Adult human exocrine pancreas differentiation to hepatocytes – potential source of a human hepatocyte progenitor for use in toxicology research
Emma A.
Fairhall†
a,
Karen
Wallace†
ab,
Steven A.
White
a,
Guo C.
Huang
c,
James A.
Shaw
a,
Sid C.
Wright
a,
Keith A.
Charlton
b,
Alastair D.
Burt
a and
Matthew C.
Wright
*a
aInstitute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK. E-mail: M.C.Wright@ncl.ac.uk
bSchool of Medical Sciences, University of Aberdeen, Aberdeen, UK
cDepartment Diabetes and Endocrinology, Rayne Institute, Kings College London, London, UK
First published on 25th October 2012
Abstract
Reduction in the use of animals in toxicology is an important goal despite the continued need to assess drug and chemical safety in man. However, a limitation to in vitro screening for drug and chemical toxicity is the lack of available human hepatocytes and the difficulties associated with generating fully functional hepatocytes from stem cells. Previously, we have shown that a rat pancreatic acinar cell line is capable of trans-differentiating into fully functional hepatocyte-like cells in response to glucocorticoid via a serine/threonine protein kinase mechanism alone. Here we demonstrated that differentiation only occurs with glucocorticoids, not other steroids. We also investigated the potential of human pancreatic cells to undergo the same process. Analysis of adult human pancreata at the level of mRNA, protein and by immunohistochemical staining demonstrated that long term systemic exposure to glucocorticoid therapy resulted in differentiation of exocrine tissue to hepatocyte-like tissue. Glucocorticoid treatment of human pancreatic acinar cells in culture also resulted in trans-differentiation to hepatocyte-like cells. Both in vivo and in vitro, trans-differentiation of pancreas cells to hepatocytes was associated with an induction of SGK1 variant transcripts that have been previously shown to drive B-13 differentiation to hepatocytes. Adult exocrine human pancreas therefore responds in a similar qualitative fashion to that previously observed in rodents exposed to elevated glucocorticoid – that of a differentiation into hepatocyte-like cells. Understanding the enhanced response of B-13 cells to glucocorticoid and engineering this response in a replicating human acinar cell could generate an unlimited supply of functional human hepatocytes in vitro that could be useful in a variety of applications, including screening drugs and chemicals for hepatic metabolism and toxicity.
Introduction
The B-13 rat pancreatic cell line is an expandable progenitor-like cell with the capacity to generate an unlimited supply of functional rat hepatocyte-like cells (termed B-13/H cells) in vitro, without the need for animal donors.1–6 The cell line appears to be unique in that it differentiates into B-13/H cells that are both qualitatively and quantitatively comparable to freshly isolated hepatocytes.1–6 Furthermore, B-13 cells undergo a change to B-13/H cells in response to the addition of a single glucocorticoid hormone, providing a simple and highly reproducible system for hepatocyte generation.6 In contrast to primary hepatocytes, B-13/H cells remain differentiated for many weeks on plastic substrata, whereas primary hepatocytes rapidly de-differentiate.6 This latter quality could make B-13/H cells a valuable model for studying hepatic metabolism and toxicity in vitro, since they offer a stable phenotype for studying toxic processes which may take long periods of time to manifest their effects (e.g. low level chronic exposure effects, carcinogenesis).We have spent the last few years both defining the mechanisms that regulate the differentiation process and putting the B-13 response to glucocorticoid into a physiological context. We sought to investigate whether the trans-differentiation of acinar cells to hepatocytes is a physiologically feasible outcome in normal cells, both in vitro and in vivo. In a series of papers, we have shown that the B-13 to B-13/H trans-differentiation is regulated by Wnt signalling and serine/threonine protein kinase (SGK1) induction4,5 and is related to a pathophysiological response of the rodent pancreas to glucocorticoid.7,8 B-13 cells appear to be hyper-responsive to glucocorticoid with respect to hepatic differentiation, but the response occurs in normal rodent pancreas acinar cells as a result of physiologically abnormally high concentrations of glucocorticoid, both in vitro and in vivo.6 It has yet to be established precisely why pancreatic acinar cells should specifically trans-differentiate into hepatocytes in response to glucocorticoid (in contrast to other tissues which are relatively unaffected). However, it is likely associated with the acinar cell's propensity to revert to a proliferative embryonic ductal phenotype (as seen in vitro;9,10 the close developmental relationship between liver and pancreas11 and the promotion of tissue maturation by glucocorticoids.6
Since a long term aim of this program of research is to isolate (or engineer) a human equivalent of the B-13 cell, we examined whether the B-13 response to dexamethasone (DEX) – a synthetic glucocorticoid – is specific to glucocorticoids or whether the response occurs to other steroids. In addition, we sought evidence that an hepatocyte phenotype occurs in human pancreatic cells in vivo and in vitro.
We show that B-13 cells differentiate into B-13/H cells in response to several glucocorticoids but not to a range of other steroid classes. We also demonstrate that long term treatment with glucocorticoid in man results in liver levels of expression of hepatocyte markers in the human pancreas, and that culturing human acinar cells with glucocorticoid also results in expression of genes specific to hepatocytes. These changes were associated with an induction in SGK1 variant gene expression and suggest a human B-13 equivalent is a realistic prospect if the glucocorticoid hyper-responsive mechanism in B-13 cells can be replicated in human cells.
Results
The trans-differentiation of B-13 cells to B-13/H cells is a specific response to glucocorticoid steroids
The trans-differentiation of pancreatic B-13 cells to hepatocyte-like B-13/H cells in response to dexamethasone includes a change in morphology (Fig. 1A) and expression of liver-specific mRNAs (cytochrome P450 2E1 [CYP2E1] and carbamoyl phosphate synthase [CPSI]) from undetectable levels as determined by RT-PCR (Fig. 1B). However, expression of the pancreatic acinar cell marker amylase was retained in B-13/H cells (Fig. 1B), as previously reported.1,2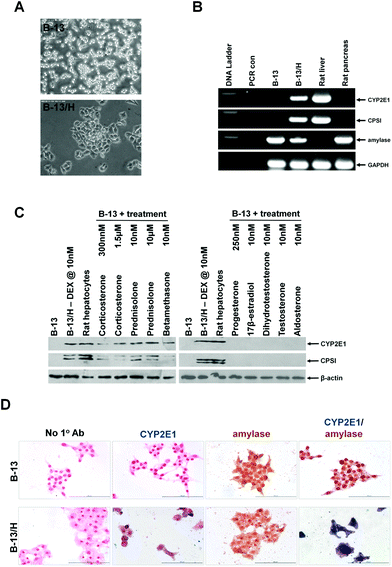 | ||
| Fig. 1 The model B-13 rat pancreatic acinar cell line trans-differentiates into hepatocytes in response to glucocorticoid treatments but not in response to other steroids or nuclear receptor agonists. A, typical light micrographs of parent B-13 cell line (upper) and B-13/H cells (lower) observed after 14 days treatment with 10 nM DEX. B, RT-PCR analysis for the indicated transcript. PCR con, amplification reaction in the absence of input RNA during the reverse transcription step. C, Western blot for the indicated protein after treatment with the indicated concentration of glucocorticoids or other compounds for 14 days. Note, that trans-differentiation to B-13/H cells results in a block in mitosis (and these cells remain viable for at least 60 days in this state.2–4 In the absence of trans-differentiation, B-13 cells require sub-culture to prevent cell death and so cells treated with other compounds required sub-culturing. The normal endogenous concentrations in rats were used when greater than 10 nM, to compare with dexamethasone. For the non-glucocorticoids the rat serum levels are reported to be: progesterone, 250 nM in pregnancy;32 17β-estradiol, 40 pM;32 dihydrotestosterone and testosterone, <15–30 nM, based on the concentration of testosterone;33 aldosterone, 0.2 nM34 1,25 dihydroxyvitamin D, based on circulating 25 hydroxyvitamin D of 80 nM in humans35 and thyroxine, 10 nM.36 These approx. concentrations were used or increased to 10 nM to avoid false negative results. D, immunohistochemical staining for CYP2E1 and/or amylase. No 1° Ab, staining under identical conditions without the addition of primary antibodies. All data typical of at least 10 separate experiments. | ||
To establish whether trans-differentiation is a response that can occur on exposure to other steroids, the B-13 cell was screened for its response to a range of glucocorticoids and other steroids. Fig. 1C demonstrates that B-13 cells responded to the endogenous rat glucocorticoid corticosterone at both normal endogenous (300 nM)12 and elevated Cushing's disease levels (1.5 μM)8 as well as to a range other synthetic clinically-employed glucocorticoids: prednisolone, DEX and betamethasone (Fig. 1C). In contrast, no other steroids or nuclear receptor activators directed the B-13 cells to a B-13/H phenotype (data not shown) or induced the expression of hepatocyte genes (Fig. 1C). Fig. 1C also indicates that B-13/H cells express similar levels of CYP2E1 and CPSI as freshly isolated rat hepatocytes, confirming their quantitative comparability to normal liver hepatocytes in terms of expression levels.
Immunocytochemical staining confirmed that cells B-13/H cells retained expression of both amylase and CYP2E1 and that it was not a sub-set of cells within the culture expressing CYP2E1 only (Fig. 1D). These data indicate that the appearance of B-13/H cells in vitro is a specific response to glucocorticoids that does not occur in response to other steroids or ligands of other closely-related nuclear receptors, and that B-13/H appearance is dependent on trans-differentiation from acinar cells.
Adult human acinar cells express hepatocyte markers in vivo and in vitro in response to glucocorticoid exposure
Previous work has shown that markers of liver gene expression appear in rodent pancreata13–16 and human fetal acinar cells.17 To establish whether adult human pancreatic tissue is similarly plastic in vivo and responsive to glucocorticoid, tissue from a patient treated for at least 20 years with systemic glucocorticoid (NP10) was compared to tissue from patients with no history of long term systemic glucocorticoid therapy (Fig. 2). Fig. 2A demonstrates that 3 mRNA transcript markers expressed at high levels in hepatocytes (cytochrome P450 – CYP3A4) or specifically in hepatocytes (carbamyl phosphate synthase [CPSI] and albumin) were readily detectable in tissue from patient NP-10, but were undetectable in 4 control pancreas samples (patients with no history of systemic glucocorticoid therapy). Examination at the protein level using Western blotting confirmed expression of liver proteins in the NP10 pancreas, and indicate that the levels of expression are similar to that observed in human liver (Fig. 2B). Immunohistochemical analyses confirmed that hepatocyte gene expression was present in the acinar regions of the NP10 pancreas (Fig. 2C). These data confirm the observations made on archived pancreatic tissue isolated from 2 other patients maintained on systemic glucocorticoid (Fig. 3).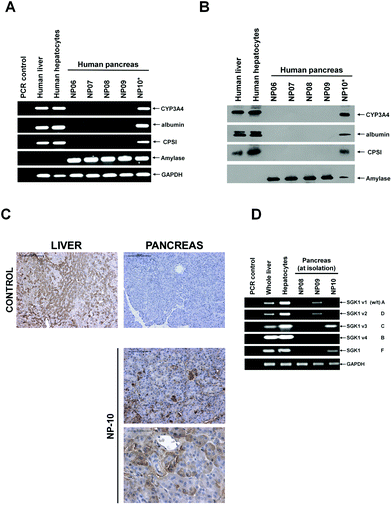 | ||
| Fig. 2 Screening human pancreas samples for the expression of hepatic markers. A, RT-PCR for the indicated transcript. PCR control, amplification in the absence of RNA in the 1st strand cDNA synthesis step. B, Western blot for the indicated protein. C, Immunohistochemical staining for CYP3A4 in sections from control human liver and pancreas and in pancreas sections from NP10, no primary control – identical staining without primary antibody incubation. | ||
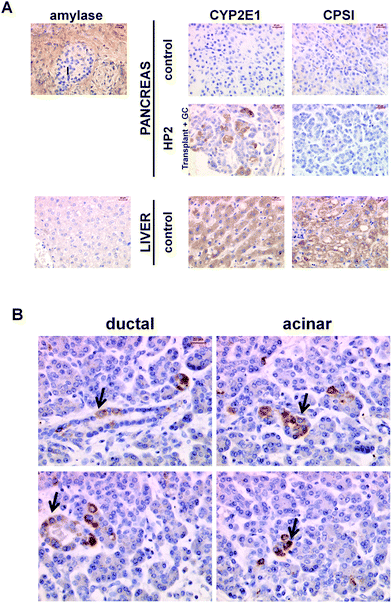 | ||
| Fig. 3 Immunohistochemical staining of human pancreas and liver sections. (A) Sections were stained for the exocrine pancreas marker amylase or liver markers CYP2E1 and CPS-I. Control pancreas was obtained from a patient with no record of extended systemic glucocorticoid therapy and no overt pancreatic abnormalities in H and E-stained sections. Two human pancreas samples from patients (HP1 and HP2) maintained on systemic glucocorticoid were available as archived paraffin blocks only. HP1 was a 65 year old male that had been maintained on prednisolone for 8 months for the treatment of pemphigoid. The patient underwent a pancreaticoduodenectomy for a pancreatic adenocarcinoma. HP2 was a 35 year old female recipient who received a simultaneous pancreas kidney transplant for end stage diabetic nephropathy. Her immunosuppressive regimen included prednisolone. Approximately 1 year after implantation the pancreas had to be removed because of sepsis and pancreas graft rejection/thrombosis. Liver sections were prepared from normal tissue at the margins of an hepatectomy for a secondary tumour. Mild steatosis observed is common. (B) Further high powered fields from HP2 immunostained for CYP2E1, demonstrating positive staining in both acinar and ductal cells. In general, positive cells were observed in acinar regions in occasionally in duct cells. Similar results were observed in sections from HP1, data not included. | ||
Previous work has identified that SGK1 is markedly up-regulated in B-13 cells when treated with glucocorticoid, most notably an alternatively transcribed variant 3 (also termed variant C).4 Transfecting B-13 cells with expression vectors encoding the different human SGK1 variants demonstrated that SGK1 v3 (and an unassigned human-specific F variant) directed B-13 cells into hepatocytes without glucocorticoid treatment, demonstrating a pivotal role for specific SGK1 transcript induction in the trans-differentiation response.4Fig. 2D demonstrates that transcripts for SGK1 v3 and SGK1 variant F were detectable in NP08 and NP10 pancreata, with the highest expression in NP10 (the patient maintained long term on systemic glucocorticoid and showing extensive evidence of hepatocyte gene expression in their pancreas at resection).
To establish whether adult human pancreatic acinar cells are capable of trans-differentiation to hepatocyte-like cells in vitro, acinar cells were isolated and cultured with or without the addition of 10 μM dexamethasone (DEX), a synthetic glucocorticoid. Fig. 4A indicates that cells from patient NP8 expressed a range of hepatic markers at the mRNA level (CYP2E1, CPSI, albumin, CYP3A4) in response to DEX treatment, with no expression detected in cells cultured in basal media. Cells from patient NP10 – which already expressed hepatic markers – retained the expression of the hepatic markers when cultured with DEX, except for albumin mRNA. CYP1A1 mRNA, which was not induced in NP08 cells by DEX, was induced from undetectable levels by DEX in NP10 cells (Fig. 4A). Fig. 4B demonstrates that cultured human acinar cells retained the expression of amylase and that treatment with DEX resulted in the co-expression of CYP2E1 and amylase as previously observed with B-13 cells.1–4 Thus, DEX treatment likely results in the trans-differentiation of acinar cells to hepatocyte-like cells, rather than hepatic differentiation of a progenitor cell present within the culture. Furthermore, whereas acinar cells became more fibroblastic in morphology with time in culture, the addition of DEX promoted a more epithelial morphology. Fig. 4C shows the levels of expression of SGK1 variants after culture for 14 days with or without the addition of glucocorticoid. DEX treatment induced all SGK1 variants in cells isolated from patient NP08 (which trans-differentiated into hepatocytes in response to DEX), whereas cells from patient NP09 (which trans-differentiated into hepatocytes in response to DEX with low efficiency – data not shown) only showed induction of SGK1 v1 transcript and no expression at any time of SGK1 v3 and F (Fig. 4C). Acinar cells from patient NP10 (which already contained hepatocytes due to prolonged therapy with glucocorticoid) showed retained or further induction of SGK1 variant transcripts with DEX treatment (Fig. 4C).
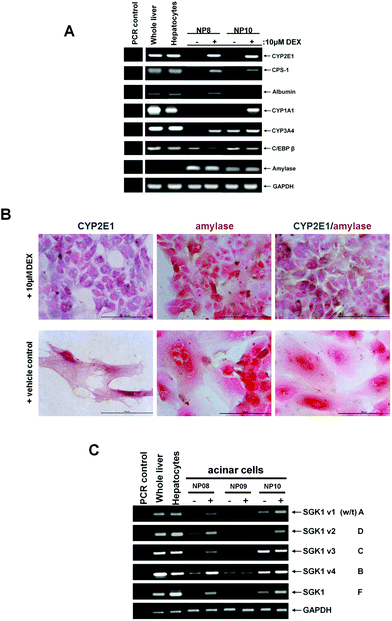 | ||
| Fig. 4 Human pancreatic acinar cells in culture express hepatocyte marker genes in response to treatment with glucocorticoid – trans-differentiation correlates with SGK1 v3 and F induction. Human pancreatic acinar cells were isolated as outlined in the methods section and where indicated, treated with 10 μM dexamethasone (DEX) or ethanol vehicle as control. After 14 days, cells were harvested and analyzed. (A) RT-PCR for the indicated transcript. PCR control, amplification in the absence of RNA in the 1st strand cDNA synthesis step. (B) Immunohistochemical staining for amylase and CYP2E1 in human acinar cells cultured in control medium or medium supplemented with 10 μM DEX for 14 days. Cells were fixed and co-stained amylase (brown) and CYP2E1 (blue) – data typical of staining from cells isolated from 5 individuals. C, RT-PCR for SGK1 variant transcript expression in human acinar cells treated for 14 days with (+) or without (−) 10 μM DEX for 14 days. | ||
Discussion
The data in this paper demonstrate for the first time that hepatocyte-like cells appear in the adult human pancreas in response to prolonged glucocorticoid exposure. Thus, the pathophysiological response of this tissue to glucocorticoid in rodents (and the mechanisms which regulate it), also exists in man. It is therefore reasonable to predict that if the changes that have occurred in B-13 cells can be replicated in a human pancreatic acinar cell, they will behave similarly to B-13 cells. Such a resource would have extensive utility in a range of toxicity investigations.An intriguing question is why hepatocyte-like cells appear in the pancreas in response to excessive glucocorticoid exposure? In addition to their role in playing a regulatory role in metabolism, glucocorticoids have an important role in cellular differentiation. Glucocorticoid levels are low in the developing fetus because placental 11β hydroxysteroid dehydrogenase 2 inactivates maternal glucocorticoid.18 In humans and many animal species, there is a rise in glucocorticoid concentrations during late pregnancy that parallels the increased maturity of fetal organs.19 The importance of glucocorticoids in development is clearly illustrated in the lung, where therapeutic use reduces the incidence of respiratory distress in premature birth.20,21 It is likely therefore, that normal glucocorticoid concentrations promote the maturation of tissues toward a neonatal phenotype. This is supported by empirical observations with stem cell differentiation to mature cell types and has resulted in glucocorticoid frequently being incorporated into media designed to promote differentiation to mature phenotypes.22–26 Since pancreatic tissue is a default state of differentiation for embryonic pancreatic cells capable of being directed toward an hepatic phenotype,27 it is likely that the mechanism(s) which promotes this process remains functional in adult cells and/or may modulate differentiation when normal adult cells are exposed to high levels of glucocorticoid.
Recent data from this laboratory has shown that B-13 cells have a number of chromosomal changes (but retain a 4n complement of chromosomes),28 a feature typical of many dividing cells that have been propagated for some time in vitro but not undergone transformation, such as embryonic stem cell lines.29 However, B-13 do not grow in soft agar or form tumours in NOD/SCID mice, indicating that they retain a requirement for anchorage-dependent growth and responsiveness to factors which prevent un-controlled cell growth.28 Interestingly, B-13 cells only engraft in to the liver and pancreas.28
The importance of understanding the genetic changes that have occurred in the B-13 cells as well as the mechanisms that control differentiation lies in the fact that a human equivalent would have extensive basic research and clinical value. Human hepatocytes are a scarce resource and a human B-13 equivalent would provide a plentiful supply of functional cells to be generated in vitro. Also, because the B-13 progenitor cell is proliferative, recombinant DNA technologies can be applied to manipulate the cells and to generate hepatocytes with genetic alterations. Hepatocytes are a valuable resource for screening drugs and chemical metabolism and toxicity.6 Stably transfecting variant drug metabolizing enzyme or drug transporter genes in human B-13 cell lines would significantly advance testing for drug and chemical metabolism and toxicity in man.
Materials and methods
Human tissues
Human liver and pancreas tissue was obtained with patient consent and with approval of the Newcastle & North Tyneside 2 Research Ethics Committee and the Wales Research Ethics Committee respectively (see Table 1 for patient details). Human liver tissue was prepared from the margins of tissue removed from patients having a resection for both benign and malignant tumours.| Patient code | Age/Sex | Disease status |
|---|---|---|
| Tissue at the margins of the resection with a normal macroscopic appearance, and as far away from the tumor as possible, were sampled in these studies. For pathology archived block samples – HP1 and HP2 – which were only available as fixed tissue – see Fig. 3 legend. | ||
| NP06 | 78/♂ | Pancreaticoduodectomy for an ampullary adenocarcinoma. Pancreatic parenchyma demonstrated mild focal atrophy but no evidence of chronic pancreatitis. No history of systemic glucocorticoid therapy. |
| NP07 | 47/♀ | Pancreaticoduodectomy for distal cholangiocarcinoma. Pancreatic parenchyma lobular structure preserved with patchy atrophy, fibrosis and inflammation consistent with obstructive pancreatitis. Islets of Langerhans appear normal. No history of systemic glucocorticoid therapy. |
| NP08 | 52/♂ | Pancreaticoduodectomy for a pancreatic ductal adenocarcinoma. Background pancreatic parenchyma preserved with a normal lobular architecture. Islets of Langerhans appear normal. No history of systemic glucocorticoid therapy. |
| NP09 | 75/♂ | Pancreaticoduodectomy and liver resection for a gall bladder adenocarcinoma. Pancreatic parenchyma shows normal lobular architecture with no metaplastic or dysplastic changes in the pancreatic ducts. Islets of Langerhans show normal distribution and size. No history of systemic glucocorticoid therapy. |
| NP10 | 87/♀ | Distal pancreatectomy, splenectomy and nephrectomy for ductal adenocarcinoma of the tail of the pancreas. Background parenchyma shows pancreatic atrophy, patchy chronic inflammation. Patient had diabetes and polymyalgia rheumatica. Received prednisolone therapy for at least 20 years. |
RT-PCR
Total RNA was purified using Trizol (Invitrogen, Paisley, UK) and RT-PCR performed and analysed essentially as previously outlined.7 Primer sequences are given in Table 2. Primer sequences for the amplification of rat sequences have been published previously.7| Oligo ID | 5′-3′ sequence | An'ling conditions (°C) | Comments |
|---|---|---|---|
| SGK1 Isoform 1 (NM_005627.3) is the wild type form SGK1A along with the 3 other forms identified by Simon et al.37 – isoform 2 (NM_001143676.1) is SGK1D; isoform 3 (NM_001143677.1) is SGK1C and isoform 4 (NM_001143678.1) is SGK1B. The SGK1F form sequence has been directly submitted to NCBI only – acc# CAI19718 and # FM205710. | |||
| RT-PCR | |||
| hCYP2E1US | TCCTTCACCCGGTTGGCCCA | 58 | Will amplify human CYP2E1 (NM_000773.3) cDNA sequence of 93 bp. |
| hCYP2E1DS | CACCGCCTTGTAGCCGTGCA | ||
| hCYP3A4US | ACGGGACTATTTCCACCACCCCC | 56 | Will amplify human CYP3A4 (NM_017460.3) cDNA sequence of 322 bp. |
| hCYP3A4DS | CTGACAAAGGCCCCACGCCAA | ||
| hCYP1A1US | TTCCCTGATCCTTGTGATCCCAGGC | Will amplify human CYP1A1 (NM_000499.3) cDNA sequence of 781 bp. | |
| hCYP1A1DS | AGAGCCAACCACCTCCCCGAA | 56 | |
| hC/EBPβUS | CCAGCCACCAGCCCCCTCACTAATA | 58 | Will amplify human CAAT/EBPβ (NM_005194.2) cDNA sequence of 264 bp. |
| hC/EBPβDS | CCAAGCAGTCCGCCTCGTAGT | ||
| hALBUMINUS | AGCTGCCTGCCTGTTGCCAAA | 56 | Will amplify human albumin (NM_000477.5) cDNA sequence of 135 bp. |
| hALBUMINDS | AGGCGAGCTACTGCCCATGC | ||
| hCPSIUS | TTTAGCCGAGGCCCATGCCACA | 58 | Will amplify human CPSI variant 1 (NM_001122633.1) cDNA sequence of 238 bp. US primer does not hybridise to variant 2 (NM_001875.3) |
| hCPSIDS | CCAGCAACAGAGGATGGATGGCC | ||
| hAMYLASEUS | ACATGGGGCTGGAGGAGCCT | 55 | Will amplify human amylase – alpha 2A (NM_000699.2) and human amylase alpha 2B (NM_020978.3) cDNAs sequences of 170 and 172 bp respectively. |
| hAMYLASEDS | TGGTGGCCCAACCCAATCAT | ||
| rmhGAPDHUS | TGACATCAAGAAGGTGGTGAAG | 50 | Will amplify rat (NM_017008), human (NM_002046) or mouse (NM_008084) glyceraldehyde 3 phosphate dehydrogenase cDNA sequence of 243 bp. |
| rmhGAPDHUS | TGACATCAAGAAGGTGGTGAAG | ||
| hSGK1v1US | CGAGCCGGTCTTTGAGCGCTAAC | 55 | Will amplify human SGK1 variant 1 (NM_005627.3) cDNA sequence of 118 bp. Also referred to as SGK1A and wild type sequence. |
| hSGK1v1DS | GAATTGCCACCATGCCCCTCATCC | ||
| hSGK1v2US | CCCTCTGCCTTTCTGGCGCTGTTC | 55 | Will amplify human SGK1 variant 2 (NM_001143676.1) cDNA sequence of 140 bp. Also referred to as SGK1D. |
| hSGK1v2DS | CTGGAGGCGGCTTGAGAGAGGAG | ||
| hSGK1v3US | GAACAGGGATAGCCGTCTCTGGC | 55 | Will amplify human SGK1 variant31 (NM_001143677.1) cDNA sequence of 121 bp. Also referred to as SGK1C. |
| hSGK1v3DS | TTCTGGAGGCTGGAGGTAGAGCC | ||
| hSGK1v4US | AAAAGGCGTTTTCGGAAGCGACCC | 55 | Will amplify human SGK1 variant 4 (NM_001143678.1) cDNA sequence of 140 bp. Also referred to as SGK1B. |
| hSGK1v4DS | CAGACGAGAGCGACCGGCGAG | ||
| hSGK1FUS | TCTCCTCCTTCATCCACAGCTTTCA | 55 | Will amplify a novel human SGK1 variant (CAI19718) cDNA sequence of 211 bp. Also referred to as SGK1F. |
| hSGK1FDS | TGGACGACGGGCCAAGGTTG | ||
Western blotting
Western blotting was performed essentially as previously outlined30 with antibodies described previously.6–8 Detection was achieved using an ECL kit (Amersham, UK).Immunohistochemistry
Tissues were fixed in formalin and processed for immunohistochemistry as previously outlined with antigen retrieval using 0.01 M citrate buffer.30 Tissue sections were then incubated with 3% H2O2 for 10 minutes at room temperature (RT) to quench endogenous peroxide activity, and then washed for 5 minutes in 1 × PBS. Cultured cells were prepared by permeabilising in ice cooled methanol for 10 minutes before washing in 1 × PBS and fixing in 2% w/v formaldehyde and 0.2% glutaraldehyde in 1 × PBS pH 7.4. Non-specific binding of antibodies was blocked in all samples through incubation with 20% (v/v) bovine foetal calf serum (FCS) in 1 × PBS for 20 minutes at room temperature. Samples were then incubated with primary antibodies diluted in 0.05% (v/v) FCS; anti-CYP2E1 (Abcam – ab28146 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 dilution); anti-amylase (Santa-Cruz – SC12821 1
200 dilution); anti-amylase (Santa-Cruz – SC12821 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 dilution), and left to incubate overnight at 4 °C. Samples were washed twice in 1 × PBS before applying secondary antibodies for 1 hour at RT – alkaline phosphatase conjugated donkey polyclonal anti-rabbit IgG (Abcam – ab97061) and peroxidise conjugated rabbit anti-goat IgG (Sigma – A5420) both at a 1
300 dilution), and left to incubate overnight at 4 °C. Samples were washed twice in 1 × PBS before applying secondary antibodies for 1 hour at RT – alkaline phosphatase conjugated donkey polyclonal anti-rabbit IgG (Abcam – ab97061) and peroxidise conjugated rabbit anti-goat IgG (Sigma – A5420) both at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 dilution. Samples were washed twice in 1 × PBS before colour development was carried out using BCIP®/NBT substrate system followed by Liquid DAB + substrate chromogen system for CYP2E1 (alkaline peroxidase activity) and Amylase (HRP activity) expression respectively. In all cases control sections were stained without primary antibody incubation for each individual antibody used. After colour development slides were rinsed briefly in H2O and counterstained using nuclear fast red (Vector laboratories) when required. Samples were sequentially dehydrated through 50%, 75%, 95%, 100% ethanol and finally into xylene before mounting in depex™.
200 dilution. Samples were washed twice in 1 × PBS before colour development was carried out using BCIP®/NBT substrate system followed by Liquid DAB + substrate chromogen system for CYP2E1 (alkaline peroxidase activity) and Amylase (HRP activity) expression respectively. In all cases control sections were stained without primary antibody incubation for each individual antibody used. After colour development slides were rinsed briefly in H2O and counterstained using nuclear fast red (Vector laboratories) when required. Samples were sequentially dehydrated through 50%, 75%, 95%, 100% ethanol and finally into xylene before mounting in depex™.
Cell isolation and culture
Acinar cells were obtained as by-products of human islet isolation as described previously31 with minor modification. Briefly, human pancreas was distended with a freshly prepared solution consisting of ∼2000 units of collagenases NB1 (Serva, Germany) and 0.15 mg ml−1 Pefabloc SC Plus serine protease inhibitor (Roche, Germany) and followed up with ∼25 units of neutral protease NB (Serva, Heidelberg, Germany). The pancreas was then digested at 37 °C. Human islets were purified using a cooling COBE 2991 machine. The remaining enriched exocrine tissues were retrieved and washed by re-suspending in MEM solution centrifuged at 1000 rpm in a bench top centrifuge for 1 min. The supernatant was discarded and the cells washed a further two times. The cells where then cultured in Dulbecco's minimum essential medium supplemented with 10% (v/v) fetal calf serum, 80 μg ml−1 penicillin and 80 μg ml−1 streptomycin.Rat and human hepatocytes were prepared by collagenase perfusion essentially as previously described.30 B-13 cells were routinely cultured in Dulbecco's minimum essential medium supplemented with 10% (v/v) FCS, 80 μg ml−1 penicillin and 80 μg ml−1 streptomycin. All cells were incubated at 37 °C in an humidified incubator gassed with 5% CO2 in air. Steroids and other compounds were all purchased from the Sigma Chem Co. (Poole, UK) and were added to medium from 1000-fold concentrated ethanol vehicle solvated stocks, control cells were treated with 0.1% (v/v) ethanol alone as control.
Acknowledgements
Supported in part by a Medical Research Council ITTP PhD studentship to EAF and by European Commission FP7 program grant ‘D-Liver’ (EC Contract No. 287596; http://www.D-LIVER.eu/).References
- C. N. Shen, J. M. Slack and D. Tosh, Molecular basis of transdifferentiation of pancreas to liver, Nat. Cell Biol., 2000, 2, 879–887 CrossRef CAS.
- C. J. Marek, G. A. Cameron, L. J. Elrick, G. M. Hawksworth and M. C. Wright, Generation of hepatocytes expressing functional cytochromes P450 from a pancreatic progenitor cell line in vitro, Biochem. J., 2003, 370, 763–769 CrossRef CAS.
- K. Wallace, C. J. Marek, S. Hoppler and M. C. Wright, Glucocorticoid-dependent transdifferentiation of pancreatic progenitor cells into hepatocytes is dependent on transient suppression of WNT signalling, J. Cell Sci., 2010, 123, 2103–2110 CrossRef CAS.
- K. Wallace, Q. Long, E. A. Fairhall, K. A. Charlton and M. C. Wright, Serine/threonine protein kinase SGK1 in glucocorticoid-dependent trans-differentiation of pancreatic acinar cells to hepatocytes, J. Cell Sci., 2011, 124, 405–413 CrossRef CAS.
- D. Eberhard, K. O'Neill, Z. D. Burke and D. Tosh, In vitro reprogramming of pancreatic cells to hepatocytes, Methods Mol. Biol., 2010, 636, 285–292 CAS.
- K. Wallace, E. A. Fairhall, K. A. Charlton and M. C. Wright, AR42J-B-13 cell: an expandable progenitor to generate an unlimited supply of functional hepatocytes, Toxicology, 2010, 278, 277–287 CrossRef CAS.
- K. Wallace, C. J. Marek, R. A. Currie and M. C. Wright, Exocrine pancreas trans-differentiation to hepatocytes – a physiological response to elevated glucocorticoid in vivo, J. Steroid Biochem. Mol. Biol., 2009, 116, 76–85 CrossRef CAS.
- K. Wallace, P. A. Flecknell, A. D. Burt and M. C. Wright, Disrupted pancreatic exocrine differentiation and malabsorption in response to chronic elevated systemic glucocorticoid, Am. J. Pathol., 2010, 177, 1225–1232 CrossRef CAS.
- P. A. Hall and N. R. Lemoine, Rapid acinar to ductal transdifferentiation in cultured human exocrine pancreas, Am. J. Pathol., 1992, 166, 97–103 CAS.
- M. R. Vila, J. Lloreta and F. X. Real, Normal human pancreas cultures display functional ductal characteristics, Lab. Invest., 1994, 71, 423–431 CAS.
- K. S. Zaret and M. Grompe, Generation and regeneration of cells of the liver and pancreas, Science, 2008, 322, 1490–1494 CrossRef CAS.
- S. L. Lightman, C. C. Wiles, H. C. Atkinson, D. E. Henley, G. M. Russell, J. A. Leendertz, M. A. McKenna, F. Spiga, S. A. Wood and B. L. Conway-Campbell, The significance of glucocorticoid pulsatility, Eur. J. Pharmacol., 2008, 583, 255–262 CrossRef CAS.
- M. S. Rao, R. S. Dwivedi, V. Subbarao, M. I. Usman, D. G. Scarpelli, M. R. Nemali, A. Yeldandi, S. Thangada, S. Kumar and J. K. Reddy, Almost total conversion of pancreas to liver in the adult rat: a reliable model to study transdifferentiation, Biochem. Biophys. Res. Commun., 1988, 156, 131–136 CrossRef CAS.
- A. V. Yeldandi, X. D. Tan, R. S. Dwivedi, V. Subbarao, D. D. Smith Jr, D. G. Scarpelli, M. S. Rao and J. K. Reddy, Coexpression of glutamine synthetase and carbamoylphosphate synthase I genes in pancreatic hepatocytes of rat, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 881–885 CrossRef CAS.
- M. L. Krakowski, M. R. Kritzik, E. M. Jones, T. Krahl, J. Lee, M. Arnush, D. Gu and N. Sarvetnick, Pancreatic expression of keratinocyte growth factor leads to differentiation of islet hepatocytes and proliferation of duct cells, Am. J. Pathol., 1999, 154, 683–691 CrossRef CAS.
- T. Yamaoka, K. Yoshino, T. Yamada, M. Yano, T. Matsui, T. Yamaguchi, M. Moritani, J. Hata, S. Noji and M. Itakura, Transgenic expression of FGF8 and FGF10 induces transdifferentiation of pancreatic islet cells into hepatocytes and exocrine cells, Biochem. Biophys. Res. Commun., 2002, 292, 138–143 CrossRef CAS.
- S. Sumitran-Holgersson, G. Nowak, S. Thowfeequ, S. Begum, M. Joshi, M. Jaksch, A. Kjaeldgaard, C. Jorns, B. G. Ericzon and D. Tosh, Generation of hepatocyte-like cells from in vitro transdifferentiated human fetal pancreas, Cell Transplant., 2009, 18, 183–193 CrossRef.
- J. R. Seckl and M. C. Holmes, Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology, Nat. Clin. Pract. Endocrinol. Metab., 2007, 3, 479–488 CrossRef CAS.
- V. E. Murphy, R. Smith, W. B. Giles and V. L. Clifton, Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus, Endocr. Rev., 2006, 27, 141–169 CrossRef.
- G. C. Liggins and R. N. Howie, A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants, Pediatrics, 1972, 50, 515–525 CAS.
- P. L. Schmidt, M. E. Sims, H. T. Strassner, R. H. Paul, E. Mueller and D. McCart, Effect of antepartum glucocorticoid administration upon neonatal respiratory distress syndrome and perinatal infection, Am. J. Obstet. Gynecol., 1984, 148, 178–186 CAS.
- T. Hamazaki, Y. Iiboshi, M. Oka, P. J. Papst, A. M. Meacham, L. I. Zon and N. Terada, Hepatic maturation in differentiating embryonic stem cells in vitro, FEBS Lett., 2001, 497, 15–19 CrossRef CAS.
- Y. Yajima, M. Sato, M. Sumida and S. Kawashima, Mechanism of adult primitive mesenchymal ST-13 preadipocyte differentiation, Endocrinology, 2003, 144, 2559–2565 CrossRef CAS.
- A. S. Srivastava, S. Kaushal, R. Mishra, T. A. Lane and E. Carrier, Dexamethasone facilitates erythropoiesis in murine embryonic stem cells differentiating into hematopoietic cells in vitro, Biochem. Biophys. Res. Commun., 2006, 346, 508–516 CrossRef CAS.
- W. L. Randle, J. M. Cha, Y. S. Hwang, K. L. Chan, S. G. Kazarian, J. M. Polak and A. Mantalaris, Integrated 3-dimensional expansion and osteogenic differentiation of murine embryonic stem cells, Tissue Eng., 2007, 13, 2957–2970 CrossRef CAS.
- M. Ren, L. Yan, C. Z. Shang, J. Cao, F. P. Li, J. Y. Li, H. Cheng and J. Min, Sodium butyrate and dexamethasone promote exocrine pancreatic gene expression in mouse embryonic stem cells, Acta Pharmacol. Sin., 2009, 30, 1289–1296 CrossRef CAS.
- D. J. Elbaum, E. M. Bender, J. M. Brown and P. L. Keyes, Serum progesterone in pregnant rats with ectopic or in situ corpora lutea: correlation between amount of luteal tissue and progesterone concentration, Biol. Reprod., 1975, 13, 541–545 CrossRef CAS.
- E. A. Fairhall, K. Wallace, C. J. Schwab, C. J. Harrison, K. A. Charlton and M. C. Wright, The B-13 hepatic progenitor cell line specifically engrafts to the liver and pancreas, Manuscript submitted, 2012.
- D. E. Baker, N. J. Harrison, E. Maltby, K. Smith, H. D. Moore, P. J. Shaw, P. R. Heath, H. Holden and P. W. Andrews, Adaptation to culture of human embryonic stem cells and oncogenesis in vivo, Nat. Biotechnol., 2007, 25, 207–215 CrossRef CAS.
- E. L. Haughton, S. J. Tucker, C. J. Marek, E. Durward, V. Leel, Z. Bascal, T. Monaghan, M. Koruth, E. Collie-Duguid, D. A. Mann, J. E. Trim and M. C. Wright, Pregnane X receptor activators inhibit human hepatic stellate cell transdifferentiation in vitro, Gastroenterology, 2006, 131, 194–209 CrossRef CAS.
- G. C. Huang, M. Zhao, P. Jones, S. Persaud, R. Ramracheya, K. Löbner, M. R. Christie, J. P. Banga, M. Peakman, P. Sirinivsan, M. Rela, N. Heaton and S. Amiel, The development of new density gradient media for purifying human islets and islet-quality assessments, Transplantation, 2004, 77, 143–145 CrossRef CAS.
- D. Alvaro, G. Alpini, P. Onori, L. Perego, G. Svegliata Baroni, A. Franchitto, L. Baiocchi, S. S. Glaser, G. LeSage, F. Folli and E. Gaudio, Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats, Gastroenterology, 2000, 119, 1681–1691 CrossRef CAS.
- R. M. Gow, M. K. O'Bryan, B. J. Canny, G. T. Ooi and M. P. Hedger, Differential effects of dexamethasone treatment on lipopolysaccharide-induced testicular inflammation and reproductive hormone inhibition in adult rats, J. Endocrinol., 2001, 168, 193–201 CrossRef CAS.
- M. Wagner, E. Rudakova and T. Volk, Aldosterone-induced changes in the cardiac L-type Ca2+ current can be prevented by antioxidants in vitro and are absent in rats on low salt diet, Pfluegers Arch., 2008, 457, 339–349 CrossRef CAS.
- N. Binkley, R. Novotny, D. Krueger, T. Kawahara, Y. G. Daida, G. Lensmeyer, B. W. Hollis and M. K. Drezner, Low vitamin D status despite abundant sun exposure, J. Clin. Endocrinol. Metab., 2007, 92, 2130–2135 CrossRef CAS.
- Y. Kato, H. Suzuki, S. Ikushiro, S. Yamada and M. Degawa, Decrease in serum thyroxine level by phenobarbital in rats is not necessarily dependent on increase in hepatic UDP-glucuronosyltransferase, Drug Metab. Dispos., 2005, 33, 1608–1612 CrossRef CAS.
- P. Simon, M. Schneck, T. Hochstetter, E. Koutsouki, M. Mittelbronn, A. Merseburger, C. Weigert, A. Niess and F. Lang, Differential regulation of serum- and glucocorticoid-inducible kinase 1 (SGK1) splice variants based on alternative initiation of transcription, Cell Physiol. Biochem., 2007, 20, 715–728 CrossRef CAS.
Footnote |
| † Joint first authors. |
| This journal is © The Royal Society of Chemistry 2013 |
