A long wavelength hydrophobic probe for intracellular lipid droplets†
Jingying
Zhai
,
Yawen
Zhang
,
Chenye
Yang
,
Yanmei
Xu
and
Yu
Qin
*
Department of Chemistry, Nanjing University, Nanjing, China. E-mail: qinyu75@nju.edu.cn; Fax: +86-25-83592562; Tel: +86-25-83592562
First published on 8th November 2013
Abstract
A highly stable, long wavelength polarity sensitive probe, 8-nitrophenyl-3,5-dipiperidine-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (NPBDP), was developed for living cell imaging of intracellular lipid droplets by fluorescence microscopy.
During the last few years, intracellular lipid droplets have become the focus of intense study. Lipid droplets are composed of a neutral lipid core surrounded by a phospholipid monolayer, within which proteins reside.1 Once thought to be simple storage vesicles for neutral lipids, it is now clear that lipid droplets serve as reservoirs for lipids and proteins involved in many cellular processes.2 The fluorescence-based imaging techniques have been proven to be among the most powerful and commonly used approaches in biophysical studies for examining membrane structure, phase separation, and domain dynamics.3
Although numerous fluorescent molecules have been developed and extensively applied in cell imaging, the probes for selective detection of intracellular lipid droplets are very limited.4 Oil red O has been a commonly used fat stain for several decades.5 In 1985, Greenspan and coauthors reported that Nile red can be a selective fluorescent probe for lipid droplets.4,6 Such probes usually require sufficient hydrophobicity to remain in the lipid and certain water solubility to be used in buffers for living cell staining. The fluorescent dyes with long excitation and emission wavelengths are desirable due to small interference from the background autofluorescence and less damage to the cell. The fat stains that have all these merits are barely seen.
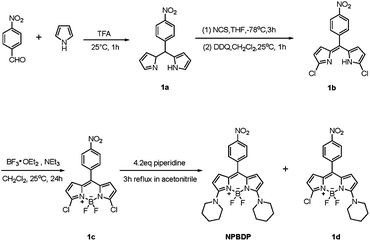
Boron dipyrromethene (BODIPY) dyes are well-known to be highly fluorescent, very stable, having narrow emission bandwidths and amenable to structure modification.7 In our recent work, the extension of the conjugated BODIPY core structure by a traditional auxochromic substituent to the 5-position of the BODIPY through Knoevenagel condensation resulted in a limited wavelength change of about 70 nm, but associated with complicated synthetic routes and poor water solubility.8 Here we designed and synthesized a novel hydrophobic probe based on a BODIPY fluorophore 8-nitrophenyl-3,5-dipiperidin-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene, to which two piperidine groups were attached by using a facile synthetic method as shown in Scheme 1.
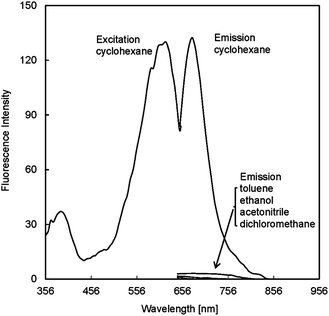
NPBDP has the maximum excitation at 618 nm, and a long wavelength emission peak at 677 nm in cyclohexane because the piperidine units linked to 3 and 5 positions of the BODIPY core and the conjugation of the electron pair on the nitrogen atom with the BODIPY core lead to nearly 160 nm red shift of the emission wavelength compared to the unsubstituted compound. Near infrared dyes are usually very hydrophobic molecules that require further structure modification to make them water soluble for cell imaging. The readily synthesized NPBDP is small in size, and an aqueous buffer solution with 10−5 M NPBDP can be prepared.
BODIPY derivatives with no nitro group in the meso benzene ring are intrinsically highly fluorescent and the dyes are insensitive to the solvent polarity change, because both the lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) largely centered on the BODIPY core.9 In contrast, NPBDP is inherently non-fluorescent due to the photo-induced electron transfer (PET) quenching process. The electron-withdrawing ability of the nitro aromatic moiety at the 8 position of the BODIPY core lowers the LUMO energy level of the BODIPY fluorophore. Meanwhile, the HOMO energy level localizes unchangedly on the boron-dipyrromethane component. The minimal orbital overlap between the nitro aromatic moiety and the fluorophore, because of the uncoupled and orthogonal aligned two π rings, definitely leads to internal quenching through the PET process. The quantum yield of NPBDP in cyclohexane was measured to be 0.03 with Nile blue as the reference dye. The compound is highly sensitive to the solvent polarity as shown in Fig. 1. The fluorescence emission intensity in cyclohexane is 127 times that in ethanol under the same experimental conditions (Fig. 1), while the fluorescence intensity of the commercially available hydrophobic probe 3,3′-dioctadecyloxacarbocyanine perchlorate (DiOC18) increases only 1.6 times from polar ethanol to non-polar cyclohexane (Fig. S6†). To mimic the hydrophobic environment in lipid droplets, we further incorporated NPBDP into lipophilic plasticized membranes with different polarities. The plasticizers used were bis(2-ethylhexyl)sebacate (DOS), dibutyl phthalate (DBP), dimethyl phthalate (DMP), and tripropyl phosphate (TPP). These plasticizers, DOS, DBP, DMP, and TPP, have increasing dielectric constants from 4.6, 6.4, 8.5, and 13.2,10 and consequently higher polarity. As expected, the fluorescent intensity of the entrapped NPBDP decreased when the non-polar plasticizer DOS was replaced with other plasticizers with higher polarity, and the ratios of the maximum fluorescence intensity of the dye in DOS over the maximum intensity in other plasticizers as shown in Fig. S7, S8 and Table S1.† The maximum emission peak of NPBDP is 650 nm in the DOS film which is slightly blue shifted compared with the one in cyclohexane.
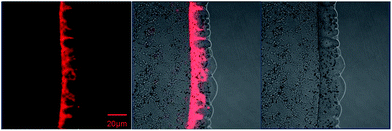
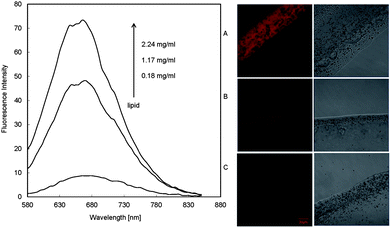
The fluorescence property of NPBDP in multilamellar lipids was investigated by spreading phospholipid multilayers on planar glass supports.11 When small unilamellar vesicles prepared from the phospholipid 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) with 0.4 μmol of NPBDP dissolved in pH 7.4 phosphate buffer are dropped on the microscope cover glass, supported lipid layers are formed rapidly. As shown in Fig. 2, the red emission can be observed in the lipid using a confocal microscope, and the fluorescence became stronger for the thicker lipid layer that entraps more NPBDP. More quantitative studies were performed by adding the same concentration of NPBDP (0.4 μmol) to various amounts of lipid in 1 mL PBS (pH 7.4). The fluorescence spectrum rather than the single peak intensity was measured using the Pariss Hyperspectral Imaging System as shown in Fig. 3. The emission wavelength at 665 nm was larger than the one in the DOS film due to their slightly different hydrophobicity. The fluorescence intensity at 665 nm enhanced with more lipid applied. For lipid concentrations lower than 0.18 mg mL−1, there was no measurable fluorescence at the micromolar concentration of the dye. The results suggested that NPBDP can be used as a specific fluorescent dye for the intracellular lipid droplets with a large amount of neutral lipid in the core, but not for lipid bilayers such as cell membranes.
The pH response of the probe has been measured and the fluorescence of NPBDP barely changed at different pH values (in Fig. S9†). The interference from inorganic salts, reactive oxygen species, glucose, vitamin C, amino acids, human serum albumin and bovine serum albumin were studied by measuring the signal in the presence of the above species. The results showed no fluorescence change under these conditions (in Fig. S10†), which suggested that NPBDP is a highly selective polarity probe.
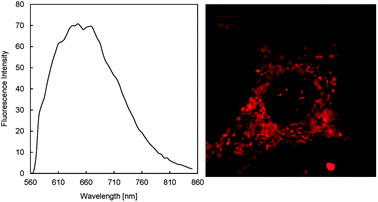
Fig. 4 showed the fluorescence images and spectra of NPBDP (10 μM) loaded-MCF7 cells. It is clearly shown that there are numerous red punctate spots indicating the intracellular lipid droplets bound by cell membranes. The fluorescence of NPBDP is highly dependent on the polarity of the environment. The probe is non-fluorescent in aqueous solution and polar organic solvents; therefore, the emission of NPBDP observed in the cell is due to its location in lipophilic domains including the membranes and the lipid droplets. The lipid layers experiment showed that the dye is only fluorescent in thick lipid layers, which makes a reasonable conclusion that NPBDP is located in the lipid droplet in the cell, and not in the lipid bilayers. Similar results from the Nile red dye were previously reported.4 However, the fluorescence image of Nile red stained cells had significant background fluorescence, while NPBDP gave a much improved signal-to-noise ratio and no fluorescence in the cytoplasmic matrix was observed. The fluorescence intensity ratio of the lipid droplet and the cytolymph with the same size as shown in Fig. 4 is calculated to be 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. NPBDP also exhibited good photo-stability. The fluorescence was stable after the dye was loaded in the cell and the spectrum did not change after 1.5 hours' of measurement as shown in Fig. S11.† After incubation in the medium containing NPBDP for one week, MCF7 cells still displayed similar localization phenomenon and biological activity. The cell toxicity experiments (MTT assay) were performed and the results (Fig. S12†) showed that NPBDP had a small influence on the cell activity; 90% cell activity remained when the micromolar concentration of the dye was used compared to the control sample without any dye.
000. NPBDP also exhibited good photo-stability. The fluorescence was stable after the dye was loaded in the cell and the spectrum did not change after 1.5 hours' of measurement as shown in Fig. S11.† After incubation in the medium containing NPBDP for one week, MCF7 cells still displayed similar localization phenomenon and biological activity. The cell toxicity experiments (MTT assay) were performed and the results (Fig. S12†) showed that NPBDP had a small influence on the cell activity; 90% cell activity remained when the micromolar concentration of the dye was used compared to the control sample without any dye.
In conclusion, we designed NPBDP as a long wavelength fluorescent stain for selective detection of intracellular lipid droplets. The new dye has several advantages over other fat stains. It only exhibits emission in a highly hydrophobic environment with low polarity that eliminates the interference from the cytoplasmic matrix and cell membrane. The facile synthesis and avoiding the introduction of long alkyl chains and conjugated structures lead to the achievement of an appropriate lipophilicity of NPBDP that is beneficial for cell incubation in aqueous medium. The dye has low toxicity, and the excitation and emission in the near-infrared region further reduce damage to the cell and the background interference.
Acknowledgements
This work was supported by the National Special Fund for Major Research Instrumentation Development (No. 2011YQ17006711) and the National Natural Science Foundation of China (No. 21075062).Notes and references
- (a) K. Tauchi-Sato, S. Ozeki, T. Houjou, R. Taguchi and T. Fujimoto, J. Biol. Chem., 2002, 277, 44507 CrossRef CAS PubMed; (b) D. J. Murphy and J. Vance, Trends Biochem. Sci., 1999, 24, 109 CrossRef CAS.
- (a) M. A. Welte, Trends Cell Biol., 2007, 17, 363 CrossRef CAS PubMed; (b) T. C. Walther and R. V. Farese, Annu. Rev. Biochem., 2012, 81, 687 CrossRef CAS PubMed; (c) D. J. Murphy, Prog. Lipid Res., 2001, 40, 325 CrossRef CAS; (d) S. Martin and R. G. Parton, Nat. Rev. Mol. Cell Biol., 2006, 7, 373 CrossRef CAS PubMed; (e) N. A. Ducharme and P. E. Bickel, Endocrinology, 2008, 149, 942 CrossRef CAS PubMed; (f) T. Fujimoto, Y. Ohsaki, J. Cheng, M. Suzuki and Y. Shinohara, Histochem. Cell Biol., 2008, 130, 263 CrossRef CAS PubMed; (g) J. M. Goodman, J. Biol. Chem., 2008, 283, 28005 CrossRef CAS PubMed; (h) S. O. Olofsson, P. Boström, L. Andersson, M. Rutberg, M. Levin, J. Perman and J. Borén, Curr. Opin. Lipidol., 2008, 19, 441 CrossRef CAS PubMed; (i) C. Thiele and J. Spandl, Curr. Opin. Cell Biol., 2008, 20, 378 CrossRef CAS PubMed; (j) S. Murphy, S. Martin and R. G. Parton, Biochim. Biophys. Acta, 2009, 1791, 441 CrossRef CAS PubMed; (k) T. C. Walther and R. V. Farese, Biochim. Biophys. Acta, 2009, 1791, 459 CrossRef CAS PubMed; (l) M. Beller, K. Thiel, P. J. Thul and H. Jäckle, FEBS Lett., 2010, 584, 2176 CrossRef CAS PubMed.
- (a) H. H. An, S. J. Lee, H. S. Kim, W. B. Han and C. S. Yoon, Biochim. Biophys. Acta, 2012, 1818, 2884 CrossRef CAS PubMed; (b) A. Anantharam, D. Axelrod and R. W. Holz, J. Neurochem., 2012, 122, 661 CrossRef CAS PubMed; (c) J. Otterstrom and A. M. V. Oijen, Biochemistry, 2013, 52, 1654 CrossRef CAS PubMed; (d) J. B. Miller and D. E. Koshland, Jr, Nature, 1978, 272, 83 CrossRef CAS; (e) J. Karolin, L. B. A. Johansson, L. Strandberg and T. Ny, J. Am. Chem. Soc., 1994, 116, 7801 CrossRef CAS; (f) J. Korlach, P. Schwille, W. W. Webb and G. W. Feigenson, Proc. Natl. Acad. Sci., 1999, 96, 8461 CrossRef CAS; (g) F. G. Prendergast, R. P. Haugland and P. J. Callahan, Biochemistry, 1981, 20, 7333 CrossRef CAS.
- P. Greenspan, E. P. Mayer and S. D. Fowler, J. Cell Biol., 1985, 100, 965 CrossRef CAS.
- R. Koopman, G. Schaart and M. K. C. Hesselink, Histochem. Cell Biol., 2001, 116, 63 CAS.
- P. Greenspan and S. D. Fowler, J. Lipid Res., 1985, 26, 781 CAS.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed.
- J. Zhai, T. Pan, J. Zhu, Y. Xu, J. Chen, Y. Xie and Y. Qin, Anal. Chem., 2012, 84, 10214 CrossRef CAS PubMed.
- M. Landrum, A. Smertenko, R. Edwards, P. J. Hussey and P. G. Steel, Plant J., 2010, 62, 529 CrossRef CAS PubMed.
- D. R. Lide, CRC Handbook of chemistry & physics, 86th edn, CRC Press, Taylor & Francis, 2005 Search PubMed.
- P. S. Cremer and S. G. Boxer, J. Phys. Chem. B, 1999, 103, 2554 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3an01461d |
| This journal is © The Royal Society of Chemistry 2014 |