca, F. J. L. Aragao and L. F. Cooper, Biomaterials, 2008, 29, 3822–3835 CrossRef CAS PubMed.
- R. J. vanKlaveren, M. Demedts and B. Nemery, Eur. Respir. J., 1997, 10, 1392–1400 CrossRef CAS.
- M. Chatzinikolaidou, T. K. Lichtinger, R. T. Muller and H. P. Jennissen, Acta Biomater., 2010, 6, 4405–4421 CrossRef CAS PubMed.
- A. N. Zelikin, Q. Li and F. Caruso, Chem. Mater., 2008, 20, 2655–2661 CrossRef CAS.
- S. Pavlukhina and S. Sukhishvili, Adv. Drug Delivery Rev., 2011, 63, 822–836 CrossRef CAS PubMed.
- U. Hersel, C. Dahmen and H. Kessler, Biomaterials, 2003, 24, 4385–4415 CrossRef CAS PubMed.
- S. Verrier, S. Pallu, R. Bareille, A. Jonczyk, J. Meyer, M. Dard and J. Amedee, Biomaterials, 2002, 23, 585–596 CrossRef CAS PubMed.
- J. S. Park, H. N. Yang, S. Y. Jeon, D. G. Woo, K. Na and K. H. Park, Biomaterials, 2010, 31, 6239–6248 CrossRef CAS PubMed.
- H. Zreiqat, F. A. Akin, C. R. Howlett, B. Markovic, D. Haynes, S. Lateef and L. Hanley, J. Biomed. Mater. Res., 2003, 64A, 105–113 CrossRef CAS PubMed.
- M. C. Siebers, P. J. ter Brugge, X. F. Walboomers and J. A. Jansen, Biomaterials, 2005, 26, 137–146 CrossRef CAS PubMed.
- J. M. Wozney, V. Rosen, A. J. Celeste, L. M. Mitsock, M. J. Whitters, R. W. Kriz, R. M. Hewick and E. A. Wang, Science, 1988, 242, 1528–1534 CAS.
- V. Kartsogiannis and K. W. Ng, Mol. Cell. Endocrinol., 2004, 228, 79–102 CrossRef CAS PubMed.
- G. R. Beck Jr., E. C. Sullivan, E. Moran and B. Zerler, J. Cell. Biochem., 1998, 68, 269–280 CrossRef CAS.
- G. S. Stein and J. B. Lian, Endocr. Rev., 1993, 14, 424–442 CrossRef CAS PubMed.
- M. L. Macdonald, R. E. Samuel, N. J. Shah, R. F. Padera, Y. M. Beben and P. T. Hammond, Biomaterials, 2011, 32, 1446–1453 CrossRef CAS PubMed.
- N. J. Shah, M. L. Macdonald, Y. M. Beben, R. F. Padera, R. E. Samuel and P. T. Hammond, Biomaterials, 2011, 32, 6183–6193 CrossRef CAS PubMed.
- T. Luo, W. Zhang, B. Shi, X. Cheng and Y. Zhang, Clin. Oral. Implants. Res., 2012, 23, 467–473 CrossRef PubMed.
- L. B. Shields, G. H. Raque, S. D. Glassman, M. Campbell, T. Vitaz, J. Harpring and C. B. Shields, Spine, 2006, 31, 542–547 CrossRef PubMed.
- D. A. Puleo and R. Bizios, Bone, 1991, 12, 271–276 CrossRef CAS PubMed.
- G. Wu, Y. Liu, T. Iizuka and E. B. Hunziker, Biomaterials, 2010, 31, 7485–7493 CrossRef CAS PubMed.
- A. Lochmann, H. Nitzsche, S. von Einem, E. Schwarz and K. Mader, J. Controlled Release, 2010, 147, 92–100 CrossRef CAS PubMed.
- O. A. Arosarena, F. E. Del Carpio-Cano, R. A. Dela Cadena, M. C. Rico, E. Nwodim and F. F. Safadi, J. Cell. Physiol., 2011, 226, 2943–2952 CrossRef CAS PubMed.
- Y. T. Sul, B. S. Kang, C. Johansson, H. S. Um, C. J. Park and T. Albrektsson, J. Biomed. Mater. Res., Part A, 2009, 89, 942–950 CrossRef PubMed.
- J. W. Park, Y. J. Kim, J. H. Jang, T. G. Kwon, Y. C. Bae and J. Y. Suh, Acta Biomater., 2010, 6, 1661–1670 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: ESI includes the sequences of primers for target genes, FT-IR of the HA derivatives (HA-GRGDSPC-(SH) and HA-GRGDSP), the representative SFM images of AE-Ti group, the detection of C1s and N1s spectra of the coating and the C and N contents of the coating in the BEC-CL-Ti group before and after crosslinking via XPS, the detailed assay procedures of ALP activity, cytoplasmic total protein concentration and OC production, the representative SFM images of the AE-Ti group, and the surgical procedures of the animal experiment. This material is available free of charge. See DOI: 10.1039/c3bm60254k |
| ‡ These two authors contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2014 |