11-Mercaptoundecanoic acid directed one-pot synthesis of water-soluble fluorescent gold nanoclusters and their use as probes for sensitive and selective detection of Cr3+ and Cr6+†
Jian
Sun
,
Jie
Zhang
and
Yongdong
Jin
*
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, Jilin, P.R. China. E-mail: ydjin@ciac.jl.cn; Fax: +86-431-85262661; Tel: +86-431-85262661
First published on 22nd October 2012
Abstract
We report a one-pot approach to prepare fluorescent gold nanoclusters (AuNCs) from HAuCl4 by simply using 11-mercaptoundecanoic acid (11-MUA) as a reducing and capping agent, in an aqueous solution of NaOH at room temperature. The as-prepared water-soluble AuNCs with average diameters of 1.8 ± 0.4 nm exhibit a unique fluorescence excitation at 285 nm, a maximum emission at 608 nm, and a quantum yield of 2.4%. We find that the fluorescence of 11-MUA-AuNCs can be quenched by several metal ions but selectively by Cr3+ ions when using EDTA as the masking agents for other metal ions. This phenomenon is further exploited as a “turn-off” fluorescent sensor for sensitive and selective detection of Cr3+ ions. Upon the quantitative addition of Cr3+ in the presence of EDTA, the fluorescence intensity quenches linearly within the range of 25 nM to 10 μM with high sensitivity (LOD = 26 nM, S/N = 3). Furthermore, the 11-MUA-AuNCs could be used to detect Cr6+ indirectly by using ascorbic acid to reduce Cr6+ into Cr3+ in aqueous solution.
Introduction
Gold nanoparticles (AuNPs), typically with sizes of ∼2 to 100 nm, are well-studied and widely-employed for sensing, bioassays, and nanomedicine owing to their tunable electronic structures and broad material properties, especially the size dependent surface plasmon absorption properties.1 But over the past decade, ultrasmall AuNPs, or the quantum sized gold nanoclusters (AuNCs), which vary in smaller sizes from several atoms to a few nanometers (typical <2 nm), have been developed and stimulated extensive interest.2 The ultra-small size of these AuNCs induces distinctive quantum confinement effects, which result in discrete energy levels, molecular-like properties such as fluorescence and lacking an apparent SPR band compared to the relatively larger AuNPs.3 Therefore, the AuNCs are promising for applications in sensing and bioimaging because of the unique fluorescence characteristics.4Several approaches have been developed to synthesize fluorescent AuNCs with different emission and ligands through the use of a variety of reductants and protecting agents. For example, Zheng et al.2a,b used NaBH4 as the reductant and dendrimers as templates to encapsulate few-atoms AuNCs with different emission maxima in the blue to near-IR regions. Duan et al.2c developed an etching method to synthesize polyethylenimine coated AuNCs with blue emission. Recently, Bovine serum albumin (BSA)2e and other biomolecules5 have also been used as a capping and reducing agent to prepare fluorescent AuNCs. Moreover, because of their predominant water-solubility and functionalization, several small thiolated ligands, including glutathione,6D-penicillamine,7L-3,4-dihydroxyphenylalanine4c and dihydrolipoic acid8 have been introduced in the synthesis of fluorescent AuNCs. Although the above-mentioned methods proved to be efficient, it still remains a challenge for facile one-step synthesis of fluorescent AuNCs.9
AuNCs have been successfully used as fluorescent probes for sensitive and selective detection of several heavy metal ions, such as Fe3+,4c Hg2+,10 Cu2+,11 and Pb2+.12 But there are no reports of AuNCs so far for Cr3+ sensing. Chromium is commonly found in the environment, existing in two stable oxidation states, Cr3+ and Cr6+, which can be interchangeable.13 Cr6+ is commonly used in metallurgy, pigment manufacturing and wood treatment. It is considered a severe environmental pollutant, due to its highly cytotoxicity and carcinogenicity.14 While Cr3+ is an essential micronutrient in mammals and participated in the maintenance of an effective carbohydrates, adipose and protein metabolism by activating certain enzymes.15 Its deficient dietary intake may increases the risk of diabetes, fat metabolism disorders and cardiovascular diseases. On the other hand, exposure to high levels of Cr3+ can negatively affect cellular structures and microorganisms, like Shewanella sp. MR-4, although its toxicity observed in vivo is much lower compared to that of Cr6+.16 Therefore, under the strict rules for human health, the development of simple, rapid and highly sensitive methods for selective quantificational detection of chromium species is of great significance and remains a challenge to date.17
The classical methods for detection of Cr3+ such as atomic absorption/emission spectroscopy18 or inductively coupled plasma mass spectrometry19 are time-consuming and need expensive instruments. Recently, a variety of fluorescent chemosensors,17a–c electrochemical sensors,17d and AuNP-based colorimetric sensors17e,f were reported for Cr3+ detection. However, most of these methods are tied to the use of organic solvents, complex synthesis procedures, relatively high detection limit and/or cross-sensitivity to other metal ions.
Herein, we report a facile, one-pot strategy for preparing fluorescent AuNCs directly by reducing gold salt using 11-mercaptoundecanoic acid (11-MUA) not only as the capping agent but also as the reductant in aqueous solution. Although Lai et al.20 recently used 11-MUA as an effective surfactant and high-flux X-rays from a synchrotron source to synthesize the AuNCs with sizes lower than 2 nm, the fluorescent properties of the AuNCs were not reported. Compared to the previous reported alkanethiol-capped AuNCs,6–9 our synthetic AuNCs exhibit unique optical properties, large Stokes shift and not featuring traditional reductants (e.g., NaBH4 or tetrakis(hydroxymethyl)phosphonium chloride). Subsequently, we used the as-prepared AuNCs as fluorescent probes with excellent sensitivity and selectivity for the detection of Cr3+ ions in the presence of ethylenediaminetetraacetic acid (EDTA). To our best knowledge, the present study is the first report of a fluorescent AuNCs sensor that can selectively detect Cr3+ ions in aqueous solution with a limit of detection of about 26 nM. In addition, the potential application of the fluorescent 11-MUA-AuNCs in detection of Cr6+ was investigated and discussed.
Experimental
Reagents and materials
HNO3, NaCl, LiNO3, KCl, MgCl2, AlCl3, CaCl2, CrCl3, MnCl2, FeCl2, FeCl3, CoCl2, Ni(NO3)2, CuCl2, Zn(NO3)2, AgNO3, CdCl2, HgCl2, PbCl2, K2Cr2O7 and NaOH were purchased from Beijing Chemical Works. Hydrogen tetrachloroaurate trihydrate (HAuCl4·3H2O), ascorbic acid and 11-mercaptoundecanoic acid (11-MUA) were purchased from Sigma-Aldrich. Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) and HEPES were purchased from DingGuo Biotech. Ltd. (Beijing, China). All chemicals were of analytical grade and used as received without further purification. The water used throughout all the experiments was purified through a Millipore system.Characterizations
TEM and high resolution TEM (HRTEM) measurements were carried out by using a FEI TECNAI F20 EM with an accelerating voltage of 200 kV equipped with an energy dispersive spectrometer. XPS measurements were performed with Thermo ESCALAB VG Scientific 250 and equipped with monochromatized Al Kα excitation. The fluorescence intensity (FL) spectra were recorded by a Perkin-Elmer LS-55 Luminescence Spectrometer (Perkin-Elmer Instruments U.K.). UV-Vis absorption spectra were recorded by a CARY 500 UV-Vis-near-IR Varian spectrophotometer using a 1 cm path length quartz cell at room temperature. The fluorescence lifetime measurements were recorded on a Horiba-Jobin-Yvon Fluorolog-3 spectrofluorometer equipped with a time-correlated single photon counting (TCSPC) system.Preparation and purification of AuNCs
All glassware was thoroughly cleaned with aqua regia (HNO3/HCl, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and rinsed extensively with Millipore water prior to use. The fluorescent 11-MUA-AuNCs were synthesized following a one-pot method through 11-MUA-mediated reduction of HAuCl4. Briefly, an aqueous solution of HAuCl4 (250 μL, 1% by mass) and NaOH (100 μL, 1 M) were added to the solution that was prepared by adding 6.6 mg 11-MUA to water (10 mL). The thorough mixture was left to react for 5 h at room temperature during which the colorless solution slowly turned pale yellow. The solution was purified by adding 5 mL ethanol into the aqueous solution (H2O/ethanol, 2
3) and rinsed extensively with Millipore water prior to use. The fluorescent 11-MUA-AuNCs were synthesized following a one-pot method through 11-MUA-mediated reduction of HAuCl4. Briefly, an aqueous solution of HAuCl4 (250 μL, 1% by mass) and NaOH (100 μL, 1 M) were added to the solution that was prepared by adding 6.6 mg 11-MUA to water (10 mL). The thorough mixture was left to react for 5 h at room temperature during which the colorless solution slowly turned pale yellow. The solution was purified by adding 5 mL ethanol into the aqueous solution (H2O/ethanol, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and centrifuged at 5000g for 10 min to remove the supernatant containing the free 11-MUA and gold ions. The precipitates were then washed twice by the mixed solvent (H2O/ethanol, 2
1) and centrifuged at 5000g for 10 min to remove the supernatant containing the free 11-MUA and gold ions. The precipitates were then washed twice by the mixed solvent (H2O/ethanol, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and finally re-suspended readily in water. The as-obtained purified AuNCs solution and freeze-drying powder were stored at 4 °C for further use.
1) and finally re-suspended readily in water. The as-obtained purified AuNCs solution and freeze-drying powder were stored at 4 °C for further use.
Detection of Cr3+ ions using fluorescent 11-MUA-AuNCs
The as-prepared AuNCs solutions were diluted 100 times in the HEPES buffer (10 mM, pH 7.0) containing various concentrations of Cr3+ ions in the absence or presence of EDTA (100 μM). The fluorescence quenching spectra were then recorded (excitation 280 nm; maximum emission 608 nm). To evaluate the detection selectivity, other ions such as H+, Li+, Na+, K+, Al3+, Mg2+, Ca2+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Ag+, Cd2+, Hg2+, Pb2+ and Cr2O72− were individually investigated and recorded.Detection of Cr6+ ions using fluorescent 11-MUA-AuNCs
The proton or metal ions containing H+, Li+, Na+, K+, Al3+, Mg2+, Ca2+, Mn2+, Cr3+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Ag+, Cd2+, Hg2+, Pb2+ and Cr2O72− were individually mixed with 5 times the concentration of ascorbic acid, incubated at ambient temperature for 30 min, and then added to the diluted AuNCs solutions in the HEPES buffer (10 mM, pH 7.0) in the presence of EDTA (100 μM). The fluorescence spectra of the AuNCs in the absence or presence of proton or metal ions mixed with ascorbic acid were recorded (excitation 280 nm; maximum emission 608 nm).Results and discussion
Characterization of AuNCs
Fluorescent AuNCs can be facilely prepared in aqueous solutions incorporating 11-MUA as both a reducing and protecting agent. The as-prepared 11-MUA-AuNCs were pale yellow in visible light, no fluorescence under a UV light of 365 nm wavelength, but excitedly an orange-red luminescence was observable by the naked eye under a UV light source with wavelength of 254 nm (the inset of Fig. 1A).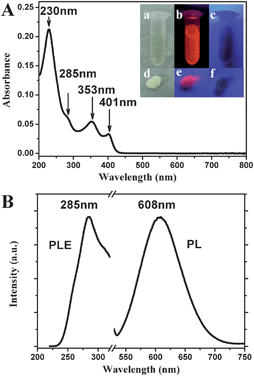 | ||
| Fig. 1 (A) UV-vis absorption spectrum of the as-prepared 11-MUA-AuNCs. The inset shows the photographs of 11-MUA-AuNCs in aqueous solution (top) or the freeze-dried powder (bottom) under irradiation of visible light (a and d), 254 nm light (b and e) and 365 nm light (c and f), respectively. (B) Fluorescence excitation and emission spectrum of 11-MUA-AuNCs. | ||
The absorption of the AuNCs solution lacks the characteristic surface plasmon resonance (SPR) peak of gold nanoparticles.21 It is suggested that highly purified AuNCs can be obtained without the formation of larger AuNPs or bulk metals as byproducts, which also could be confirmed by our TEM and HRTEM observations. Therefore, no further purification process to remove larger AuNPs is necessary in our experiments. Instead, there is a series of absorption peaks centered at about 401 nm, 353 nm, 285 nm and 230 nm, respectively, in the UV-vis absorption spectrum (Fig. 1A) due to molecular-like properties of the AuNCs.3
The fluorescence excitation spectrum of the 11-MUA-AuNCs showed a peak at about 285 nm (Fig. 1B), which was consistent with the UV-vis absorption spectrum. When the AuNCs were excited at varying wavelength from 230 to 400 nm, the emission spectra were always centered at 608 nm (Fig. 2). Meanwhile, the decrease or increase in the excitation wavelengths causes a gradual decrease in the maximum fluorescence intensity, which is observed to be excited at 285 nm. The as-prepared 11-MUA-AuNCs should be a single component owing to the inherent peaks in the excitation and emission spectra.
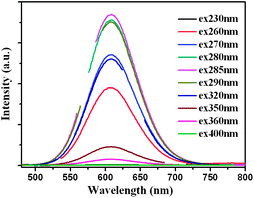 | ||
| Fig. 2 Emission spectra of 11-MUA-AuNCs at different excitation wavelengths from 230 to 400 nm. The break region of each line in the graphs is the frequency-doubled peak of the excitation wavelengths. | ||
Additional experiments containing the different molar ratios of 11-MUA to HAuCl4 were performed to find the optimal synthetic conditions for the fluorescent AuNCs. The UV-vis absorption spectra and fluorescence emission spectra (Fig. S1†) showed that there was almost no fluorescent AuNC and plasmonic AuNP formation below the ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Fluorescent AuNCs were formed with molar ratios of MUA to HAuCl4 higher than 2
1. Fluorescent AuNCs were formed with molar ratios of MUA to HAuCl4 higher than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the slight effect of the MUA/HAuCl4 ratio on emission peak (i.e. NC size) and intensity was observed. The optimal molar ratio of MUA/HAuCl4 for the highest fluorescent intensity was found to be 4
1, and the slight effect of the MUA/HAuCl4 ratio on emission peak (i.e. NC size) and intensity was observed. The optimal molar ratio of MUA/HAuCl4 for the highest fluorescent intensity was found to be 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
The as-prepared 11-MUA-AuNCs have a very large Stokes shift (more than 320 nm), quite similar to the behavior of the luminescent Au(I) complexes or polynuclear Au(I) clusters, where hybrid S–Au charge transfer states are involved in luminescence transitions.3,22 The X-ray photoelectron spectroscopy (XPS) shows that the binding energy of Au 4f7/2 is located at 84.45 eV (Fig. 3A), which is between the Au(0) film (84 eV) and the Au(I) of gold thiolate (86 eV). This result suggested that both Au(0) and Au(I) coexist in the surface of the 11-MUA-AuNCs,23 which is similar to the reported surface states of several other thiol-stabilized AuNCs.7,9
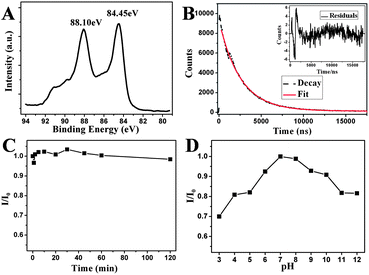 | ||
| Fig. 3 (A) XPS spectra of Au 4f for the as-prepared 11-MUA-AuNCs. (B) Fluorescence decays for 11-MUA-AuNCs in aqueous solution. The red line gives a biexponential fit and the inset is the residuals of the fits. (C) Photostability of the 11-MUA-AuNCs system measured with the relative fluorescence intensity at 608 nm of the AuNCs in water as a function of the UV irradiation time (254 nm). (D) The relative fluorescence intensity at 608 nm of the 11-MUA-AuNCs in the HEPES buffer at different pHs from 3 to 12. | ||
The quantum yield (QY) of our luminescent 11-MUA-AuNCs in aqueous solution was measured to be 2.4%, using Rhodamine B (QY = 0.31 in water) as the reference. This value is a great improvement, by approximately 2 orders of magnitude, relative to the AuNPs synthesized by reduction using NaBH43a,24 and higher than most previously reported water-soluble fluorescent AuNCs by a simple one-pot synthetic method, employing L-3,4-dihydroxyphenylalanine (L-DOPA) as a reducing/capping reagent (QY = 1.7%),4c using tetrakis(hydroxymethyl)-phosphonium chloride (THPC) as a reductant with D-penicillamine (DPA) as a capping agent (QY = 1.3%),7 and with the bidentate ligand dihydrolipoic acid (DHLA) as a stabilizer (QY = 0.6%),9 respectively. The fluorescence lifetime measurements revealed that the fluorescence decay spectra (excitation at 280 nm) could be fitted with biexponential curves, suggesting the possible existence of two components with lifetimes of 4.6 μs (81%) and 8.1 μs (19%), respectively (Fig. 3B). While the detailed emission mechanisms of these thiol-capped luminescent AuNCs are still not clear, Zheng et al.3b had a hypothesis of optical mechanisms based on previous studies on electronic structures of polynuclear Au(I) clusters, AuNCs and fully reduced Au(0) NPs. It is suggested that thiolate ligands can form hybrid states with sp orbitals of surface gold atoms Au(0), constructing the HOMO of the NPs. The microsecond lifetimes are the same with the aforementioned large Stokes shifts, which suggest that S–Au charge transfer is involved in the emission.7,11 The emission likely arises from the decay of excited electrons from higher energy states in the sp band to hybrid electronic states of surface gold atoms and thiolated ligands.
Fig. 4 displays the HRTEM images of the as-prepared, well-monodispersed 11-MUA-AuNCs. The close-up in the top inset shows lattice planes separated by 0.235 nm, corresponding to the (111) lattice spacing of the face-centered cubic Au.25 The average diameter of the AuNCs is 1.8 ± 0.4 nm, as judged from image analysis of 200 individual particles. The photostability of 11-MUA-functionalized AuNCs was also assessed using a UV light irradiation experiment. Fig. 3C shows that the fluorescence intensity did not change obviously under UV light (254 nm) irradiation after 120 minutes, meanwhile, the quantum yield of the AuNCs decreased only 10% (Fig. S2†). This indicates that the as-prepared 11-MUA-AuNCs have good photostability.
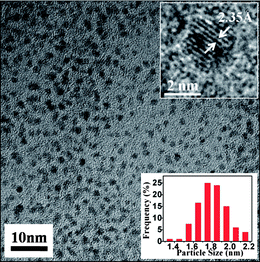 | ||
| Fig. 4 Typical TEM image of the 11-MUA-AuNCs. The insets show the HRTEM image (top) and the size distribution histogram (bottom). | ||
Detection of Cr3+ using 11-MUA-AuNCs as fluorescent probes
Before applying the 11-MUA-AuNCs for detection of metal ions in buffer solutions, the pH effect of the fluorescent AuNCs was investigated in the HEPES buffer. As shown in Fig. 3D, the low or high pH values cause a gradual decrease on the maximum fluorescence intensity, which is obtained at around neutral pH. Comprehensively considering the fluorescence intensity, metal ions properties and practical sensing applications, we chose pH 7.0 as the optimum pH value in our detection experiments.We first investigated the changes in fluorescence of the 11-MUA-AuNCs that induced with 10 μM of the following different types of metal ions and proton (H+, Li+, Na+, K+, Al3+, Mg2+, Ca2+, Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Ag+, Cd2+, Hg2+, Pb2+ and Cr2O72−), respectively in pH 7.0, 10 mM HEPES buffer. Fig. 5A displays the relative fluorescence intensity of AuNCs (I0/I). The fluorescence emission of our probe showed an obvious decrease in the presence of Cr3+, Fe2+, Fe3+, Co2+, Ni2+, and Cu2+. Recent reports4c,10,11 showed that several metal ions containing Cu2+, Fe3+, Hg2+, Pb2+ and Cd2+ could quench the fluorescence of AuNCs due to the interaction with the surface ligands outside of the AuNCs, such as the coordination of these metal ions with the carboxyl group of 11-MUA, which might block the charge transfer from the complex of S–Au to AuNCs.
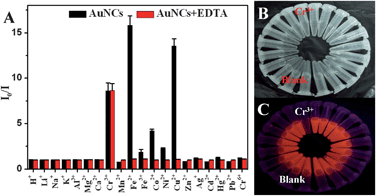 | ||
| Fig. 5 (A) Fluorescence intensity ratios (I0/I at 608 nm, where I and I0 are the corresponding fluorescence intensities at 608 nm in the presence and absence of proton or metal ions, respectively) for the 11-MUA-AuNCs measured in the absence (black) and presence (red) of EDTA (100 μM) after the addition of 10 μM proton or various individual metal ions in 10 mM HEPES buffer at pH 7.0. The photograph of the AuNCs after the addition of different metal ions under the irradiation of visible (B) and UV of 254 nm (C) light in the presence of EDTA (100 μM). | ||
To further improve the detection selectivity of metal ions of the AuNCs probe, we added a common chelating ligand, EDTA, to the solutions, which showed the effective masking ability for Fe2+, Fe3+, Co2+, Ni2+, and Cu2+ as a result. Compared to the 11-MUA with a simple carboxyl group, EDTA has the stronger affinity to the metal ions as a common poly-toothed ligand and chelating agent based on its two amines and four carboxylates.26 The fluorescence intensity of the 11-MUA-AuNCs containing 100 μM EDTA still showed a significant decrease in the presence of Cr3+, but no obvious change in the presence of Fe2+, Fe3+, Co2+, Ni2+, Cu2+ along with other metal ions (Fig. 5A). As a control, the fluorescence of metal-ion-free 11-MUA-AuNCs has a negligible change in the presence of 100 μM EDTA (data not shown). Although the detailed reasons why EDTA addition could not prevent Cr3+ from quenching the fluorescence of 11-MUA-AuNCs are still not clear, we supposed that it may be related to the paramagnetic property of Cr3+ ions27 or the affinity change of Cr3+ to the surface-bound 11-MUA owing to the electronic structure transitions after coating onto the AuNCs.3,11
As such, we found experimentally that the as-prepared 11-MUA-AuNCs, in the presence of EDTA, can be used as an efficient fluorescent probe for selective detection of Cr3+. We further quantitatively describe the response sensitivity and linearity of the current sensing system. As indicated in Fig. 6A, the sensitivity and linearity of the 11-MUA-AuNCs-Cr3+ system was evaluated by varying the Cr3+ concentrations in the presence of 100 μM EDTA. With the increase of the concentrations of Cr3+, the fluorescence emission intensity at 608 nm of the 11-MUA-AuNCs decreased gradually. There is an excellent linear relationship between the loss in fluorescence intensity and the concentration of the Cr3+ within the range of 25 nM–10 μM (Fig. 6B). The fitted linear data could be expressed as I0/I = 1 + 0.00077[Cr3+] (R2 = 0.9993). Under the current experimental conditions, the limit of detection (LOD) of Cr3+, at a signal-to-noise ratio of 3, was estimated to be 26 nM, which is approximately 1–2 orders of magnitude lower than most previously reported AuNP-based colorimetric sensors17e,f and at the same order of magnitude as several fluorescent chemosensors.17a,b The results suggested that the present 11-MUA-AuNCs system could be successfully used to detect Cr3+ in aqueous solution selectively and sensitively.
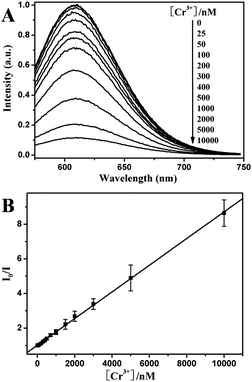 | ||
| Fig. 6 (A) Fluorescence emission spectra of the AuNCs with the addition of different Cr3+ concentrations increasing from 0 to 10 μM (top to bottom, excitation at 280 nm). (B) Fluorescence intensity ratios (I0/I at 608 nm) for AuNCs versus the Cr3+ concentrations. Measurements were performed in pH 7.0, 10 mM HEPES buffer containing 100 μM EDTA. | ||
Detection of Cr6+ using fluorescent 11-MUA-AuNCs
Since Cr6+ sensing is of great environmental interest due to its high toxicity, we further investigate the possibility of the AuNCs for Cr6+ sensing. In the previous reports,14b,28 ascorbic acid was investigated as a reductant in the highly efficient reduction of Cr6+ to Cr3+. However, the fluorescence intensity of our AuNCs would be quenched in the presence of higher concentration of ascorbic acid, because both Au(0) and Au(I) coexist in the surface of the 11-MUA-AuNCs, where the Au(I) is closely related to fluorescence and could be partly reduced by ascorbic acid. Therefore, the potential ability of fluorescent 11-MUA-AuNCs in detection of Cr6+ was investigated in the presence of lower concentration of ascorbic acid. The proton or metal ions were individually incubated with ascorbic acid at ambient temperature for 30 min, and then added to the 11-MUA-AuNCs solutions in the HEPES buffer (10 mM, pH 7.0) in the presence of EDTA (100 μM), where the concentration of metal ions and ascorbic acid were 2 μM and 10 μM, respectively. As a control, the fluorescence intensity of metal-ion-free 11-MUA-AuNCs in the buffer has only approximate 5% decrease in the presence of 10 μM ascorbic acid (data not shown). Fig. 7 shows that only Cr3+ and Cr6+ incubated with ascorbic acid could substantially quench the fluorescence of 11-MUA-AuNCs, where the concentration of metal ions is nearly to the maximum contaminant level (MCL) in drinking water for total chromium limit (100 ppb, ∼2 μM) set by the US Environmental Protection Agency (EPA). Therefore, these results suggested that our 11-MUA-AuNCs, except for Cr3+ detection, could also be used to detect Cr6+ in aqueous solution by using ascorbic acid to reduce Cr6+ into Cr3+.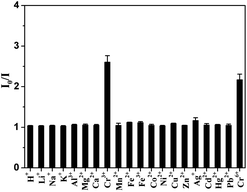 | ||
Fig. 7 Fluorescence intensity ratios (I0/I at 608 nm) for the 11-MUA-AuNCs measured after the addition of 2 μM protons or various individual metal ions in 10 mM HEPES buffer at pH 7.0 containing 100 μM EDTA. The protons or metal ions were treated beforehand with ascorbic acid for 30 min where the molar ratios of ascorbic acid to metal ions were 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
Conclusions
In summary, we describe here an 11-MUA-directed, facile one-pot method for the synthesis of water-soluble fluorescent AuNCs. The approach is simple and more environmentally friendly than previously reported etching-based or organic phase-based strategies. Along with being commonly used as a capping agent, in this study 11-MUA has been used for the first time as the reducing agent to direct synthesis of water-soluble AuNCs from HAuCl4. The unique fluorescence properties and good photostability of the as-prepared 11-MUA-AuNCs were applied for the detection of metal ions and used for highly sensitive and selective detection of Cr3+ ions in the presence of EDTA. The fluorescence intensity of the AuNCs quenched linearly with the addition of Cr3+ within the range of 25 nM to 10 μM with high sensitivity (LOD = 26 nM). The 11-MUA-AuNCs could also be used to detect Cr6+ in the presence of ascorbic acid as a reductant in aqueous solution. The study would also be extended to use other functional thiolated ligands to prepare fluorescent AuNCs with surprising optical properties and potential sensing applications in chemistry and biology.Acknowledgements
This work was supported by start-up funds from the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences and the State Key Laboratory of Electroanalytical Chemistry (no. 110000R387).Notes and references
- (a) Y. J. Kim, R. C. Johnson and J. T. Hupp, Nano Lett., 2001, 1, 165 CrossRef CAS; (b) E. Katz and I. Willner, Angew. Chem., Int. Ed., 2004, 43, 6042 CrossRef CAS; (c) P. K. Jain, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Acc. Chem. Res., 2008, 41, 1578 CrossRef CAS; (d) C. M. Cobley, J. Y. Chen, E. C. Cho, L. V. Wang and Y. N. Xia, Chem. Soc. Rev., 2011, 40, 44 RSC; (e) J. Li, X. Li, H. J. Zhai and L. S. Wang, Science, 2003, 299, 864 CrossRef CAS.
- (a) J. Zheng, J. T. Petty and R. M. Dickson, J. Am. Chem. Soc., 2003, 125, 7780 CrossRef CAS; (b) J. Zheng, P. R. Nicovich and R. M. Dickson, Annu. Rev. Phys. Chem., 2007, 58, 409 CrossRef CAS; (c) H. W. Duan and S. M. Nie, J. Am. Chem. Soc., 2007, 129, 2412 CrossRef CAS; (d) H. Tsunoyama and T. Tsukuda, J. Am. Chem. Soc., 2009, 131, 18216 CrossRef CAS; (e) J. P. Xie, Y. G. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888 CrossRef CAS; (f) R. C. Jin, Nanoscale, 2010, 2, 343 RSC.
- (a) Z. K. Wu and R. C. Jin, Nano Lett., 2010, 10, 2568 CrossRef CAS; (b) J. Zheng, C. Zhou, M. Yu and J. Liu, Nanoscale, 2012, 4, 4073 RSC.
- (a) L. Shang, S. J. Dong and G. U. Nienhaus, Nano Today, 2011, 6, 401 CrossRef CAS; (b) Y. Yue, T. Y. Liu, H. W. Li, Z. Y. Liu and Y. Q. Wu, Nanoscale, 2012, 4, 2251 RSC; (c) J. A. Ho, H. C. Chang and W. T. Su, Anal. Chem., 2012, 84, 3246 CrossRef.
- (a) H. Wei, Z. D.Wang, J. Zhang, S. House, Y. G. Gao, L. M. Yang, H. Robinson, L. H. Tan, H. Xing, C. J. Hou, I. M. Robertson, J. M. Zuo and Y. Lu, Nat. Nanotechnol., 2011, 6, 92 CrossRef; (b) F. Wen, Y. H. Dong, L. Feng, S. Wang, S. C. Zhang and X. R. Zhang, Anal. Chem., 2011, 83, 1193 CrossRef CAS.
- (a) M. X. Yu, C. Zhou, J. B. Liu, J. D. Hankins and J. Zheng, J. Am. Chem. Soc., 2011, 133, 11014 CrossRef CAS; (b) M. A. H. Muhammed, S. Ramesh, S. S. Sinha, S. K. Pal and T. Pradeep, Nano Res., 2008, 1, 333 CrossRef CAS.
- L. Shang, R. M. Dorlich, S. Brandholt, R. Schneider, V. Trouillet, M. Bruns, D. Gerthsen and G. U. Nienhaus, Nanoscale, 2011, 3, 2009 RSC.
- C. A. J. Lin, T. Y. Yang, C. H. Lee, S. H. Huang, R. A. Sperling, M. Zanella, J. K. Li, J. L. Shen, H. H. Wang, H. I. Yeh, W. J. Parak and W. H. Chang, ACS Nano, 2009, 3, 395 CrossRef CAS.
- L. Shang, N. Azadfar, F. Stockmar, W. Send, V. Trouillet, M. Bruns, D. Gerthsen and G. U. Nienhaus, Small, 2011, 7, 2614 CrossRef CAS.
- (a) C. C. Huang, Z. Yang, K. H. Lee and H. T. Chang, Angew. Chem., Int. Ed., 2007, 46, 6824 CrossRef CAS; (b) M. C. Paau, C. K. Lo, X. P. Yang and M. M. F. Choi, J. Phys. Chem. C, 2010, 114, 15995 CrossRef CAS.
- (a) H. Y. Liu, X. A. Zhang, X. M. Wu, L. P. Jiang, C. Burda and J. J. Zhu, Chem. Commun., 2011, 47, 4237 RSC; (b) Y. M. Guo, Z. Wang, H. W. Shao and X. Y. Jiang, Analyst, 2012, 137, 301 RSC.
- Z. Q. Yuan, M. H. Peng, Y. He and E. S. Yeung, Chem. Commun., 2011, 47, 11981 RSC.
- (a) D. M. Proctor, J. M. Otani, B. L. Finley, D. J. Paustenbach, J. A. Bland, N. Speizer and E. V. Sargent, J. Toxicol. Environ. Health, Part A, 2002, 65, 701 CrossRef CAS; (b) D. R. Lovley and J. D. Coates, Curr. Opin. Biotechnol., 1997, 8, 285 CrossRef CAS.
- (a) K. Ashley, A. M. Howe, M. Demange and O. Nygren, J. Environ. Monit., 2003, 5, 707 RSC; (b) Y. J. Lai and W. L. Tseng, Analyst, 2011, 136, 2712 RSC.
- (a) D. Bagchi, S. J. Stohs, B. W. Downs, M. Bagchi and H. G. Preuss, Toxicology, 2002, 180, 5 CrossRef CAS; (b) G. Pagano, P. Manini and D. Bagchi, Environ. Health Perspect., 2003, 111, 1699 CrossRef CAS.
- R. Bencheikh-Latmani, A. Obraztsova, M. R. Mackey, M. H. Ellisman and B. M. Tebo, Environ. Sci. Technol., 2007, 41, 214 CrossRef CAS.
- (a) P. Mahato, S. Saha, E. Suresh, R. Di Liddo, P. P. Parnigotto, M. T. Conconi, M. K. Kesharwani, B. Ganguly and A. Das, Inorg. Chem., 2012, 51, 1769 CrossRef CAS; (b) S. H. Liu, F. Lu and J. J. Zhu, Chem. Commun., 2011, 47, 2661 RSC; (c) Z. G. Zhou, M. X. Yu, H. Yang, K. W. Huang, F. Y. Li, T. Yi and C. H. Huang, Chem. Commun., 2008, 3387 RSC; (d) K. Huang, H. Yang, Z. Zhou, M. Yu, F. Li, X. Gao, T. Yi and C. Huang, Org. Lett., 2008, 10, 2557 CrossRef CAS; (e) Y. Q. Dang, H. W. Li, B. Wang, L. Li and Y. Q. Wu, ACS Appl. Mater. Interfaces, 2009, 1, 1533 CrossRef CAS; (f) T. Wu, C. Liu, K. J. Tan, P. P. Hu and C. Z. Huang, Anal. Bioanal. Chem., 2010, 397, 1273 CrossRef CAS.
- K. S. Subramanian, Anal. Chem., 1988, 60, 11 CrossRef CAS.
- A. J. Bednar, R. A. Kirgan and W. T. Jones, Anal. Chim. Acta, 2009, 632, 27 CrossRef CAS.
- S. F. Lai, W. C. Chen, C. L. Wang, H. H. Chen, S. T. Chen, C. C. Chien, Y. Y. Chen, W. T. Hung, X. Cai, E. Li, I. M. Kempson, Y. Hwu, C. S. Yang, E. S. Tok, H. R. Tan, M. Lin and G. Margaritondo, Langmuir, 2011, 27, 8424 CrossRef CAS.
- K. C. Grabar, R. G. Freeman, M. B. Hommer and M. J. Natan, Anal. Chem., 1995, 67, 735 CrossRef CAS.
- (a) V. W. W. Yam, C. L. Chan, C. K. Li and K. M. C. Wong, Coord. Chem. Rev., 2001, 216, 173 CrossRef; (b) Y. A. Lee and R. Eisenberg, J. Am. Chem. Soc., 2003, 125, 7778 CrossRef CAS.
- (a) Y. Negishi, K. Nobusada and T. Tsukuda, J. Am. Chem. Soc., 2005, 127, 5261 CrossRef CAS; (b) Z. Y. Huo, C. K. Tsung, W. Y. Huang, X. F. Zhang and P. D. Yang, Nano Lett., 2008, 8, 2041 CrossRef CAS.
- Y. Negishi, Y. Takasugi, S. Sato, H. Yao, K. Kimura and T. Tsukuda, J. Am. Chem. Soc., 2004, 126, 6518 CrossRef CAS.
- P. A. Buffat, M. Flueli, R. Spycher, P. Stadelmann and J. P. Borel, Faraday Discuss., 1991, 92, 173 RSC.
- Y. R. Kim, R. K. Mahajan, J. S. Kim and H. Kim, ACS Appl. Mater. Interfaces, 2010, 2, 292 CAS.
- K. Rurack, Spectrochim. Acta, Part A, 2001, 57, 2161 CrossRef CAS.
- X. R. Xu, H. B. Li, X. Y. Li and J. D. Gu, Chemosphere, 2004, 57, 609 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2tc00021k |
| This journal is © The Royal Society of Chemistry 2013 |
