Hydrogel-based bioassay sheets for in vitro evaluation of contraction-dependent metabolic regulation in skeletal muscle cells†
Kuniaki
Nagamine
ab,
Kohei
Okamoto
a,
Shingo
Otani
a,
Hirokazu
Kaji
ab,
Makoto
Kanzaki
bc and
Matsuhiko
Nishizawa
*ab
aDepartment of Bioengineering and Robotics, Graduate School of Engineering, Tohoku University, 6-6-01 Aramaki, Aoba-ku, Sendai 980-8579, Japan. E-mail: nishizawa@biomems.mech.tohoku.ac.jp
bJST, CREST, Sanbancho, Chiyoda-ku, Tokyo 102-0075, Japan
cDepartment of Biomedical Engineering, Graduate School of Biomedical Engineering, Tohoku University, Biomedical BLD, 2-1 Seiryo-machi, Aoba-ku, Sendai 980-8575, Japan
First published on 24th October 2013
Abstract
Two types of hydrogel-based bioassay sheets were developed for in vitro evaluation of contraction-dependent metabolic regulation in skeletal muscle cells: one is an oxygen sensor sheet and the other is an immunocapture sheet for a myokine, interleukin-6 (IL-6). These soft, molecularly permeable hydrogel-based bioassay sheets were directly laminated to another hydrogel on which myotubes were micropatterned, and displayed usefully measurable changes in local oxygen consumption and IL-6 secretion of myotubes upon electrically-induced contraction.
Introduction
In vitro bioassay systems with cultured cells have been developed as an alternative to whole animal experiments for molecular and cellular physiological analysis. Skeletal muscle cells are one of the important types of cells used to study the complex mechanisms of type 2 diabetes because the disease is associated with a disorder of insulin- or exercise-induced glycemic control in skeletal muscles. In addition, recent lines of evidence have suggested that the skeletal muscle functions as an endocrine organ that secretes a number of cytokines (myokines) in response to exercise in order to regulate the physiological activities of the whole body including glucose metabolism of the muscle itself.1–3 To examine the regulation of cellular metabolic activity, the compounds secreted (or consumed) by the cells have been analyzed in vitro. However, most conventional bioassays have been designed to evaluate the average secretion (or consumption) activity of randomly cultured myotubes by analyzing the concentration of these compounds in a bulk medium.Cell micropatterning techniques enable site-specific physiological analysis,4–6 which has been difficult to achieve using conventional random culture-based assays. In previous work, we have developed hydrogel-supported micropatterns of aligned skeletal muscle cells,7,8 and demonstrated spatiotemporally controlled contraction-induced GLUT4 translocation by combining them with microelectrodes to stimulate a selected part of the patterned muscle cells.8 The benefits of the micropatterning approach will be further increased if site-specific analysis functions could be integrated for in situ assay of cellular metabolic activity. In recent years, 2D fluorescent imaging sensor sheets have been actively developed.9 Wolfbeis et al. demonstrated the applicability of the oxygen-responsive sheet for wound healing assays in vivo.10
In the present work, we prepared two types of hydrogel-based bioassay sheets: one was an oxygen sensor sheet embedding oxygen-responsive phosphorescent beads; the other was an immunocapture sheet functionalized with anti-IL-6 antibody-modified beads for in situ assay of IL-6. Oxygen consumption and IL-6 secretion are typical markers of muscular metabolic activity. The hydrogel-based bioassay sheet is soft enough for direct lamination to contracting myotubes on a fibrin gel. Owing to the molecular permeability of the hydrogel, continuous cultivation with the laminated bioassay sheet is possible. A monolayer of functional microbeads was immobilized on the surface of the hydrogel to be positioned in the vicinity of the cells for local detection of consumed oxygen or secreted IL-6.
Experimental section
Preparation of an oxygen sensor hydrogel sheet
An oxygen-responsive phosphorescent dye, platinum octaethylporphyrin (PtOEP, Sigma-Aldrich), was encapsulated in a polystyrene microbead (5 μm diameter, Micromod) as described in our previous report.11 The beads were dispersed in a polyacrylamide prepolymer solution composed of 5 wt% acrylamide (Wako Pure Chemical Industries) as a monomer, 0.24 wt% N,N-methylene-bis-acrylamide (Wako Pure Chemical Industries) as a crosslinker, and 1 mg mL−1 Irgacure 2959 (BASF Japan Ltd.) as a photo-initiator. The bead suspension was poured into a silicone rubber chamber (1 mm thickness) attached to a glass substrate and the chamber was left undisturbed for 4 h at room temperature to allow the beads to settle on the substrate (Fig. 1a). Then, UV irradiation was employed to polymerize the prepolymer solution, resulting in encapsulation of the beads in the gel sheet (Fig. 1b). The resulting sensor gel was washed five times with distilled water to remove unreacted monomers. The sensor gel was stored in the electrical pulse stimulation (EPS) medium at 37 °C under a 5% CO2 atmosphere for 1 day to replace PBS in the gel by the medium. The EPS medium was composed of DMEM containing 2% calf serum (Gibco), 1% MEM amino acids solution (Gibco), 1% MEM non-essential amino acids solution (Gibco), 100 units mL−1 penicillin, and 100 μg mL−1 streptomycin (Gibco).Imaging of oxygen concentration gradient around myotubes
A band pattern of aligned C2C12 myotubes on a conventional culture dish were transferred onto a fibrin gel containing the EPS medium as described in our previous report.7,8 The oxygen sensor gel was laminated on the myotube/fibrin gel (Fig. 1c) and the resulting assembly was immobilized in a carbon electrode chamber (C-Pace 100, IonOptix) with fibrin glue (15 mg mL−1 fibrinogen (Sigma-Aldrich), 10 units mL−1 thrombin (Sigma-Aldrich) dissolved in the EPS medium) (Fig. 1d). The sensor beads were embedded within 50 μm of the surface (from the cells), as assessed from the defocusing Z distance using a confocal microscope (Fig. 1e). Pre-exercise using periodic pulses (0.7 V mm−1 amplitude, 1 Hz frequency, 2 ms duration) was performed for 4 days in the EPS medium to induce maximum cellular contraction.7 Then, various conditions of electric pulses were applied to the cells for an additional 3 h followed by observation of phosphorescent images by a microscope. The beads were excited at 520–550 nm using a mercury lamp with a bandpass filter, and a broad phosphorescence emission was observed at around 650 nm. We conducted most of the experiments, including the electrical stimulation, in a 5% CO2 incubator at 37 °C. Only the occasional microscope observations (within 1 min) were conducted outside the incubator. In the absence of the cell sheets, the phosphorescence of the sensor sheet did not show change even during continuous application of the electrical pulses (amplitude, 0.7 V mm−1; duration, 2 ms; frequency, 1 Hz or 10 Hz (1 s train, 9 s interval)) for 5 h, suggesting that the oxygen concentration in a bulk medium was not significantly changed by the electrochemical reactions (reduction of oxygen and hydrolysis of the medium) at the stimulation carbon electrodes.The phosphorescent intensity I was converted to oxygen concentration using the Stern–Volmer relationship,12I0/I = 1 + Ksv [O2], where I0 is the intensity in the absence of oxygen, and Ksv is the Stern–Volmer constant. Two-point calibration at oxygen concentrations of 0 mM (N2-saturated condition) and 0.22 mM (air-saturated condition)13 was carried out to determine Ksv as 4.0 × 10−3 μM−1.
Preparation of an immunocapture hydrogel sheet
Amino-polystyrene microbeads (5 μm diameter, Micromod) were treated with 8% glutaraldehyde dissolved in PBS (pH 7.0) for 4 h at room temperature. The suspension of the glutaraldehyde-modified beads was poured onto a glass substrate with a silicone rubber chamber and evaporated overnight. After peeling the chamber off, 5 wt% polyacrylamide gel sheet (10 mm × 10 mm × 0.1 mm) was put on the bedded beads and incubated overnight at room temperature to obtain covalent immobilization of the beads on the polyacrylamide gel via cross-linking with glutaraldehyde (Fig. 1a and 1b). Then, 0.1 mg mL−1 of anti-mouse IL-6 antibody (clone: MP5-20F3, eBioscience) was poured onto the beads-immobilized gel and reacted for 1 h at 37 °C. Finally, the gel sheet was immersed in a blocking solution (5 vol% calf serum in PBS) for 1 h at room temperature to prevent nonspecific adsorption during the immunoassay. The number of antibody molecules immobilized on a bead was found to be ca. 1 × 106 (ESI Fig. S1†) which is more than enough to capture all the IL-6 secreted from a myotube during 12 h of contraction (2 × 105 molecules per myotube).14 The resulting gel sheet was immersed in a serum-free DMEM at 37 °C under a 5% CO2 atmosphere for 1 day to replace PBS in the gel by the medium.Mapping of IL-6 secretion from myotubes
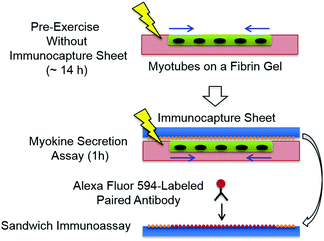
The myotube/fibrin gel was placed in a carbon electrode chamber and was immobilized with fibrin glue. As illustrated in Fig. 2, periodic pulses (amplitude, 0.7 V mm−1; frequency, 1 Hz; duration, 2 ms) were applied for an arbitrary time as a pre-exercise without the immunocapture sheet in the EPS medium at 37 °C maintained by a 5% CO2 incubator. Then, the immunocapture sheet was placed on the myotube/fibrin gel and the assembly was lightly fixed by applying a small amount of fibrin glue to its outer periphery, followed by in situ capture of the IL-6 secretion during an additional 1 h of twitch contraction at 37 °C under a 5% CO2 atmosphere. After the assay time, the immunocapture sheet can be peeled from the myotube/fibrin gel because a polyacrylamide hydrogel does not chemically adhere to a fibrin gel. The immunocapture sheet was treated with a biotin-conjugated anti-mouse IL-6 antibody (clone: MP5-32C11, eBioscience) for a paired-antibody sandwich immunoassay overnight at 4 °C. Finally, Alexa Fluor 594-conjugated streptavidin (Invitrogen) was bound to the biotinylated antibody and observed using a LSM 700 laser scanning fluorescent microscope (Carl Zeiss MicroImaging Co., Ltd). In the assay using a fibrin gel with a random myotube monolayer (monolayer area, 1 cm2), the fluorescent intensity is used to produce an average intensity of the sensor beads in a given microscopic field (340 × 450 μm2).Results and discussion
Oxygen consumption assay
Fig. 3a shows an optical micrograph of an assembly composed of a myotube band pattern, indicated by white dotted lines (250 μm width), and a sensor sheet embedding oxygen-responsive PtOEP beads, whose phosphorescent intensity shows an inverse relationship with the oxygen concentration.11 It is worthwhile to note that the phosphorescent signal is not interfered by the pH change in the range from 3 to 9 (ESI Fig. S2†), even though acidic compounds such as lactic acid are secreted during the contraction of myotubes. The sensor beads on the gel showed motion synchronized with the directed contraction of the aligned myotubes, as shown in ESI Movie S1,† proving that the soft hydrogel-based bioassay sheet supports the myotube contraction.15Fig. 3b shows the phosphorescent image around the resting myotubes without stimulation and its intensity profile along the cross section a–b of the image. Importantly, the phosphorescent response reversibly appeared only when the sensor gel was put on the cells, proving that the images are caused by cellular respiration. The profile was almost constant for an additional 2 h of resting time, suggesting that the hemicylindrical diffusion of oxygen toward the cellular band pattern was at a steady-state. Then, the oxygen consumption rate (F) of the myotube band pattern (250 μm width, 3 mm length) can be assumed to be expressed by F = πrxLDΔCx,16 where rx is the distance from the cells, L is the length of the myotube band patern, and D is the diffusion coefficient of oxygen in a hydrogel (D = 8 × 10−6 [cm2 s−1]).17 ΔCx is the concentration gradient of oxygen at rx, and can be calculated from the phosphorescent intensity using the Stern–Volmer relationship (see the Experimental section).12 We used the concentration gradient in the vicinity (within 1 mm) of the cell patterns because the ideal profile of the diffusion layer would be disturbed with increasing the distance from the cell patterns. The resulting F value per cellular unit area was ca. 1.7 × 10−13 mol s−1 mm−2 (area of the pattern: 0.75 mm2), the value being in agreement with that previously reported for the resting myotubes cultured in a small sealed well using a Flux Analyzer, ca. 1.1 × 10−13 mol s−1 mm−2 (500 pmol min−1 for 75 mm2 culture).18
The oxygen consumption rate during electrically-induced contractions was measured in the same way as above. Fig. 3c shows the results for the myotubes with twitch (1 Hz frequency, 2 ms duration) and tetanic contraction (10 Hz frequency, 2 ms duration, 1 s train, 9 s interval). The phosphorescent intensity profile was at steady state even during contraction experiments. The oxygen consumption rate of myotubes under both types of contractions (twitch and tetanus) was ca. 2.4 × 10−13 mol s−1, which was an increase above that of the resting cells by about 2-fold. This increase indicates that the contracting myotubes produce more ATP for continuous contraction through the process of oxidative phosphorylation in mitochondria than that of the resting cells. The harsh tetanic contraction showed a similar oxygen consumption rate to that of twitch contraction, probably because of the long interval between the contractions. The previous studies using skeletal muscle fibers19,20 revealed that a long interval switches the ATP synthesis process from oxidative phosphorylation in mitochondria to an oxygen-independent pathway such as phosphocreatinine hydrolysis. Therefore, the cellular oxygen requirement in the tetanic contracting cells would be decreased to the same level as the twitching cells. Unfortunately, the tetanic contraction with a shorter interval (1 s) cannot be studied by our system because such a harsher contraction did not continue for more than 1 h. To our knowledge, the present data are the first to quantify the oxygen consumption rate of myotubes during exercise. It was possible to make a quantitative measurement of the oxygen concentration gradient around the contracting myotubes because of the relatively slow response of the sensor beads (6 s for 100% response).11 Additionally, the reversibility of the sensor response will allow longer-term monitoring of oxygen consumption activity of the cells.
Myokine secretion assay
In addition to oxygen consumption, the secretion of myokines such as IL-6 is another typical marker of muscular metabolic activity. We studied the effect of pre-exercise time on the ensuing IL-6 secretion activity detected using the immunocapture sheet. Note that the assay time is equally 1 h for each experiment. Fig. 4a shows that the fluorescent intensity (amount of secreted IL-6 during 1 h of assay time) clearly depends on the length of pre-exercise time; the IL-6 secretion activity after 14 h of pre-exercise approached 2-fold above the case without pre-exercise. These IL-6 secretion tendencies of myotubes agree with the previous batch experiment using a conventional ELISA kit,21 in which a myotube monolayer twitching on a dish showed a gradual increase in IL-6 secretion depending on the period of pre-exercise. For the quantitative analysis of molecular flux of IL-6 secretion, the assay system requires improvements in the sensitivity.Fig. 4b shows a photograph of myotube pattern assembly and the immunocapture sheet, and a typical fluorescent image after the detection of IL-6 secreted from the patterned myotubes pre-exercised for 14 h. As can be seen, the immunocapture sheet displays the stripe patterns of fluorescence corresponding to the myotube band, suggesting the possible in situ mapping of local IL-6 secretion from the contracting myotubes. Since the monolayer of immunobeads was set close to the cells by a direct lamination of the gel sheet, the beads on the cells can capture the secreted IL-6 most effectively, increasing the contrast of images. For the longer experiment over 2 h, the fluorescence images tended to show diffusive spreading. The local stimulation (actuation) of a focused myotube band pattern using a microelectrode array8 will allow a more detailed, quantitative analysis of local myokine secretion.
Conclusions
In this study, we have demonstrated in vitro evaluation of local oxygen consumption and IL-6 secretion activity of contractile myotubes on a hydrogel sheet for the first time by direct lamination of a hydrogel-based bioassay sheet. Specifically, the oxygen sensor sheet enabled the quantitative measurement of contraction-dependent change in the oxygen consumption rate of the myotubes. This bioassay system will allow the study of IL-6-dependent glycemic control via mitochondrial energy production in exercised skeletal muscle cells1–3 and will help us to understand the mechanism of exercise therapy for type-2 diabetes.Notes and references
- B. K. Pedersen and M. A. Febbraio, Nat. Rev. Endocrinol., 2012, 8, 457–465 CrossRef CAS PubMed.
- W. Aoi and K. Sakuma, BioDiscovery, 2013, 7, 2–9 Search PubMed.
- B. K. Pedersen, J. Exp. Biol., 2011, 214, 337–346 CrossRef CAS PubMed.
- A. M. Ghaemmaghami, M. J. Hancock, H. Harrington, H. Kaji and A. Khademhosseini, Drug Discovery Today, 2012, 17, 173–181 CrossRef CAS PubMed.
- A. Tourovskaia, N. Z. Li and A. Folch, Biophys. J., 2008, 95, 3009–3016 CrossRef CAS PubMed.
- M. S. Sakar, D. Neal, T. Boudou, M. A. Borochin, Y. Q. Li, R. Weiss, R. D. Kamm, C. S. Chen and H. H. Asada, Lab Chip, 2012, 12, 4976–4985 RSC.
- K. Nagamine, T. Kawashima, T. Ishibashi, H. Kaji, M. Kanzaki and M. Nishizawa, Biotechnol. Bioeng., 2010, 105, 1161–1167 CAS.
- K. Nagamine, T. Kawashima, S. Sekine, Y. Ido, M. Kanzaki and M. Nishizawa, Lab Chip, 2011, 11, 513–517 RSC.
- M. I. J. Stich, L. H. Fischer and O. S. Wolfbeis, Chem. Soc. Rev., 2010, 39, 3102–3114 RSC.
- R. J. Meier, S. Schreml, X. D. Wang, M. Landthaler, P. Babilas and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2011, 50, 10893–10896 CrossRef CAS PubMed.
- K. Nagamine, S. Ito, M. Takeda, S. Otani and M. Nishizawa, Electrochemistry, 2012, 80, 318–320 CrossRef CAS PubMed.
- K. Montagne, K. Komori, F. Yang, T. Tatsuma, T. Fujii and Y. Sakai, Photochem. Photobiol. Sci., 2009, 8, 1529–1533 CAS.
- M. Nishizawa, K. Takoh and T. Matsue, Langmuir, 2002, 18, 3645–3649 CrossRef CAS.
- T. Nedachi, H. Fujita and M. Kanzaki, Am. J. Physiol. Endocrinol. Metab., 2008, 295, E1191–E1204 CrossRef CAS PubMed.
- S. Sekine, Y. Ido, T. Miyake, K. Nagamine and M. Nishizawa, J. Am. Chem. Soc., 2010, 132, 13174–13175 CrossRef CAS PubMed.
- T. Saito, C.-C. Wu, H. Shiku, T. Yasukawa, M. Yokoo, T. Ito-Sasaki, H. Abe, H. Hoshi and T. Matsue, Analyst, 2006, 131, 1006–1011 RSC.
- S. J. Hepworth, M. O. Leach and S. J. Doran, Phys. Med. Biol., 1999, 44, 1875–1884 CrossRef CAS.
- L. Ye, B. Varamini, D. W. Lamming, D. M. Sabatini and J. A. Baur, Front. Genet., 2012, 3, 177–186 CrossRef PubMed.
- D. A. Hood, J. Gorski and R. L. Terjung, Am. J. Physiol. Endocrinol. Metab., 1986, 250, E449–E456 CAS.
- R. A. Howlett, C. A. Kindig and M. C. Hogan, J. Appl. Physiol., 2007, 102, 1456–1461 CrossRef PubMed.
- A. Farmawati, Y. Kitajima, T. Nedachi, M. Sato, M. Kanzaki and R. Nagatomi, Endocrinol. J., 2013, 60, 137–147 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Quantitative analysis of the amount of antibody on a bead, performance of O2-responsive beads at various pH conditions, and a movie of synchronous contraction of a myotube band pattern with O2-responsive beads. See DOI: 10.1039/c3bm60179j |
| This journal is © The Royal Society of Chemistry 2014 |