Construction and characterization of Cy3- or Cy5-conjugated hairpin pyrrole–imidazole polyamides binding to DNA in the nucleosome
Yong-Woon
Han
*ab,
Yasuo
Tsunaka
ac,
Hiroaki
Yokota
a,
Tomoko
Matsumoto
ad,
Gengo
Kashiwazaki
e,
Hironobu
Morinaga
e,
Kaori
Hashiya
e,
Toshikazu
Bando
e,
Hiroshi
Sugiyama
*abe and
Yoshie
Harada
*a
aInstitute for Integrated Cell-Materials Science (iCeMS), Kyoto University, Yoshida Honmachi, Sakyo, Kyoto 606-8501, Japan. E-mail: han.yongwoon.4u@kyoto-u.ac.jp; hs@kuchem.kyoto-u.ac.jp; harada.yoshie.4r@kyoto-u.ac.jp
bCREST, Japan Science and Technology Corporation (JST), Sanbancho, Chiyoda-ku, Tokyo 102-0075, Japan
cPREST, Japan Science and Technology Corporation (JST), Sanbancho, Chiyoda-ku, Tokyo 102-0075, Japan
dDepartment of Human Life Studies, Doshisha Women's College of Liberal of Arts, Teramachi Nishiiru, Imadegawa-dori, Kamigyo, Kyoto 602-0893, Japan
eDepartment of Chemistry, Graduate School of Science, Kitashirakawa-oiwakecho, Kyoto University, Sakyo, Kyoto 606-8502, Japan
First published on 24th October 2013
Abstract
Sequence-specific DNA-binding modules, N-methylpyrrole (Py)-N-methylimidazole-(Im) polyamides have been recently conjugated with fluorophores, and some of these conjugates could be used for the detection of specific DNA sequences. In this study, we synthesized two Py–Im polyamides 1 and 2, which interact with the 145-bp nucleosome positioning sequence 601. We conjugated the cyanine dye Cy3 or Cy5 with 1 or 2. In the absence of target DNA, the fluorescent conjugate of a Py–Im polyamide had lower fluorescence intensity compared with Cy3 or Cy5 alone. In the presence of either the target DNA or the nucleosome, the fluorescence intensity of the conjugates increased. Furthermore, we observed a Förster resonance energy transfer between the Cy3–Py–Im polyamide and the Cy5–Py–Im polyamide on the nucleosome. These results open up the possibilities that fluorescent conjugates of Py–Im polyamides can be used for characterization of the dynamic interactions within protein–DNA complexes.
Introduction
N-Methylpyrrole (Py)–N-methylimidazole-(Im) polyamides are small molecules that can recognize specific DNA sequences in the minor groove of the B-form DNA with DNA recognition rules.1,2 Py favors the T, A, and C bases, excluding G and Im favors G. A lone pair of N3 in Im forms a hydrogen bond with 2 amino hydrogen of guanine. Anti-parallel pairings of Im/Py and Py/Im bind to the G·C and C·G sequence in DNA, respectively. Anti-parallel pairing of Py/Py binds to A·T and T·A degenerately.1,2 Aliphatic β-alanine (β) can be substituted for Py. Anti-parallel pairings of Py/β and β/Py bind to A·T and T·A degenerately, and anti-parallel pairings of Im/β and β/Im specify G·C and C·G, respectively.3,4A Py–Im polyamide can be used as a synthetic sequence-specific DNA-binding module. Py–Im polyamides that can bind to a promoter region have been designed to inhibit gene expression,5–9 and a conjugate of a Py–Im polyamide with a peptide or a small organic molecule can serve as a synthetic transcriptional activator.10–13 Various types of the alkylating moiety-conjugated Py–Im polyamides have also been developed as sequence-specific DNA-alkylating agents, and their chemical and biological properties have been investigated.14–20 In addition to these applications of Py–Im polyamides to gene expression and sequence-specific DNA alkylation, to date, many of the fluorescent conjugates of Py–Im polyamides have been synthesized, and some of them can be used as effective sequence-specific fluorescent probes.21–26 Thus, it is also possible that fluorescent conjugates of Py–Im polyamides could be used for characterization of dynamic interactions within protein–DNA complexes. At present, there are no data in the literature regarding the binding of this sort of conjugates to protein–DNA complexes.
The nucleosome is a basic unit of eukaryotic chromatin and consists of approximately 150 bp DNA wrapped in 1.7 superhelical turns around a histone octamer. The histone octamer consists of two copies each of four histone proteins, H2A, H2B, H3, and H4.27 Genetic processes such as transcription, DNA replication, recombination and repair and chromatin segregation depend on changes of chromatin structures.28–31 The histone chaperone and chromatin remodeler are the enzymes involved in chromatin assembly/disassembly and in nucleosome movement, ejection or exchange, which are crucial for the above mentioned genetic processes.30 The Dervan, Gottesfeld, and Luger groups have designed some Py–Im polyamides binding to nucleosomes and characterized them by biochemical and X-ray crystal structure analysis. They showed that DNA sites facing away from the histone octamer or even partially facing the histone octamer were fully accessible for the Py–Im polyamides.32,33 They also synthesized some bivalent hairpin Py–Im polyamides consisting of hairpin Py–Im polyamide dimers bridged in a head-to-head manner with ethylene glycol molecules and showed that the Py–Im polyamide bound to a nucleosomal “supergroove” and clamping the nucleosome by the Py–Im polyamide prevented nucleosome dissociation.34
In this work, to investigate how fluorescent conjugates of Py–Im polyamides bind to DNA in a nucleosome, we synthesized two Py–Im polyamides targeting a nucleosome positioning sequence 60135 and conjugated the two Py–Im polyamides with a cyanine dye, either Cy3 or Cy5. Using the conjugates Cy3–Py–Im polyamide and Cy5–Py–Im polyamide as a fluorescence donor–acceptor pair, we investigated a Förster resonance energy transfer (FRET) between the Cy3 conjugated and the Cy5 conjugated Py–Im polyamide on the nucleosome.
Experimental section
General methods
The following abbreviations are used in this article: Fmoc, fluorenylmethoxycarbonyl; DMSO, dimethylsulfoxide; DMF, dimethylformamide; DIEA, N,N-diisopropylethylamine; TFA, trifluoroacetic acid; Dp, 3-(dimethylamino)propylamine; EDTA, ethylenediaminetetraacetic acid; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; β,β-alanine; γ,γ-aminobutyric acid; Py, N-methylpyrrole; Im, N-methylimidazole; PEG, polyethylene glycol.Electrospray ionization time-of-flight mass spectrometry (ESI-TOFMS) was performed using a BioTOF II (Bruker Daltonics) mass spectrometer to determine the molecular weight of Py–Im polyamides 1, 2, and 5–8.
Polyamide synthesis
Py–Im polyamides 1 and 2 were synthesized in a stepwise reaction using a Fmoc solid-phase protocol as described previously.36 The synthesis was performed on a Pioneer Peptide Synthesizer, PSSM-8 (Shimadzu, Japan) in a computer-assisted mode on a 100 μmol scale (270 mg of Fmoc-β-alanine Wang resin, 120 mmol g−1). After the synthesis, Dp was mixed with the resin and the mixture was incubated for 4 h at 55 °C on a shaker at 550 rpm to detach the Py–Im polyamides from the resin. Purification of Py–Im polyamides 1 and 2 was performed on high-performance liquid chromatography (HPLC) PU-2080 Plus series system (JASCO Inc., Japan) using a 10 mm × 150 mm Chemcopak Chemcobond 5-ODS-H reverse-phase column in 0.1% TFA in water with acetonitrile as an eluent at a flow rate of 3 mL min−1, and linear-gradient elution with acetonitrile (20%–60% acetonitrile in water) lasting 20 min with detection at 254 nm. The collected fractions were analyzed using ESI-TOFMS.Cy3- and Cy5-conjugated Py–Im polyamides
Fluorescent conjugates of Py–Im polyamides 5–8 were synthesized using a coupling reaction between the Py–Im polyamide 1 or 2 and either Cy3-N-hydroxysuccinimidyl (NHS) ester 3 or Cy5-NHS ester 4. The coupling reactions were performed in DMF containing DIEA, which was used as a coupling agent. Approximately 1.0 mg of 3 or 4 was dissolved in 45 μL of DMF and then Py–Im polyamide 1 or 2 (approximately 1.5 mg) was dissolved in the mixture. Next, 0.57 μL of DIEA was added to the reaction mixtures and the mixtures were incubated with shaking at 550 rpm for 2 h at room temperature. After the coupling reaction, 1 μL of the mixture was diluted with 9 μL DMF, and 1 μL of the diluted mixture was analyzed using the HPLC PU-2080 Plus series system with a 4.6 mm × 150 mm Chemcopak Chemcobond 5-ODS-H reverse-phase column in 0.1% TFA in water with acetonitrile as an eluent at a flow rate of 1 mL min−1, using linear-gradient elution with acetonitrile (0% to 100% in water) lasting 20 min with detection at 254 nm. We obtained fractions for two major peaks and the red (Cy3) or blue (Cy5) solution corresponding to the later peak was further investigated. Those fractions were analyzed using ESI-TOFMS, and we confirmed that the fluorophore (Cy3 or Cy5) was conjugated with the Py–Im polyamide. After that, purification of Py–Im polyamides 5–8 was performed by HPLC using a PU-2080 Plus series system employing a 10 mm × 150 mm Chemcopak Chemcobond 5-ODS-H reverse-phase column in 0.1% TFA in water with acetonitrile as an eluent at a flow rate of 3 mL min−1, using linear-gradient elution with acetonitrile (20%–60% in water) for 20 min with detection at 254 nm. The collected fractions were analyzed using ESI-TOFMS.DNA preparation
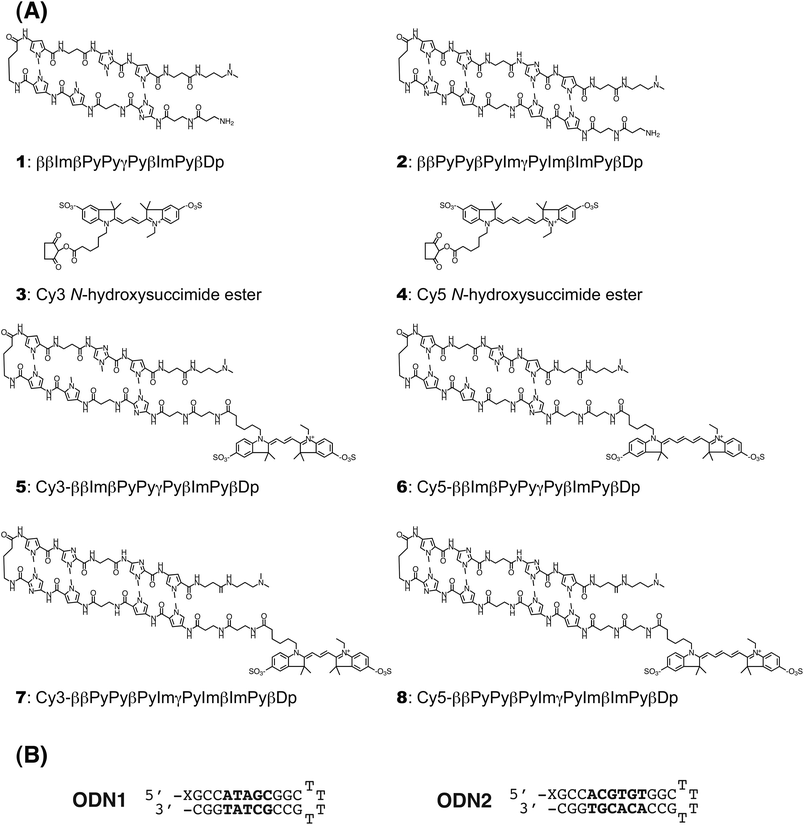
Biotinylated DNA oligonucleotides, ODN1 and ODN2 (Fig. 1B) were used for the measurement of the DNA binding activities of the Py–Im polyamides by SPR assays.Two other DNA oligos, ODN3 and ODN4, were used as primers in PCR for amplification of a 145-bp DNA fragment containing nucleosome positioning sequence 601 from the plasmid pGEM3Z-601.35,37 The sequences of ODN3 and ODN4 are as follows: ODN3, 5′-AGATCTTCGCGAGAAGACGATATCAGAATCCCGGTGCCGAGG-3′, and ODN4, 5′-TCGCGACGTCTCAGATATCGATGTATATATCTGACAC-3′. As shown in Fig. 2, the 145 bp amplicon was then inserted into T7 Blue Vector (Novagen). The ligated DNAs were introduced into the E. coli strain DH5α. We purified plasmid DNAs using the Wizard Plus SV Minipreps DNA Purification System (Promega, USA) according to the vendor's manual and confirmed that the purified plasmid DNA, pYT145-1, contained the 145-bp insert by 0.8% agarose gel electrophoresis. Next, pYT145-1 was digested with BbsI and BsmBI restriction endonucleases, and the resulting BbsI–BsmBI fragment containing the 145-bp DNA region of interest was inserted into BbsI-digested dephosphorylated pYT145-1. The ligated DNA was introduced into DH5α, and after plasmid DNA purification, we checked the DNA using 0.8% agarose gel electrophoresis. Most of the purified plasmid DNA contained two copies of the 145-bp DNA region, and we used the plasmid, pYT145-2 to construct pYT145-4 (Fig. 2). The pYT145-2 was digested with BbsI and BsmBI and the BbsI–BsmBI fragment containing the 145-bp DNA region was inserted into BbsI-digested dephosphorylated pYT145-2. The ligated DNAs were introduced into DH5α, and after plasmid DNA purification, we checked the DNA using 0.8% agarose gel electrophoresis. Most of the purified plasmid DNA contained four copies of the 145-bp DNA region, and we named this plasmid pYT145-4 (Fig. 2). After that, pYT145-4 was digested with BbsI and BsmBI and the fragment containing the 145-bp DNA of interest was inserted into BbsI-digested dephosphorylated pYT145-4. The ligated DNA was introduced into DH5α, and after plasmid DNA purification, we checked the DNA using 0.8% agarose gel electrophoresis. Most of the purified plasmid DNA contained eight copies of the 145-bp DNA region. Some of the purified plasmid DNA, however, contained more copies unexpectedly and, among them, we found pYT145-24, which contained 24 copies of the 145-bp DNA fragment. We used pYT145-24 for purification of the 145-bp DNA region as follows.
To obtain a large amount of pYT145-24, we introduced the plasmid into DH5α and one of the resultant colonies was inoculated into 4 mL of terrific broth (TB).38 The culture was incubated for 4 h at 37 °C with shaking. Next, equal amounts (0.5 mL) of the culture were transferred to eight conical flasks containing 100 mL TB and carbenicillin at 100 μg mL−1. These flasks were incubated for approximately 16 h at 37 °C with shaking. After that, pYT145-24 was purified using Nucleo Bond Xtra Maxi (Takara Bio, Japan) according to the vendor's manual.
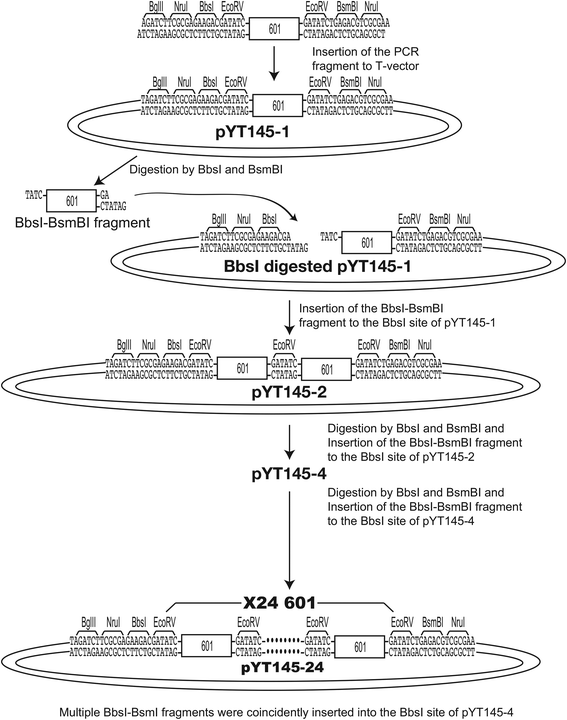
For isolation of the 145-bp DNA fragment, the mixture (1 mL) containing 1 mg of pYT145-24 was mixed with EcoRV (600 units) and incubated at 37 °C for approximately 16 h (Fig. 3A). Then, the 145-bp DNA was separated from the linearized vector DNA by means of PEG precipitation. We added 342 μL of 40% PEG4000 and 152 μL of 4 M NaCl to the 1 mL mixture and the mixture was incubated on ice for 1 h. The linearized vector DNA was pelleted by centrifugation at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g and 4 °C for 20 min. The 145-bp DNA in the supernatant was precipitated using a standard ethanol precipitation method, and the resulting DNA pellet was dissolved in 100 μL of HE buffer (10 mM HEPES, 1 mM EDTA, pH 8.0).
000g and 4 °C for 20 min. The 145-bp DNA in the supernatant was precipitated using a standard ethanol precipitation method, and the resulting DNA pellet was dissolved in 100 μL of HE buffer (10 mM HEPES, 1 mM EDTA, pH 8.0).
The earlier pellet from PEG precipitation was dissolved in 1 mL of HE buffer and checked using 1.2% agarose gel electrophoresis. Because the 145-bp DNA was still present in that pellet, 342 μL of 40% PEG4000 and 152 μL of 4 M NaCl were added to the 1 mL mixture and the 145-bp DNA was separated from the linearized vector DNA as described above (Fig. 3A). The 145-bp DNA in the supernatant was collected using ethanol precipitation and the pellet was dissolved in 100 μL of HE buffer. We confirmed that only trace amounts of the 145-bp DNA were present in the pellet from the second PEG precipitation (Fig. 3A). The yield of the 145-bp DNA was approximately 0.32 mg.
Mononucleosome preparation
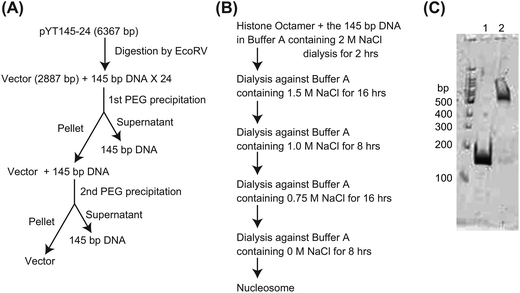
A histone octamer was prepared as described previously.37 Mononucleosomes were assembled using a salt dialysis method from the histone octamer and the 145-bp DNA fragment as follows. The histone octamer and the 145-bp DNA were mixed in 300 μL of buffer A (20 mM HEPES, 0.1 mM EDTA, pH 7.5) containing 2 M NaCl. The final concentration of the histone octamer and DNA was 0.5 μM. The mixture was transferred to the dialysis tube, Oscillatory Cup MWCO 8000 (Cosmo Bio Co., Ltd, Japan), and the dialysis against 500 mL of the buffer A containing 2 M NaCl was started at 4 °C under constant stirring. After 2 h, the buffer was changed to 500 mL of fresh buffer A containing 1.5 M NaCl and the dialysis was continued for approximately 16 h additionally. Several more stages of dialysis, with changes to a fresh buffer, were performed as shown in Fig. 3B. After the dialysis, the mixture was checked using 5% polyacrylamide gel electrophoresis and we confirmed that >95% of DNA formed complexes with the histone octamer (Fig. 3C). The concentration of the nucleosome was determined spectrophotometrically using ε260 = 1.88 × 106 cm−1 M−1.Surface plasmon resonance (SPR) assay
All SPR experiments were performed on a BIACORE X instrument at 25 °C as described previously.36,39–41 The sequences of biotinylated hairpin DNAs containing target sequences are described above. The hairpin DNAs were immobilized on a streptavidin-coated SA sensor chip at a flow rate of 20 μL min−1 to obtain the required immobilization level (up to approximately 1400 resonance units (RU) rise). The experiments were performed using HBS-EP (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.005% surfactant P20) buffer with 0.1% DMSO at 25 °C, pH 7.4. A series of sample solutions were prepared in HBS-EP buffer with 0.1% DMSO and injected at a flow rate of 20 μL min−1. To measure association and dissociation rate constants (KD, ka and kd), data processing was performed with an appropriate fitting model using the BIAevaluation 4.1 program. The sensorgrams of all data were fitted using the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model with mass transfer. The values of KD, ka and kd for all data are summarized in Table 1.
1 binding model with mass transfer. The values of KD, ka and kd for all data are summarized in Table 1.
| Py–Im polyamidea | K D (10−7 M) | K (104 M−1 s−1) | k (10−3 s−1) |
|---|---|---|---|
| a Closed circle, open circle, and β indicate Im, Py, and β-alanine, respectively. | |||

|
0.010 ± 0.0012 | 830 ± 410 | 8.7 ± 3.9 |

|
0.0059 ± 0.0037 | 290 ± 130 | 1.7 ± 1.4 |

|
3.5 ± 0.88 | 11 ± 3.7 | 38 ± 6.2 |

|
0.64 ± 0.50 | 59 ± 44 | 38 ± 19 |

|
3.1 ± 0.71 | 6.4 ± 0.13 | 20 ± 5.0 |

|
5.1 ± 0.32 | 5.5 ± 0.94 | 28 ± 3.9 |
Steady state fluorescence spectroscopy experiments
All steady-state fluorescence spectra measurements were made using a spectrofluorometer FP-6500 (JASCO Inc., Japan), equipped with a Xenon 150-W lamp. Excitation and emission wavelengths are indicated in the figure legend and all data were collected at 25 °C.Results and discussion
Synthesis of the conjugates of Py–Im polyamides with Cy3 or Cy5
As described in the Experimental section, the 145-bp DNA of nucleosome positioning sequence 601 was used for the preparation of the nucleosome. To characterize the binding of Py–Im polyamides to the nucleosome, we chose two DNA sites, TAAGC and ACGTGT (Fig. 4). Without a histone octamer, the distance between the two DNA sites was 75 bp, which corresponded to approximately 25 nm. In contrast, within the nucleosome (Fig. 4), N-terminals of each Py–Im polyamide face each other and the distance between two N-terminals is approximately 4.5 nm. The two DNA sites are not completely blocked by interactions with the histone octamer. Consequently, in this study, we synthesized two Py–Im polyamides 1 and 2 (Fig. 1A), which contain two β-alanine residues at the N-terminus. We synthesized several Py–Im polyamides with this kind of N-terminus and demonstrated that β-Dp linker at the C-terminus had a slight steric preference for A·T or T·A over G·C or C·G.40 Accordingly, the target DNA sequences of 1 and 2 were set as 5′-WWWGC-3′ and 5′-WCGWGW-3′, respectively (Fig. 4). | ||
| Fig. 4 Structure of the mononucleosome and the Py–Im polyamide binding sites. (A) The ribbon model is the crystal structure of a Xenopus laevis nucleosome core particle (Protein Data Bank code 3LZ0).58 The white arrow above the mononucleosome indicates a pseudo 2-fold axis passing through the center of the structure. The right panel displays an axial view of the mononucleosome. The target DNA sites for 1 and 2 are colored green and red, respectively. (B) DNA sequence of the 145-bp DNA fragment. The target DNA sites for 1 and 2 are colored green and red, respectively. (C) The model structure of the nucleosome-5–8 complex is shown in a CPK representation. The Py–Im polyamide structures were constructed by modification of Py–Im polyamide structures reported previously (Protein Data Bank code 1M18).34 Top and bottom strands of the 145-bp DNA in (B) are colored black and grey, respectively. Histones, Py–Im polyamides, Cy3, and Cy5 are colored blue, yellow, green, and red, respectively. Cy3-conjugated Py–Im polyamide and Cy5-conjugated Py–Im polyamide indicate Py–Im polyamide 5 and 8, respectively. | ||
After the synthesis of 1 and 2, we conjugated either Cy3 or Cy5 to the Py–Im polyamides using Cy3-NHS ester 3 or Cy5-NHS ester 4 and obtained 5–8. As shown in Fig. 1A, 5 and 6 are Cy3- and Cy5-conjugated Py–Im polyamide 1, respectively, whereas 7 and 8 are Cy3- and Cy5-conjugated Py–Im polyamide 2. We confirmed that the purity of each conjugate, 5–8, was >95%, according to analytical HPLC and ESI-TOFMS.
The KDs of 5 and 6 with ODN1 were 3.5 × 10−7 M and 6.4 × 10−8 M, respectively, which were decreased by 350- and 64-fold, respectively, compared to 1 (Table 1). In the case of 7 and 8, their KDs with ODN2 were 3.1 × 10−7 M and 5.1 × 10−7 M, respectively, which were decreased by 530- and 860-fold, respectively (Table 1), compared to 2 (Table 1). The kas and kds of 5–8 were diminished by 4.3- to 75-fold, compared to 1 or 2. These data suggest that conjugation of Cy3 or Cy5 to the N-terminus of Py–Im polyamide probably weakens the binding of the Py–Im polyamide to its target DNA and lowers the stability of the Py–Im polyamide–DNA complex.
As reported previously, the KD of a pyrene-conjugated Py–Im polyamide with its target DNA is 8.3 × 10−7 M,24 which is comparable with KDs of 5–8; emission intensity of the pyrene-conjugated Py–Im polyamide increased in the presence of the target DNA. Therefore, in this study, we decided to measure the emission spectra of 5–8 as described below.
Next, we obtained the spectrum for each conjugate 5–8 in the presence of the target DNA (Fig. 6C–F). At 50 nM of the DNA, the fluorescence intensities of 5–8 increased by 3.9-, 1.3-, 5.8- and 1.6-fold, respectively, compared with those without the DNA. At 200 nM of the DNA, the fluorescence intensities of 5–8 increased by 4.8-, 1.5-, 6.9- and 1.9-fold, respectively, compared with those without the DNA. From these data, the KDs of 5–8 against the target DNA were determined at 50–500 nM, and these constants are mostly consistent with the SPR data described above.
In the case of Cy3-conjugated Py–Im polyamides 5 and 7, their fluorescence intensities increased significantly as a result of addition of their target DNA. In contrast, the fluorescence intensities of Cy5-conjugated Py–Im polyamides 6 and 8 increased insignificantly after the addition of their target DNA. The peaks of emission spectra for the Py–Im polyamides in the presence of the target DNA were similar to those in the absence of the target DNA (Fig. 6C–F). These results suggest that Cy3- or Cy5-conjugated Py–Im polyamides could be useful as a fluorescent probe for detection of specific DNA sequences.
The Förster resonance energy transfer between the Cy3–Py–Im polyamide and Cy5–Py–Im polyamide on the nucleosome
As shown in Fig. 4, the distance between the two target sequence sites in the 145-bp DNA is approximately 25 nm. But this distance decreased to approximately 4.5 nm when the DNA formed a nucleosome with the histone octamer (Fig. 4). Accordingly, we measured the emission spectrum of the mixture containing 5 and 8 with either DNA or nucleosome (Fig. 7) to demonstrate whether FRET occurs between 5 and 8 on the nucleosome. In the presence of the naked 145-bp DNA, the fluorescence intensity of the mixture containing 5 and 8 decreased slightly by 5% (between 550 nm and 620 nm), compared with the mixture containing only 5 and the naked DNA (Fig. 7A). On the other hand, in the presence of the nucleosome, the fluorescence intensity of the mixture containing 5 and 8 decreased significantly by 28% (between 550 nm and 620 nm), compared with the mixture containing only 5 and the nucleosome (Fig. 7B). These results suggest that emitted fluorescence by Cy3 in 5 was absorbed by Cy5 in 8 on the same nucleosome because the distance between the Cy3 and Cy5 moieties was approximately 4.5 nm, which is shorter than the Förster distance.42 After that, we determined the emission spectra for 8 in the mixture containing 5, 8, and the nucleosome in order to demonstrate whether the fluorescence intensity of Cy5 in 8 increased as a result of the energy transfer from Cy3 in 5 on the same nucleosome. To determine the emission spectrum of 8, the emission spectrum of 5 in the mixture containing 5, 8, and the nucleosome was subtracted from the emission spectrum of the mixture. Because the fluorescence intensity between 550 nm and 620 nm of the mixture containing 5 and 8 decreased by 28%, compared with that containing 5, the decrease by 28% from the emission spectrum of the mixture containing 5 was defined as the spillover from 5 in the mixture containing 5, 8, and the nucleosome. As shown in Fig. 7C, the emission spectra from 5 in the mixture containing 5 and the spillover were almost identical between 550 nm and 620 nm. The calculated emission spectrum from 8 in the mixture containing 5, 8, and the nucleosome was determined by subtracting the spillover from the emission spectrum of the mixture containing 5, 8, and the nucleosome (Fig. 7C and D). As shown in Fig. 7D, the fluorescence intensity of 8 increased in the presence of the nucleosome, and furthermore, in the presence of both 5 and the nucleosome, the fluorescence intensity of 8 increased synergistically.In the case of 7 and 6, the fluorescence intensity of the mixture containing 7 and 6 decreased slightly by 5% (between 550 nm and 620 nm), compared with the mixture containing 7 in the presence of the naked 145-bp DNA (Fig. 8A). In contrast, in the presence of the nucleosome, the fluorescence intensity of the mixture containing 7 and 6 decreased significantly by 22% (between 550 nm and 620 nm), compared with that containing 7 (Fig. 8B). We also calculated the spillover from 7 in the mixture containing 7, 6, and the nucleosome (Fig. 8C) and determined the emission spectrum of 6 in the mixture containing 7, 6, and the nucleosome. Our data show that the fluorescence intensity of 6 was increased by the presence of 7 and the nucleosome (Fig. 8D) and FRET was observed between 7 and 6 on the nucleosome.
Previously, using a fluorescently labeled oligonucleotide, DNA fragments containing the nucleosome positioning sequence 601 have been constructed.43–46 After that, fluorescently labeled nucleosomes were constructed by mixing with the labeled DNA and the histone octamer43–46 and the properties of ATP-dependent chromatin assembly and remodeling factor (ACF) were studied by means of FRET measurements of the fluorescently labeled nucleosomes.43,44 Using fluorescence-labeled DNA fragments containing a 146-bp 5S RNA gene, the stability of the histone octamer containing the histone variant H2A.Z within the nucleosome was investigated and compared with that containing H2A.47 In the present work, using 5 and 8 or 7 and 6 as a FRET donor–acceptor pair, FRET was observed between 5 and 8 and, separately, between 7 and 6 on the nucleosome, but not on the target DNA. These results suggest that it is possible to use a fluorescent conjugate of a Py–Im polyamide for characterization of enzymes, such as the histone chaperone or chromatin remodeler or stability of various nucleosomes containing the histone variant. We are now planning to prepare some chromatin remodelers for characterization, using either spectroscopy or single-molecule fluorescence imaging techniques. If fluorophore conjugates of Py–Im polyamides can be successfully used for characterization of the histone chaperone or chromatin remodelers, then this kind of conjugate may also be applied to the characterization of longer DNA–protein complexes such as those involved in DNA-looping. DNA-looping is performed by a protein–DNA complex consisting of multiple proteins that simultaneously bind to two DNA sites that are tens to thousands of base pairs apart.48,49 DNA-looping that is mediated by transcription factors or repressors regulates gene expression.48,49 Site-specific DNA recombination by transposons such as bacteriophage Mu and retroviruses such as HIV-1 as well as V(D)J recombination also involves DNA-looping.50–53 Fluorescent conjugates of the Py–Im polyamides that bind specifically to the DNA-sites that participate in the DNA-looping process will allow us to characterize these dynamic and complicated protein–DNA complexes.
Another application of fluorescent conjugates of Py–Im polyamides is the studies of DNA-based photonic wires.54–57 W. Su et al. prepared 21-, 55- or 80-bp DNA fragments containing two fluorophores, Pacific Blue at one end and Cy3 at the other, as well as an oxazole yellow-conjugated Py–Im polyamide that binds to the internal regions of these DNAs. By measuring emission spectra of a mixture of the DNA and the Py–Im polyamide, they demonstrated that the oxazole yellow conjugate mediated the energy transfer from the Pacific Blue moiety to Cy3.57 Similarly, in the present study, we observed FRET between a Cy3-conjugated and a Cy5-conjugated Py–Im polyamide on the same nucleosome. Thus, if protein–DNA complexes whose association and dissociation processes can be regulated become available, in the future, this will help us to construct complicated controllable DNA-based photonic wires.
Conclusion
In this work, we synthesized Cy3- and Cy5-conjugated hairpin Py–Im polyamides targeting the 145-bp DNA containing nucleosome positioning sequence 601. In the presence of the nucleosome, strong fluorescence emission was observed from the Cy3 conjugate. Furthermore, we observed FRET between the Cy3-conjugated Py–Im polyamide 5 and Cy5-conjugated Py–Im polyamide 8 on the nucleosome. The same was true of 7 and 6. These results suggest that fluorophore-conjugated Py–Im polyamides can be used for studies of protein–DNA complexes.Acknowledgements
This work was supported by a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science, and Technology, an iCeMS Exploratory Grants for Junior Investigators, an iCeMS-JSPS Overseas Visit Program for Young Researchers (Y.-W.H), Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Agency (Y.-W.H. and H.S.) and Funding Program for Next Generation World-Leading Researchers (Y.H.). We thank Prof. K. Morikawa for valuable comments.Notes and references
- P. B. Dervan and B. S. Edelson, Curr. Opin. Struct. Biol., 2003, 13, 284 CrossRef CAS PubMed.
- P. B. Dervan, A. T. Poulin-Kerstien, E. J. Fechter and B. S. Edelson, Top. Curr. Chem., 2005, 253, 1 CAS.
- J. M. Turner, S. E. Swalley, E. E. Baird and P. B. Dervan, J. Am. Chem. Soc., 1998, 120, 6219 CrossRef CAS.
- C. C. Wang, U. Ellervik and P. B. Dervan, Bioorg. Med. Chem., 2001, 9, 653 CrossRef CAS PubMed.
- J. M. Gottesfeld, J. M. Turner and P. B. Dervan, Gene Expr., 2000, 9, 77 CrossRef CAS PubMed.
- E. J. Fechter and P. B. Dervan, J. Am. Chem. Soc., 2003, 125, 8476 CrossRef CAS PubMed.
- M. S. Murty and H. Sugiyama, Biol. Pharm. Bull., 2004, 27, 468 CAS.
- N. G. Nickols and P. B. Dervan, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 10418 CrossRef CAS PubMed.
- D. M. Chenoweth, D. A. Harki, J. W. Phillips, C. Dose and P. B. Dervan, J. Am. Chem. Soc., 2009, 131, 7182 CrossRef CAS PubMed.
- A. K. Mapp, A. Z. Ansari, M. Ptashne and P. B. Dervan, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 3930 CrossRef CAS.
- A. Z. Ansari, A. K. Mapp, D. H. Nguyen, P. B. Dervan and M. Ptashne, Chem. Biol., 2001, 8, 583 CrossRef CAS PubMed.
- P. S. Arora, A. Z. Ansari, T. P. Best, M. Ptashne and P. B. Dervan, J. Am. Chem. Soc., 2002, 124, 13067 CrossRef CAS PubMed.
- Y. Kwon, H. D. Arndt, Q. Mao, Y. Choi, Y. Kawazoe, P. B. Dervan and M. Uesugi, J. Am. Chem. Soc., 2004, 126, 15940 CrossRef CAS PubMed.
- T. Bando and H. Sugiyama, Acc. Chem. Res., 2006, 39, 935 CrossRef CAS PubMed.
- T. Bando, S. Sasaki, M. Minoshima, C. Dohno, K. Shinohara, A. Narita and H. Sugiyama, Bioconjugate Chem., 2006, 17, 715 CrossRef CAS PubMed.
- S. Sasaki, T. Bando, M. Minoshima, T. Shimizu, K. Shinohara, T. Takaoka and H. Sugiyama, J. Am. Chem. Soc., 2006, 128, 12162 CrossRef CAS PubMed.
- M. Minoshima, T. Bando, S. Sasaki, K. Shinohara, T. Shimizu, J. Fujimoto and H. Sugiyama, J. Am. Chem. Soc., 2007, 129, 5384 CrossRef CAS PubMed.
- G. Kashiwazaki, T. Bando, K. Shinohara, M. Minoshima, H. Kumamoto, S. Nishijima and H. Sugiyama, Bioorg. Med. Chem., 2010, 18, 2887 CrossRef CAS PubMed.
- M. Minoshima, T. Bando, K. Shinohara, G. Kashiwazaki, S. Nishijima and H. Sugiyama, Bioorg. Med. Chem., 2010, 18, 1236 CrossRef CAS PubMed.
- S. Park, T. Bando, K. Shinohara, S. Nishijima and H. Sugiyama, Bioconjugate Chem., 2011, 22, 120 CrossRef CAS PubMed.
- V. C. Rucker, S. Foister, C. Melander and P. B. Dervan, J. Am. Chem. Soc., 2003, 125, 1195 CrossRef CAS PubMed.
- E. J. Fechter, B. Olenyuk and P. B. Dervan, J. Am. Chem. Soc., 2005, 127, 16685 CrossRef CAS PubMed.
- T. Bando, J. Fujimoto, M. Minoshima, K. Shinohara, S. Sasaki, G. Kashiwazaki, M. Mizumura and H. Sugiyama, Bioorg. Med. Chem., 2007, 15, 6937 CrossRef CAS PubMed.
- J. Fujimoto, T. Bando, M. Minoshima, G. Kashiwazaki, S. Nishijima, K. Shinohara and H. Sugiyama, Bioorg. Med. Chem., 2008, 16, 9741 CrossRef CAS PubMed.
- T. Vaijayanthi, T. Bando, G. N. Pandian and H. Sugiyama, ChemBioChem, 2012, 13, 2170 CrossRef CAS PubMed.
- T. Vaijayanthi, T. Bando, K. Hashiya, G. N. Pandian and H. Sugiyama, Bioorg. Med. Chem., 2013, 21, 852 CrossRef CAS PubMed.
- K. Luger, Curr. Opin. Genet. Dev., 2003, 13, 127 CrossRef CAS PubMed.
- K. Ohta, A. Nicolas, M. Furuse, A. Nabetani, H. Ogawa and T. Shibata, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 646 CrossRef CAS.
- P. B. Becker and W. Horz, Annu. Rev. Biochem., 2002, 71, 247 CrossRef CAS PubMed.
- C. R. Clapier and B. R. Cairns, Annu. Rev. Biochem., 2009, 78, 273 CrossRef CAS PubMed.
- B. D. Price and A. D. D'Andrea, Cell, 2013, 152, 1344 CrossRef CAS PubMed.
- J. Gottesfeld, C. Melander, R. Suto, H. Raviol, K. Luger and P. Dervan, J. Mol. Biol., 2001, 309, 615 CrossRef CAS PubMed.
- R. K. Suto, R. S. Edayathumangalam, C. L. White, C. Melander, J. M. Gottesfeld, P. B. Dervan and K. Luger, J. Mol. Biol., 2003, 326, 371 CrossRef CAS PubMed.
- R. S. Edayathumangalam, P. Weyermann, J. M. Gottesfeld, P. B. Dervan and K. Luger, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 6864 CrossRef CAS PubMed.
- P. T. Lowary and J. Widom, J. Mol. Biol., 1998, 276, 19 CrossRef CAS PubMed.
- M. Minoshima, T. Bando, S. Sasaki, J. Fujimoto and H. Sugiyama, Nucleic Acids Res., 2008, 36, 2889 CrossRef CAS PubMed.
- Y. Tsunaka, J. Toga, H. Yamaguchi, S. Tate, S. Hirose and K. Morikawa, J. Biol. Chem., 2009, 284, 24610 CrossRef CAS PubMed.
- P. N. Dyer, R. S. Edayathumangalam, C. L. White, Y. Bao, S. Chakravarthy, U. M. Muthurajan and K. Luger, Methods Enzymol., 2004, 375, 23 CAS.
- W. Zhang, T. Bando and H. Sugiyama, J. Am. Chem. Soc., 2006, 128, 8766 CrossRef CAS PubMed.
- Y. W. Han, T. Matsumoto, H. Yokota, G. Kashiwazaki, H. Morinaga, K. Hashiya, T. Bando, Y. Harada and H. Sugiyama, Nucleic Acids Res., 2012, 40, 11510 CrossRef CAS PubMed.
- Y. W. Han, G. Kashiwazaki, H. Morinaga, T. Matsumoto, K. Hashiya, T. Bando, Y. Harada and H. Sugiyama, Bioorg. Med. Chem., 2013, 21, 5436 CrossRef CAS PubMed.
- P. Wu and L. Brand, Anal. Biochem., 1994, 218, 1 CrossRef CAS PubMed.
- J. G. Yang, T. S. Madrid, E. Sevastopoulos and G. J. Narlikar, Nat. Struct. Mol. Biol., 2006, 13, 1078 CAS.
- T. R. Blosser, J. G. Yang, M. D. Stone, G. J. Narlikar and X. Zhuang, Nature, 2009, 462, 1022 CrossRef CAS PubMed.
- M. G. Poirier, E. Oh, H. S. Tims and J. Widom, Nat. Struct. Mol. Biol., 2009, 16, 938 CAS.
- M. R. Duan and M. J. Smerdon, J. Biol. Chem., 2010, 285, 26295 CrossRef CAS PubMed.
- Y. J. Park, P. N. Dyer, D. J. Tremethick and K. Luger, J. Biol. Chem., 2004, 279, 24274 CrossRef CAS PubMed.
- K. S. Matthews, Microbiol. Rev., 1992, 56, 123 CAS.
- R. Schleif, Annu. Rev. Biochem., 1992, 61, 199 CrossRef CAS PubMed.
- R. Craigie and K. Mizuuchi, Cell, 1986, 45, 793 CrossRef CAS PubMed.
- K. Adzuma and K. Mizuuchi, Cell, 1989, 57, 41 CrossRef CAS PubMed.
- D. C. van Gent, K. Mizuuchi and M. Gellert, Science, 1996, 271, 1592 CAS.
- A. Engelman, K. Mizuuchi and R. Craigie, Cell, 1991, 67, 1211 CrossRef CAS PubMed.
- M. Heilemann, R. Kasper, P. Tinnefeld and M. Sauer, J. Am. Chem. Soc., 2006, 128, 16864 CrossRef CAS PubMed.
- A. L. Benvin, Y. Creeger, G. W. Fisher, B. Ballou, A. S. Waggoner and B. A. Armitage, J. Am. Chem. Soc., 2007, 129, 2025 CrossRef CAS PubMed.
- J. K. Hannestad, P. Sandin and B. Albinsson, J. Am. Chem. Soc., 2008, 130, 15889 CrossRef CAS PubMed.
- W. Su, M. Schuster, C. R. Bagshaw, U. Rant and G. A. Burley, Angew. Chem., Int. Ed., 2011, 50, 2712 CrossRef CAS PubMed.
- D. Vasudevan, E. Y. Chua and C. A. Davey, J. Mol. Biol., 2010, 403, 1 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |