Thiocholine mediated stabilization of in situ produced CdS quantum dots: application for the detection of acetylcholinesterase activity and inhibitors†
Gaizka
Garai-Ibabe
,
Laura
Saa
and
Valeri
Pavlov
*
CICbiomaGUNE, Parque Tecnológico de San Sebastián, Paseo Miramón 182, 20009, Donostia-San Sebastián, Spain. E-mail: vpavlov@cicbiomagune.es; Fax: +34 943005314; Tel: +34 943005308
First published on 22nd October 2013
Abstract
The use of acetylcholinesterase (AChE) inhibitors as chemical warfare agents or pesticides represents a strong hazard against human health. The high toxicity of these compounds arises from their ability to inhibit acetylcholinesterase from degrading acetylcholine (ACh), which could affect the physiology of the nervous system with serious or fatal consequences. Here we report a simple and fluorimetric system for a highly sensitive detection of AChE activity and inhibitors. The principle of this approach is based on the hydrolysis of acetylthiocholine (ATCh) by AChE, which yields the thiol-bearing compound thiocholine (TCh) that at trace concentrations stabilized the in situ generated CdS quantum dots (QDs). The system shows a linear relationship between the fluorescence intensity and AChE activity from 1 to 10 mU mL−1 in buffer solution. The accuracy of the proposed system was further demonstrated through the determination of AChE activity in human serum (HS) by the standard addition method. Furthermore, this novel and highly sensitive sensing system allows the detection of 80 pM of the AChE inhibitor paraoxon and 100 nM of galanthamine. The reported methodology shows potential applications for the development of a simple and inexpensive assay for the routine quantification of AChE activity and inhibitors.
Introduction
Acetylcholinesterase (AChE) inhibitors including chemical nerve agents and pesticides represent a diverse group of highly toxic compounds. AChE breaks down acetylcholine (ACh) at cholinergic synapses causing the termination of the synaptic transmission. Recently, some studies have shown that AChE could also have other important non-enzymatic neuromodulatory functions.1 The extreme toxicity of these compounds is a result of their binding to an active center of AChE leading to an inhibition of the enzymatic activity. Inhibition of AChE leads to the accumulation of ACh throughout the body, affecting the physiology of the nervous system with serious or fatal consequences.2Due to the widespread use of organophosphates as pesticides across the world and the increased threat of terrorist attacks employing nerve gases, there is an increasing interest in developing a technology which allows a simple, fast, and sensitive detection of AChE inhibitors without complex instrumentation. Until now different analytical approaches have been developed for the detection of this type of compounds using gas chromatography and mass spectroscopy.3,4 The disadvantage of such methods rely on the requirement of non-portable expensive equipment that needs to be operated by well-trained personnel. On the other hand, metallic and semiconductor nanoparticles (NPs) have been extensively used for bioanalytical applications.5 Sensors based on the enzyme modulated growth of metal NPs using AChE have been reported.6,7 However, the main drawback of these systems is that only UV-vis spectroscopy can be utilized to follow the growth of Au and Ag NPs. The discovery of enzymatic systems capable to produce fluorescent semiconductor NPs can significantly improve the bioanalytical assay by the use of a much more sensitive fluorescence spectroscopy.
Semiconductor NPs, quantum dots (QDs), can be photoexcited to generate electron–hole couples in the conduction band which recombine to yield fluorescent emission of light.8 Furthermore, their physicochemical properties are defined by their submicrometre dimensions and differ significantly from those of the corresponding bulk material.9 QDs modified with antibodies, DNA and small molecules found application as labels in bioanalytical affinity assays and imaging.10–12 More specifically, QDs have also been used for the electrochemical detection of nerve agents.13,14 QDs are also used as energy-transfer donors in assays based on fluorescence resonance energy transfer (FRET). All these analytical systems are based on presynthesized QDs and their performance is frequently hindered by high background signals, caused by nonspecific adsorption of decorated QDs on surfaces or poor quenching of a donor couple.15 We believe that the generation of QDs in situ can address these drawbacks of relevant analytical systems by decreasing the background signal. In this sense, our group introduced different unconventional approaches to achieve the biocatalytic growth of QDs.16 The first approach is based on the enzymatic generation of S2− anions followed by interaction with Cd2+ cations to yield CdS QDs.16–18 Unfortunately, a relatively high concentration of enzymatically produced S2− is required to give CdS QDs. We have also developed a more sensitive method based on the enzymatic product mediated inhibition of CdS QD formation, where the increase in enzymatic activity is related to the decrease in fluorescence intensity.19 Recently we reported glutathione and ortho-phosphate mediated stabilization of CdS QDs grown in situ.20,21
In the present paper we report a novel approach to the enzymatic generation of QDs, which has been applied for the detection of AChE activity and inhibitors (paraoxon and galanthamine). The key advantage of this system over the previous methodology is that the enzymatic activity is directly related to the increase of the intensity of fluorescence signals. The principle of the system is based on the stabilization of in situ produced QDs through the product of enzymatic reaction, for instance, thiocholine (TCh), which at trace amounts significantly influences the amount of generated QDs. The use of the proposed system in real biological samples has been validated by the determination of AChE activity in human serum (HS).
Experimental
Materials
Acetylthiocholine chloride (ATCh), AChE (from electric eel), paraoxon, galanthamine hydrobromide (from Lycoris Sp.), HS and other chemicals were obtained from Sigma-Aldrich (Spain) and used as supplied. Enzymatic activity assays were performed in black flat-well (330 μL) NUNC 96 wells microtiter plates and the fluorescence spectra were recorded with a Varioskan Flash fluorimeter (Thermo Scientific). All water used was Milli-Q ultrapure grade (EMD Millipore).AChE activity assay
Varying amounts of AChE were incubated with different amounts of ATCh in citrate–phosphate buffer (pH 7.5) at 37 °C for 30 min. Subsequently, Na2S (10 μL, 1 mM), and Cd(NO3)2 (2.5 μL, 50 mM) were added to this mixture (87.5 μL) and the fluorescence emission spectra of the resulting suspension were recorded after 5 min of incubation at room temperature (RT) at λexc = 290 nm.The AChE activity was also determined in diluted HS to evaluate the sensitivity of the assay in complex biological media. Varying known standard amounts of AChE (from 0.5 to 2 mU mL−1) were added to diluted HS samples (final dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7500, 1
7500, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 1
000, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 1
000 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per well) and incubated in the presence of ATCh (0.5 mM) at 37 °C for 30 min. The volume of the incubation mixture was 87.5 μL per well. Afterwards, 10 μL of Na2S (1 mM) and 2.5 μL of Cd(NO3)2 (50 mM) were added per well to reach the final volume of the assay mixture of 100 μL. The fluorescence emission at 560 nm of the resulting suspensions was recorded after 5 min of incubation at room temperature (RT) at λex = 290 nm.
000 per well) and incubated in the presence of ATCh (0.5 mM) at 37 °C for 30 min. The volume of the incubation mixture was 87.5 μL per well. Afterwards, 10 μL of Na2S (1 mM) and 2.5 μL of Cd(NO3)2 (50 mM) were added per well to reach the final volume of the assay mixture of 100 μL. The fluorescence emission at 560 nm of the resulting suspensions was recorded after 5 min of incubation at room temperature (RT) at λex = 290 nm.
Inhibition of AChE by paraoxon and galanthamine
Portions (10 mU mL−1) of AChE were incubated with varying concentrations of paraoxon (from 100 nM to 80 pM) and galanthamine (from 10 μM to 100 nM) in 77.5 μL of citrate–phosphate buffer pH 7.5 at 37 °C (paraoxon) or RT (galantamine) for 30 min. Next, 10 μL of 5 mM ATCh in citrate–phosphate buffer was added, and the resulting mixture was incubated at 37 °C for 30 min. After that, 10 μL of 1 mM Na2S and 2.5 μL of 50 mM Cd(NO3)2 were added to this mixture (87.5 μL) to form CdS QDs, and the fluorescence emission spectra of the resulting suspension were recorded after 5 min of incubation at RT at λexc = 290 nm.Results and discussion
The proposed operating mechanism of our assay is represented in Scheme 1. AChE breaks down ATCh to acetic acid and TCh. The latter compound has two functional moieties i.e. mercapto and tertiary amino groups. The mercapto group binds TCh through thiol linkage to the surface of growing CdS QDs generated via the interaction of Cd2+ with S2−. The hydrophilic tertiary amino group confers hydrophilicity to CdS QDs grown in situ, thereby stabilizing them in aqueous media.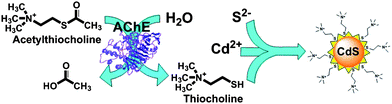 | ||
| Scheme 1 Detection of AChE activity through the thiocholine mediated stabilization of in situ produced fluorescent CdS QDs. | ||
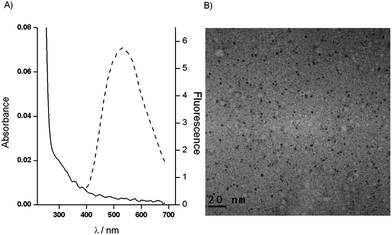
Fig. 1A demonstrates absorption and emission spectra of the in situ grown CdS QDs in the presence of TCh, obtained by the enzymatic hydrolysis of ATCh (solid and dashed lines, respectively). The UV-vis spectrum shows increasing absorption below 500 nm, and a shoulder at about 300 nm. The emission spectrum has a well shaped peak at 560 nm. The form of the peak indicates that the size distribution of the formed CdS QDs is nearly homogeneous.22 The shape and dimension of CdS QDs were determined by Transmission Electron Microscopy (TEM) (Fig. 1B). The TEM image showed spheroidal QDs with a medium diameter of 2.5 ± 0.3 nm (Fig. S1 in ESI†), which confirmed the homogeneity of CdS QDs stabilized by TCh.
In order to attest the operating mechanism of our system, a number of control experiments were carried out (Fig. S2 in ESI†). The emission peak attributed to the growing QDs was only observed in the reaction mixture containing all components of the assay: AChE, ATCh, Cd(NO3)2, and Na2S. When AChE was not added to the assay mixture the emission was significantly lower, apparently due to the decrease in the number of QDs formed in the absence of TCh. No significant fluorescence was observed in the assay mixture devoid of the enzymatic substrate ATCh. To demonstrate that the fluorescence signal was caused by CdS QDs, but not by the intrinsic fluorescence of AChE protein, the experiments were performed in the absence of TCh, Cd(NO3)2 and Na2S. Under these conditions no fluorescence was observed in the assay mixtures. Furthermore, as ATCh is decomposed enzymatically into TCh and acetic acid, to exclude the possibility that the stabilization of CdS QDs was caused by acetic acid, experiments were performed using the mixture of AChE with its natural substrate ACh. These experiments showed that the reaction products neither acetate nor choline can stabilize CdS QDs effectively. Finally, the assay performed with ACh in the absence of AChE showed no increase in fluorescence, which demonstrates that organic molecules having only tertiary amino groups are not stabilizers of in situ formed QDs, and the presence of TCh is crucial for this purpose.
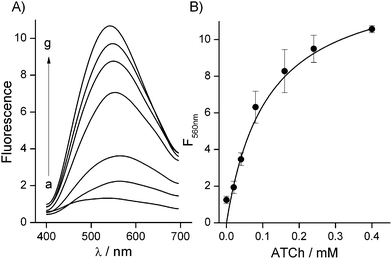
The effect of varying concentrations of ATCh on the response of the present analytical system containing a fixed amount of AChE was studied. According to Fig. 2 the response to increasing concentrations of ATCh is typical for an enzymatic system governed by the Michaelis–Menten kinetic model. One can notice the linear part of the calibration plot up to 0.05 mM of the substrate followed by the plot section approaching asymptotically the maximum response from 0.2 mM. Using these data we calculated an apparent Michaelis–Menten constant equal to 0.102 ± 0.022 mM, using software GraFit 7.2.0 (Erithacus Software), which correlates well with the literature data.23,24 For subsequent experiments aimed at design of the assay for AChE activity, we selected a concentration of ATCh equal to 0.5 mM.
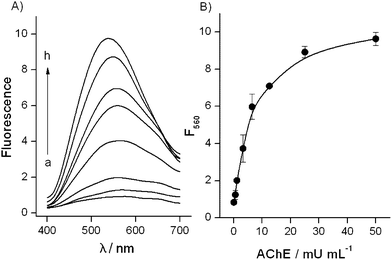
Fig. 3 shows the response of the analytical system to increasing amounts of AChE at a fixed concentration of ATCh. The system demonstrated a linear increase of the fluorescence signal when increasing the AChE concentration from 1 to 10 mU mL−1 and reached saturation starting from 40 mU mL−1.
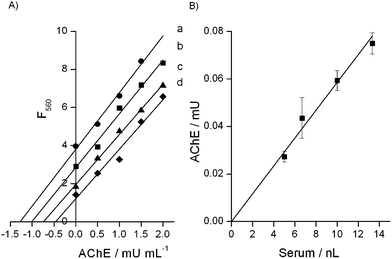
In nature, AChE is present in cholinergic brain and neuromuscular junctions and in HS proceeding from the liver. The cholinesterase readings are helpful to evaluate the acute effects of organophosphate poisoning. In order to validate the proposed system to be used in real samples we performed the standard addition procedure using commercially available HS and AChE. Different concentrations of the enzyme were added to diluted HS and the enzymatic activity was measured as previously described to calculate the AChE activity in the serum without added standard. We plotted the experimental data with the concentration standard added on the x-axis and the corresponding fluorescence signal on the y-axis (Fig. 4A). The linear regression for each serum dilution was performed to calculate the intercept of the calibration lines with the x-axis, which shows the content of AChE in the diluted real samples. The linear regression coefficients for each added standard experiment were r2 = 0.99, 0.988, 0.997 and 0.978, for 7500, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 15
000, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 20
000 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times diluted HS, respectively. It should be noted that error bars are not shown for clarity of the plots, while the RSD of any shown point represented by the medium value of three replicates was between 2.2% and 11.2%.
000 times diluted HS, respectively. It should be noted that error bars are not shown for clarity of the plots, while the RSD of any shown point represented by the medium value of three replicates was between 2.2% and 11.2%.
Fig. 4(B) shows the linear regression (r2 = 0.972) between the AChE activity and the volume of HS in the assay system. By taking into consideration the dilution factors of the serum samples, we computed the activity of AChE in commercial HS equal to 5800 mU mL−1, which is similar to that reported by Worek et al. for human plasma (5675 ± 195 mU mL−1).25 Furthermore, we calculated the recovery of the AChE activity from the standard addition experiments, demonstrating a recovery between 99.2% ± 9.3% and 103.6% ± 6.9% for dilutions between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7500 and 1
7500 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Thus, we proved the suitability of the proposed system for the measurement of AChE activity in real samples.
000. Thus, we proved the suitability of the proposed system for the measurement of AChE activity in real samples.
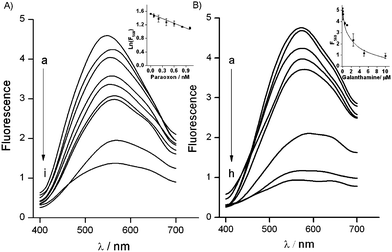
To prove the suitability of the reported system for the detection of AChE inhibitors we choose paraoxon and galanthamine. Paraoxon is a nerve agent that irreversibly inhibits the enzyme, while galanthamine is a reversible inhibitor of AChE. The mechanism of the irreversible inhibition of AChE by paraoxon is described by the equation ln[F560] = ln[Fmax560] − ki[I]t, where ki is defined as the bimolecular rate constant and is commonly used to measure the inhibitory capacity of irreversible inhibitors, [Fmax560] is the fluorescence corresponding to the initial concentration of the enzyme, [F560] is the fluorescence corresponding to the concentration of the noninhibited enzyme after a certain time (t), and [I] is the concentration of the inhibitor.26Fig. 5A shows the fluorescence spectra of AChE generated QDs in the presence of variable concentrations of paraoxon, after a fixed incubation period of 30 min. As could be noted, the fluorescence intensity decreases as the concentration of the inhibitor increases, implying that the biocatalytic enhancement of the growth of QDs was inhibited. The inset of Fig. 5A shows the linear dependence (r2 = 0.966) between the logarithm of the fluorescence emission at 560 nm and the concentration of paraoxon. From the linear regression of the irreversible inhibition equation of the obtained experimental data, we obtained a value for ki equal to 1.4 × 107 M−1 min−1. On the other hand Fig. 5B shows the fluorescence spectra of AChE generated QDs in the presence of variable concentrations of galanthamine. The inset of Fig. 5B shows the linear dependence (r2 = 0.971) between the fluorescence emission at 560 nm and the concentration of galanthamine. Galanthamine competes with the enzyme substrate for binding to the active site of AChE in a reversible manner, and its inhibitory mechanism is described by the equation F560 = Fmax560[S]/(kapm + [S]), where kapm = km(1 + [I]/ki), ki is the inhibitor's dissociation constant and [I] is the inhibitor concentration.17 Non-linear regression fitting to this equation of the obtained experimental data gave the value of ki equal to 5.8 × 10−7 M.
To the best of our knowledge, the most sensitive optical assay for nerve gases published previously is based on modulating the growth of core–shell Au–Ag NPs with enzymatically generated TCh. It demonstrates a limit of paraoxon detection equal to 4 nM.7 As could be seen in Fig. 5, by the enzyme product mediated stabilization of QDs, we were able to detect as low as 0.08 nM (80 pM) of paraoxon and 100 nM of galanthamine with a limit of detection of 0.06 nM and 115 nM, for paraoxon and galanthamine, respectively, calculated with a confidence level of 0.95.27 Thus, this new fluorogenic method is at least by 50 times more sensitive than the previously described optical assays for the detection of paraoxon, while the galanthamine detection sensitivity is in the range of previously reported techniques.7,28
Conclusions
The conventional approach to the detection of enzymatic activities based on pre-synthesized QDs would require the complicated synthesis of an enzymatic substrate modified with a quenching or fluorescent moiety for the subsequent decoration of semiconductor NPs. An enzymatic substrate tethered to the surface of QDs is not readily available to an enzyme. On the other hand, it is quite difficult to quench completely the fluorescence of QDs by quenching moieties separated through a modified enzymatic substrate. These will result in an assay suffering from high initial level of fluorescence, bad sensitivity and very low signal to noise ratio.15 Our approach does not require any additional modification of a commercially available enzymatic substrate and demonstrated good signal to noise ratio because it does not employ pre-synthesized QDs. The present study has demonstrated a new concept for the detection of enzymatic activities based on the biocatalytic generation of an organic molecule capable to stabilize fluorescent semiconductor NPs in an aqueous environment. The application of this methodology to the detection of AChE activity allowed designing a very simple and inexpensive assay to quantify the level of this enzyme in HS. The concept permits to follow the inhibition of AChE by fluorescence spectroscopy with unprecedented sensitivity and measure extremely low concentrations of paraoxon. Furthermore, we believe that the described system can serve as a model for the development of numerous biosensing techniques using enzymes as a means of signal amplification and relying on optical, electrochemical, and photoelectrochemical transduction.Acknowledgements
This work was supported by the Spanish Ministry of Economy and Competitiveness (project BIO2011-26356). V.P. acknowledges the contract Ramon y Cajal from the Spanish Ministry of Economy and Competitiveness.Notes and references
- G. Zimmerman and H. Soreq, Cell Tissue Res., 2006, 326, 655–669 CrossRef CAS PubMed.
- B. E. Mileson, J. E. Chambers, W. L. Chen, W. Dettbarn, M. Ehrich, A. T. Eldefrawi, D. W. Gaylor, K. Hamernik, E. Hodgson, A. G. Karczmar, S. Padilla, C. N. Pope, R. J. Richardson, D. R. Saunders, L. P. Sheets, L. G. Sultatos and K. B. Wallace, Toxicol. Sci., 1998, 41, 8–20 CAS.
- R. Subramaniam, C. Astot, L. Juhlin, C. Nilsson and A. Ostin, Anal. Chem., 2010, 82, 7452–7459 CrossRef CAS PubMed.
- W. A. Harris, P. T. Reilly and W. B. Whitten, Anal. Chem., 2007, 79, 2354–2358 CrossRef CAS PubMed.
- E. Katz and I. Willner, Angew. Chem., Int. Ed., 2004, 43, 6042–6108 CrossRef CAS PubMed.
- V. Pavlov, Y. Xiao and I. Willner, Nano Lett., 2005, 5, 649–653 CrossRef CAS PubMed.
- A. Virel, L. Saa and V. Pavlov, Anal. Chem., 2009, 81, 268–272 CrossRef CAS PubMed.
- N. Gaponik, S. G. Hickey, D. Dorfs, A. L. Rogach and A. Eychmuller, Small, 2010, 6, 1364–1378 CrossRef CAS PubMed.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS PubMed.
- Y. Zhang, M.-K. So, A. M. Loening, H. Yao, S. S. Gambhir and J. Rao, Angew. Chem., Int. Ed., 2006, 45, 4936–4940 CrossRef CAS PubMed.
- E. R. Goldman, A. R. Clapp, G. P. Anderson, H. T. Uyeda, J. M. Mauro, I. L. Medintz and H. Mattoussi, Anal. Chem., 2004, 76, 684–688 CrossRef CAS PubMed.
- M. Green, Angew. Chem., Int. Ed., 2004, 43, 4129–4131 CrossRef CAS PubMed.
- V. Pardo-Yissar, E. Katz, J. Wasserman and I. Willner, J. Am. Chem. Soc., 2003, 125, 622–623 CrossRef CAS PubMed.
- D. Lu, J. Wang, L. Wang, D. Du, C. Timchalk, R. Barry and Y. Lin, Adv. Funct. Mater., 2011, 21, 4371–4378 CrossRef CAS.
- R. Freeman and I. Willner, Nano Lett., 2009, 9, 322–326 CrossRef CAS PubMed.
- L. Saa, A. Virel, J. Sanchez-Lopez and V. Pavlov, Chem.–Eur. J., 2010, 16, 6187–6192 CrossRef CAS PubMed.
- L. Saa and V. Pavlov, Small, 2012, 8, 3449–3455 CrossRef CAS PubMed.
- L. Saa, J. M. Mato and V. Pavlov, Anal. Chem., 2012, 84, 8961–8965 CAS.
- G. Garai-Ibabe, M. Möller and V. Pavlov, Anal. Chem., 2012, 84, 8033–8037 CrossRef CAS PubMed.
- G. Garai-Ibabe, L. Saa and V. Pavlov, Anal. Chem., 2013, 85, 5542–5546 CrossRef CAS PubMed.
- N. Malashikhina, G. Garai-Ibabe and V. Pavlov, Anal. Chem., 2013, 85, 6866–6870 CrossRef CAS PubMed.
- P. Mandal, S. S. Talwar, S. S. Major and R. S. Srinivasa, J. Chem. Phys., 2008, 128, 114703 CrossRef CAS PubMed.
- M. Pohanka, M. Hrabinova, K. Kuca and J.-P. Simonato, Int. J. Mol. Sci., 2011, 12, 2631–2640 CrossRef CAS PubMed.
- M. Barteri, A. Pala and S. Rotella, Biophys. Chem., 2005, 113, 245–253 CrossRef CAS PubMed.
- F. Worek, U. Mast, D. Kiderlen, C. Diepold and P. Eyer, Clin. Chim. Acta, 1999, 288, 73–90 CrossRef CAS.
- F. Villatte, V. Marcel, S. Estrada-Mondaca and D. Fournier, Biosens. Bioelectron., 1998, 13, 157–164 CrossRef CAS.
- A. D. McNaught and A. Wilkinson, in Compendium of chemical terminology, Blackwell Scientific Publications, Oxford, 2nd edn, 1997 Search PubMed.
- M. Cuartero, M. S. Garcia, F. Garcia-Canovas and J. A. Ortuno, Talanta, 2013, 110, 8–14 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3an01662e |
| This journal is © The Royal Society of Chemistry 2014 |