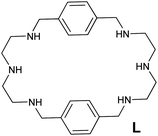
Visual colorimetric detection of TNT and 2,4-DNT using as-prepared hexaazamacrocycle-capped gold nanoparticles†
Manikkavalli
Mohan
and
Dillip Kumar
Chand
*
Department of Chemistry, Indian Institute of Technology Madras, Chennai-600036, India. E-mail: dillip@iitm.ac.in; Fax: +91-44-2257-4202; Tel: +91-44-2257-4224
First published on 22nd October 2013
Abstract
The dual role of a hexaazamacrocyclic ligand L in the effective reduction of Au3+ to Au0 and stabilization of the ensuing gold nanoparticles (AuNPs) is successfully demonstrated. The molar ratio of ligand L to Au3+ is found to be very important for the effective stabilization of the AuNPs. The as-prepared amine-capped AuNPs in the instant visual colorimetric detection of the TNT and 2,4-DNT under easy-to-adopt conditions is established. The naked eye detection of the analytes with an instant color change at concentrations as low as 476 fM is observed. Colorimetric analysis supported the detection of TNT and 2,4-DNT using the nanosensor with the LOD of 1.55 × 10−13 ± 2.02 × 10−13 M and 3.79 × 10−13 ± 3.65 × 10−13 M respectively.
Introduction
Gold nanoparticles (AuNPs) play vital roles in several research areas including the study of chemosensors,1 biosensors,2–4 cancer therapy,5–7 and optoelectronics.8 Optical properties like SPR, SERS and luminescence are monitored to comprehend the above-mentioned applications of AuNPs. These optical properties are understandably dependent on the size as well as the shape of the particles. Various parameters such as the temperature,9 pH,10 molar fraction of precursor to reducing agent11 and stabilizer10a,12 are known to modulate the size and shape of the AuNPs. Thiols are traditionally used as stabilizers during the synthesis of AuNPs.10a,12,13 However, the use of amines as stabilizers has been a recent trend. Amines could display bifunctional character in the synthesis of AuNPs by offering a dual role, i.e. as a reducing and stabilizing agent.14 Blanchard's group has studied the reduction of Au3+ to Au0 using a variety of amines as a reducing agent. It is found that amines with a reduction potential within the range of oxidation potential of Au0 to Au+ and the reduction potential of Au3+ to Au0 are suitable for the required efficient reduction of Au3+ in the formation of AuNPs.15 They have also reported the use of poly(allylamine) hydrochloride as a reducing agent in the size-controlled formation of AuNPs. The size control could be achieved by varying the ratio of PAH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HAuCl4, wherein each of the nitrogen centres functions as a one-electron donor in the reduction of Au3+ to Au0.12b
HAuCl4, wherein each of the nitrogen centres functions as a one-electron donor in the reduction of Au3+ to Au0.12b
Detection of the commonly used secondary explosives like TNT and 2,4-DNT when present in a given sample should be possible by employing suitable amine-stabilized AuNPs. Nitro derivatives are known to act as an acceptor and form Meisenheimer complexes when combined with donors such as amines.16 Thus, amine-modified AuNPs are potential candidates to study their applications as a sensor for the detection of TNT and 2,4-DNT. The interactions of donor amine groups (present in amine-functionalized AuNPs) with the acceptor nitro-compounds (that are employed as analytes) promote the aggregation of NPs. The concomitant aggregation of amine-functionalized AuNPs when exposed to nitro derivatives is actually perceived by various techniques like colorimetry,1a fluorimetry,1b,17 SERS,18 DLS,19 electrochemical methods20 and miscellaneous methods.21 Among these the colorimetric technique is considered as the least expensive and most convenient to execute and hence is suitable for application purposes. For instance, aggregation of cysteamine-modified AuNPs in the presence of TNT is reported whereupon TNT is detected colorimetrically.1a
As per previous reports, a variety of AuNPs are prepared via citrate reduction in the first step and then functionalized with amines in the next step for their use in the detection of nitro derivatives.1,18a,b,19,20b,22 In the first step, AuNPs are prepared by reducing Au3+ using an external reducing agent and an appropriate stabilizer. The AuNPs so prepared are further modified with suitable amines in a subsequent step and then used as sensors of compatible nitro derivatives. It should be possible to prepare AuNPs by using the chosen amines as stabilizers so that no post-modification is warranted. In case the chosen amine is also capable of reducing the metal ion, the use of an external reducing agent could be avoided, and thus possible contaminations are curtailed. Keeping in mind the above detail, we have used a hexaazamacrocyclic ligand L that displays a dual role of reducing and stabilizing agent during the synthesis of a new class of amine-stabilized AuNPs. The synthesis of the AuNPs could be accomplished under mild reaction conditions. Since non-functionalized amine-capped AuNPs synthesized in a one-pot reaction are utilized as a sensor, it not only reduces the number of steps in the nanosensor synthesis, but also possible sensor contaminants such as citrate or other stabilizer and reducing agents usually used prior to the functionalization step could be avoided. This new system has been successfully used in the present work for the naked-eye and colorimetric detection of TNT and 2,4-DNT under neat and easy-to-adopt conditions, even at the femtomolar level.
Experimental
Materials and characterization techniques
The hexaazamacrocyclic ligand L was prepared following a literature method.23 The metal salt HAuCl4·3H2O (99.98%) was purchased from Aldrich. UV-visible spectral studies were performed using a JASCO V-650 instrument. TEM images were recorded using a Philips CM12 TEM with (EDS) detector. Powder XRD patterns were obtained using a Bruker D8 Advance instrument.Procedure
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 0.5
1; 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1
1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 2
1; 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In all experiments the final concentration of Au was kept at 1 mM. In a typical synthesis an aliquot of 2 mL of 5 mM aqueous solution of Au3+ was added to the required volume of the solution of L in such a manner that the total volume and ratios of H2O
1. In all experiments the final concentration of Au was kept at 1 mM. In a typical synthesis an aliquot of 2 mL of 5 mM aqueous solution of Au3+ was added to the required volume of the solution of L in such a manner that the total volume and ratios of H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH were maintained as 5 mL and 1
MeOH were maintained as 5 mL and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. The reaction mixtures were allowed to stand at room temperature for 2 weeks to ensure complete growth. Among the above trials, 2 or 3 equivalents of the ligand L appears to be required for the complete reduction and stabilization of the AuNPs. When 1 equivalent of ligand L was used, although complete reduction was observed the NPs formed were deposited. We found that a minimum of 2 equivalents of ligand L is required for stabilization of the AuNPs.
1, respectively. The reaction mixtures were allowed to stand at room temperature for 2 weeks to ensure complete growth. Among the above trials, 2 or 3 equivalents of the ligand L appears to be required for the complete reduction and stabilization of the AuNPs. When 1 equivalent of ligand L was used, although complete reduction was observed the NPs formed were deposited. We found that a minimum of 2 equivalents of ligand L is required for stabilization of the AuNPs.
The preparation of AuNPs was also attempted at a low concentration of Au (e.g. 0.1 mM) by retaining the optimized ratio of ligand to metal, i.e. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. At this low concentration of Au, which is lower than the above considered 1 mM, the reaction mixture was heated at 50–60 °C for 16 h to ensure complete reduction. Further, the mixture was allowed to stand for 2 weeks to ensure complete growth.
1. At this low concentration of Au, which is lower than the above considered 1 mM, the reaction mixture was heated at 50–60 °C for 16 h to ensure complete reduction. Further, the mixture was allowed to stand for 2 weeks to ensure complete growth.
Similar experiments were also conducted using AuNPs prepared at a lower concentration of Au. To 3 mL of AuNPs (0.1 mM in Au), 0.1 mL of the required concentration of TNT and 2,4-DNT was added whereupon the concentration of the nitro-compounds in the final solution remained in the range of 47.6 μM to 476 fM. Samples were monitored for visual colour change. UV-vis spectra were recorded for each sample.
To 3 mL of the as-prepared AuNPs (0.1 mM Au), 0.1 mL of 1 mM aqueous solutions of LiCl, NaCl, KCl, MgCl2, CaCl2, MnCl2, CuCl2, FeCl2, NiCl2 and ZnCl2 were added separately in batches. UV-vis spectra were recorded for each sample.
Results and discussion
Recently, we reported the use of a hexaazamacrocyclic ligand as the sensing agent of Ag+via silver nanoparticle formation.14j In continuation of our efforts, herein we synthesized gold nanoparticles (AuNPs) using the hexaazamacrocycle, L under mild conditions and utilized the as-prepared AuNPs in the visual colorimetric detection of TNT and 2,4-DNT selectively up to the femtomolar level.In the synthesis of the AuNPs, the molar ratio of L to Au3+ is found to influence the feasibilities of reduction of the Au3+ and also stabilization of the ensuing NPs. During the synthesis of the AuNPs, the molar ratio of L to Au3+ was varied by increasing the concentration of L with respect to Au3+ ion in the samples. In the case of molar ratio values less than 2 it was observed that the solutions were turbid with a yellowish colouration. This suggests incomplete reduction of Au3+ to Au+ and Au0 due to shortage of the ligand which resulted in the yellow colouration. The stabilization of the AuNPs is also hampered due to the shortage of the ligand, and nucleated particles began to be deposited at the walls of the vial. When the molar ratio of L to the metal salt in a sample is sufficiently high, such as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or higher, the solutions were supersaturated with the Au0 nucleated particles leading to the formation of stable AuNPs. Thus the formation of stable AuNPs is facilitated in such situations and the colour changed from yellow to yellowish orange instantly and then slowly it turned to red over time. The colour change from yellow to red is attributed to the formation of spherical AuNPs.
1 or higher, the solutions were supersaturated with the Au0 nucleated particles leading to the formation of stable AuNPs. Thus the formation of stable AuNPs is facilitated in such situations and the colour changed from yellow to yellowish orange instantly and then slowly it turned to red over time. The colour change from yellow to red is attributed to the formation of spherical AuNPs.
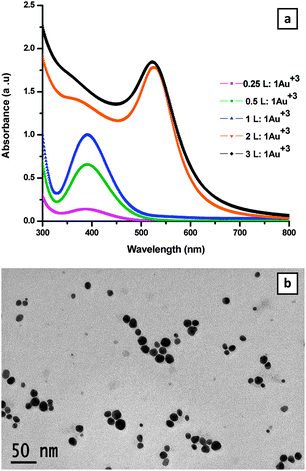
UV-visible spectra were recorded for each sample with different molar ratios as shown in Fig. 1a. The spectra showed SPR peaks at 523 nm for the samples obtained from 2L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1Au3+ and 3L
1Au3+ and 3L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1Au3+ solutions. The SPR peaks confirm the formation of AuNPs. The as-prepared AuNPs are found to have excellent stability in the solution state for more than a year. At lower molar ratios, no peak was observed at the 525 nm region; however, at 400 nm a broad peak was seen probably due to incomplete reduction, the small size of AuNPs or formation of a coordination complex with Au+. The influence of the increase of temperature for the sample at a molar ratio below 2 for L
1Au3+ solutions. The SPR peaks confirm the formation of AuNPs. The as-prepared AuNPs are found to have excellent stability in the solution state for more than a year. At lower molar ratios, no peak was observed at the 525 nm region; however, at 400 nm a broad peak was seen probably due to incomplete reduction, the small size of AuNPs or formation of a coordination complex with Au+. The influence of the increase of temperature for the sample at a molar ratio below 2 for L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au3+ was probed to check whether or not it is possible to drive the reactions favorably, but there was no improvement. This study suggested that a 2
Au3+ was probed to check whether or not it is possible to drive the reactions favorably, but there was no improvement. This study suggested that a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio is required for the effective preparation and stabilization of the AuNPs.
1 molar ratio is required for the effective preparation and stabilization of the AuNPs.
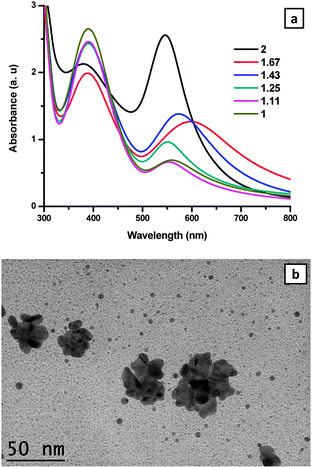
The attempts to prepare AuNPs in the range of 1–2 for the molar ratio of L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au3+, i.e. 1, 1.11, 1.25, 1.43, 1.67 and 2, resulted in the successful stabilization of AuNPs only when the ratio is 2. A colour change was observed from orange to red after 1 day for the case of the molar ratio 2
Au3+, i.e. 1, 1.11, 1.25, 1.43, 1.67 and 2, resulted in the successful stabilization of AuNPs only when the ratio is 2. A colour change was observed from orange to red after 1 day for the case of the molar ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and as the time evolved the colour of the solution became intense. UV-vis spectra were recorded for each sample after 3 days, and an intense peak at 523 nm was observed for samples containing L
1 and as the time evolved the colour of the solution became intense. UV-vis spectra were recorded for each sample after 3 days, and an intense peak at 523 nm was observed for samples containing L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au3+ of 2
Au3+ of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The AuNPs prepared in less than 2
1. The AuNPs prepared in less than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios formed flocs and the UV-vis spectra showed a broad peak in the longer wavelength region due to the unstable particles leading to flocculation/aggregation as shown in Fig. 2a.
1 molar ratios formed flocs and the UV-vis spectra showed a broad peak in the longer wavelength region due to the unstable particles leading to flocculation/aggregation as shown in Fig. 2a.
The TEM image of L-stabilized AuNPs prepared at the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was recorded after aging the sample for 2 weeks and is shown in Fig. 1b. The average size of the AuNPs was found to be 13.06 ± 3.46 nm. However, the TEM image taken for the attempted preparation of AuNPs at 1
1 ratio was recorded after aging the sample for 2 weeks and is shown in Fig. 1b. The average size of the AuNPs was found to be 13.06 ± 3.46 nm. However, the TEM image taken for the attempted preparation of AuNPs at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of L
1 molar ratio of L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au3+ showed flocculated/aggregated AuNPs as shown in Fig. 2b.
Au3+ showed flocculated/aggregated AuNPs as shown in Fig. 2b.
Powder XRD of the well stabilized AuNPs prepared at the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio showed face-centred cubic lattice planes of Au with 2θ values at 38.219°, 44.396°, 64.666°, 77.665° and 81.882° as shown in Fig. 3 corresponding to the (111), (200), (220), (311) and (222) planes. The 2θ values are comparable with the data reported in JCPDS 89-3697.
1 ratio showed face-centred cubic lattice planes of Au with 2θ values at 38.219°, 44.396°, 64.666°, 77.665° and 81.882° as shown in Fig. 3 corresponding to the (111), (200), (220), (311) and (222) planes. The 2θ values are comparable with the data reported in JCPDS 89-3697.
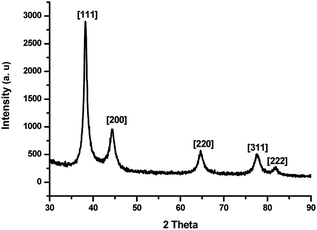 | ||
Fig. 3 Powder XRD patterns of AuNPs prepared by using the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of ligand to metal (1 mM of Au3+ was taken) after aging the AuNPs for 2 weeks. 1 molar ratio of ligand to metal (1 mM of Au3+ was taken) after aging the AuNPs for 2 weeks. | ||
The colour of the AuNPs changed spontaneously from red to blue when combined with TNT or 2,4-DNT. In addition to the SPR peak of the AuNPs another broad peak was observed at around 600 nm in both cases as shown in Fig. 4. The presence of the peak around 600 nm is a clear indication of the aggregation of AuNPs. This aggregation is attributed to Meisenheimer complex formation between the amine groups present at the surface of the AuNPs and TNT or 2,4-DNT that is used as the analyte.18a Picric acid and 3,4-DNT are analogues of TNT and 2,4-DNT, respectively. However, both of these nitro-compounds did not show any immediate colour change where the SPR of AuNPs also remained unchanged as shown in Fig. 4. In the case of picric acid, when sufficient time is provided in the order of 10–12 h then a visible colour change from red to purple was observed. The reason for this slow reactivity of picric acid may be attributed to the less electron-deficient nature of its π system compared to that of TNT, and also it can form salts with the amine – this salt formation can be evidenced from the peak observed in the 360 nm region. However, in the case of 3,4-DNT the colour of AuNPs remained unchanged even after a long reaction time. It is a well-known fact that 3,4-DNT is less electron-deficient when compared with 2,4-DNT. This could account for the behaviour of 3,4-DNT as a poor acceptor in detection.
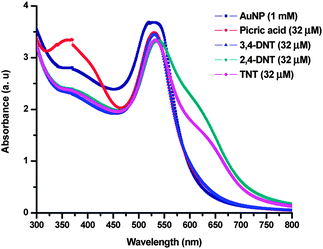 | ||
| Fig. 4 UV-vis spectra of (1 mM) AuNPs before and after addition of picric acid, 3,4-DNT, 2,4-DNT and TNT. The spectra were recorded immediately. | ||
To investigate the selectivity for nitro-compounds, we screened some other derivatives such as nitrobenzene, nitrotoluene, o-nitrophenol, and p-nitrophenol for the detection. While TNT and 2,4-DNT were detected spontaneously, picric acid required a longer time for the detection. The other compounds were not detected as concluded from the UV-vis studies, see Fig. S1, ESI.†
To further support the argument, TEM characterizations were made for the sample in the absence and presence of nitro-compounds. In the case of TNT, or 2,4-DNT, TEM samples were prepared immediately after the addition of TNT and 2,4-DNT to the AuNPs and the solvent was removed by touching the grid gently with tissue paper. In the case of the sample containing picric acid the TEM sample was prepared 1 day after the addition of picric acid to the as-prepared AuNPs.
The TEM image of the as-prepared AuNPs (prepared from 0.1 mM HAuCl4) showed complete dispersion of AuNP particles, as shown in Fig. 5a. The average size of the AuNPs was found to be 11.47 ± 2.62 nm. The TEM image of the AuNPs after the addition of TNT/2,4-DNT showed the aggregation of particles with no significant increase in the sizes of the particles. The images of samples containing 2,4-DNT, TNT and picric acid are shown in Fig. 5b–d. The results strongly suggest that the colour change observed was only due to aggregation and not because of any particle growth that can occur by coalescence of unprotected particles. Hence, the amine ligand is found to be proficient for capping the AuNPs strongly, even after the addition of TNT or 2,4-DNT. The aggregation is attributed to the formation of Meisenheimer complexes.
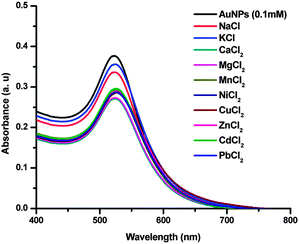
The possibility of interference from the metal ions of alkali, alkaline and transition series was probed by adding their salts to the AuNPs. The samples of LiCl, NaCl, KCl, MgCl2, CaCl2, MnCl2, CuCl2, FeCl2, NiCl2 and ZnCl2 did not show any changes in the peak positions in the UV-vis spectra as shown in Fig. 6, where the concentration of Au in the samples was 0.1 mM and with the final concentration of the analyte metal salts at 32 μM.
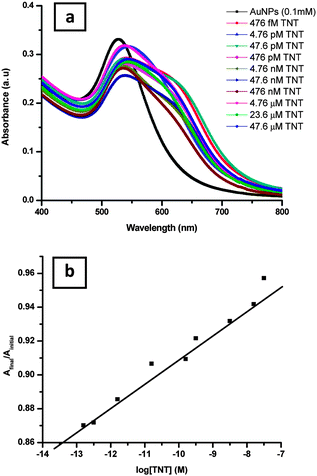
To further assess the sensitivity of the simple colorimetric assay of the nitro-compounds, a batch of experiments was carried out at various lower concentrations of the nitro-compounds. Wherein, after the addition of various concentrations of TNT or 2,4-DNT, ranging from 476 fM to 47.6 μM, to the as-prepared AuNPs, an immediate colour changed from red to blue was observed. The observed change in the colour of the samples is due to aggregation of the AuNPs. A photograph of the AuNPs with and without TNT is shown in Fig. 7. The analytes could be detected by amine-capped AuNPs with a visual color change instantly, even to a concentration as low as the 476 fM level. The UV-vis spectra of the samples with the increase in the concentration of the analyte are found to show a red shift with broadening of the SPR peak. The UV-vis study for the analyte TNT is shown in Fig. 8a whereas that of 2,4-DNT is shown in Fig. S2a, ESI.† The detection limit of the analyte was calculated from the plot of log[TNT] or [2,4-DNT] vs. Afinal/Ainitial respectively. The average detection limit values of three batches of experiments is found to be 1.55 × 10−13 ± 2.02 × 10−13 M for TNT and 3.79 × 10−13 ± 3.65 × 10−13 M for 2,4-DNT. The plot related to the detection limit study for TNT is shown in Fig. 8b whereas that of 2,4-DNT is given in Fig. S2b, ESI.†
As per previous reports, a variety of AuNPs are prepared via citrate reduction or so in the first step and then functionalized with amines in the next step for their use in the detection of nitro derivatives.18a,b,19,20b,22 The LOD for TNT and 2,4-DNT in the present study is as low as 155 fM and 359 fM respectively, using AuNPs by a simple colorimetric method. Reported methods and LOD values for TNT and 2,4-DNT detection using nanoparticles are collected in Table S1 in the ESI†. The comparison shows that our method is one of the best reported so far. There is one report with an LOD at the zeptomolar level for the detection of TNT.17e However, a fluorescence technique is required for the detection and the synthesis of the sensor involves a time-consuming multi-step process. Further, under colorimetric conditions the LOD is found to be higher than 44 μM. To the best of our knowledge all the reports using NPs as the sensor for TNT involve a functionalization/modification step of the NPs, mostly with amines. Expensive equipment is employed for the detection of the analytes in the reported methods. The novelty of the present work is that the as-prepared AuNPs were suitable for the detection of compatible nitro derivatives without any further modification.
Conclusions
In summary, AuNPs were synthesized using the hexaazamacrocycle L, and the as-prepared AuNPs were successfully utilized in the spontaneous visual and colorimetric detection of TNT and 2,4-DNT at the femtomolar level.Acknowledgements
D.K.C. thanks CSIR, India for financial support.References
- (a) Y. Jiang, H. Zhao, N. Zhu, Y. Lin, P. Yu and L. Mao, Angew. Chem., Int. Ed., 2008, 47, 8601 CrossRef CAS PubMed; (b) N. Tu and L. Wang, Chem. Commun., 2013, 49, 6319 RSC.
- C. Guo, P. Boullanger, L. Jiang and T. Liu, Biosens. Bioelectron., 2007, 22, 1830 CrossRef CAS PubMed.
- X. Mao, Y. Ma, A. Zhang, L. Zhang, L. Zeng and G. Liu, Anal. Chem., 2009, 81, 1660 CrossRef CAS PubMed.
- D. Zhang, O. Neumann, H. Wang, V. M. Yuwono, A. Barhoumi, M. Perham, J. D. Hartgerink, P. Wittung-Stafshede and N. J. Halas, Nano Lett., 2009, 9, 666 CrossRef CAS PubMed.
- A. Ambrosi, F. Airò and A. Merkoçi, Anal. Chem., 2009, 82, 1151 CrossRef PubMed.
- B. Kang, M. A. Mackey and M. A. El-Sayed, J. Am. Chem. Soc., 2010, 132, 1517 CrossRef CAS PubMed.
- S. Lee, S. Kim, J. Choo, S. Y. Shin, Y. H. Lee, H. Y. Choi, S. Ha, K. Kang and C. H. Oh, Anal. Chem., 2007, 79, 916 CrossRef CAS PubMed.
- M. E. van der Boom, Angew. Chem., Int. Ed., 2002, 41, 3363 CrossRef CAS.
- G. Palui, S. Ray and A. Banerjee, J. Mater. Chem., 2009, 19, 3457 RSC.
- (a) H. Xia, S. Bai, J. r. Hartmann and D. Wang, Langmuir, 2009, 26, 3585 CrossRef PubMed; (b) J. D. S. dos Santos, R. A. Alvarez-Puebla, J. O. N. Oliveira and R. F. Aroca, J. Mater. Chem., 2005, 15, 3045 RSC.
- J. D. S. Newman and G. J. Blanchard, J. Nanopart. Res., 2007, 9, 861 CrossRef CAS.
- (a) T. Selvam and K.-M. Chi, J. Nanopart. Res., 2011, 13, 1769 CrossRef CAS; (b) B. Kim, S. L. Tripp and A. Wei, J. Am. Chem. Soc., 2001, 123, 7955 CrossRef CAS.
- J. Song, D. Kim and D. Lee, Langmuir, 2011, 27, 13854 CrossRef CAS PubMed.
- (a) M. Aslam, L. Fu, M. Su, K. Vijayamohanan and V. P. Dravid, J. Mater. Chem., 2004, 14, 1795 RSC; (b) C. Graf and A. van Blaaderen, Langmuir, 2001, 18, 524 CrossRef; (c) A. Kumar, S. Mandal, R. Pasricha, A. B. Mandale and M. Sastry, Langmuir, 2003, 19, 6277 CrossRef CAS; (d) D. V. Leff, L. Brandt and J. R. Heath, Langmuir, 1996, 12, 4723 CrossRef CAS; (e) H. Nakao, H. Shiigi, Y. Yamamoto, S. Tokonami, T. Nagaoka, S. Sugiyama and T. Ohtani, Nano Lett., 2003, 3, 1391 CrossRef CAS; (f) P. R. Selvakannan, S. Mandal, S. Phadtare, R. Pasricha and M. Sastry, Langmuir, 2003, 19, 3545 CrossRef CAS; (g) K. G. Thomas and P. V. Kamat, J. Am. Chem. Soc., 2000, 122, 2655 CrossRef CAS; (h) K. G. Thomas, J. Zajicek and P. V. Kamat, Langmuir, 2002, 18, 3722 CrossRef CAS; (i) J. Athilakshmi and D. Chand, J. Chem. Sci., 2011, 123, 875 CrossRef CAS; (j) J. Athilakshmi and D. K. Chand, Tetrahedron Lett., 2010, 51, 6760 CrossRef CAS PubMed.
- J. D. S. Newman and G. J. Blanchard, Langmuir, 2006, 22, 5882 CrossRef CAS PubMed.
- F. Terrier, Chem. Rev., 1982, 82, 77 CrossRef CAS.
- (a) J. Geng, P. Liu, B. Liu, G. Guan, Z. Zhang and M.-Y. Han, Chem.–Eur. J., 2010, 16, 3720 CrossRef CAS PubMed; (b) L. Feng, H. Li, Y. Qu and C. Lu, Chem. Commun., 2012, 48, 4633 RSC; (c) D. Gao, Z. Wang, B. Liu, L. Ni, M. Wu and Z. Zhang, Anal. Chem., 2008, 80, 8545 CrossRef CAS PubMed; (d) R. Tu, B. Liu, Z. Wang, D. Gao, F. Wang, Q. Fang and Z. Zhang, Anal. Chem., 2008, 80, 3458 CrossRef CAS PubMed; (e) A. Mathew, P. R. Sajanlal and T. Pradeep, Angew. Chem., Int. Ed., 2012, 51, 9596 CrossRef CAS PubMed.
- (a) S. S. R. Dasary, A. K. Singh, D. Senapati, H. Yu and P. C. Ray, J. Am. Chem. Soc., 2009, 131, 13806 CrossRef CAS PubMed; (b) T. Demeritte, R. Kanchanapally, Z. Fan, A. K. Singh, D. Senapati, M. Dubey, E. Zakar and P. C. Ray, Analyst, 2012, 137, 5041 RSC; (c) M. Liu and W. Chen, Biosens. Bioelectron., 2013, 46, 68 CrossRef CAS PubMed; (d) X. Liu, L. Zhao, H. Shen, H. Xu and L. Lu, Talanta, 2011, 83, 1023 CrossRef CAS PubMed.
- (a) S. S. R. Dasary, D. Senapati, A. K. Singh, Y. Anjaneyulu, H. Yu and P. C. Ray, ACS Appl. Mater. Interfaces, 2010, 2, 3455 CrossRef CAS PubMed; (b) D. Lin, H. Liu, K. Qian, X. Zhou, L. Yang and J. Liu, Anal. Chim. Acta, 2012, 744, 92 CrossRef CAS PubMed.
- (a) S. Hrapovic, E. Majid, Y. Liu, K. Male and J. H. T. Luong, Anal. Chem., 2006, 78, 5504 CrossRef CAS PubMed; (b) M. Riskin, R. Tel-Vered, T. Bourenko, E. Granot and I. Willner, J. Am. Chem. Soc., 2008, 130, 9726 CrossRef CAS PubMed.
- (a) K. Qian, H. Liu, L. Yang and J. Liu, Analyst, 2012, 137, 4644 RSC; (b) T. Kawaguchi, D. R. Shankaran, S. J. Kim, K. Matsumoto, K. Toko and N. Miura, Sens. Actuators, B, 2008, 133, 467 CrossRef CAS PubMed; (c) S. S. R. Dasary, A. K. Singh, D. Senapati, H. Yu, M. Dubey, P. Amirtharaj and P. C. Ray, IEEE Trans. Nanotechnol., 2011, 10, 1083 CrossRef.
- X. Zhou, H. Liu, L. Yang and J. Liu, Analyst, 2013, 138, 1858 RSC.
- D. Chen and A. E. Martell, Tetrahedron, 1991, 47, 6895 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis spectra of AuNPs containing nitro-compounds, and also that of sensitivity study of 2,4-DNT. A table describing reported methods and LOD for TNT and 2,4-DNT detection using nanoparticles. See DOI: 10.1039/c3ay41824c |
| This journal is © The Royal Society of Chemistry 2014 |