Irradiation- and thermoinduced synthesis of Ag nanoparticles within amphiphilic carbosilane-thioether dendrimers†
Li
Chen
ab,
Theodore E.
Andersson
a,
Christiana
Rissing
a,
Shengyang
Yang
b,
Su
Chen
b and
David Y.
Son
*a
aDepartment of Chemistry, Center for Drug Discovery, Design, and Delivery, Southern Methodist University, Dallas, Texas 75275-0314, USA. E-mail: dson@smu.edu
bState Key Laboratory of Material-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing 210009, P. R. China
First published on 7th November 2012
Abstract
We report on the preparation of new amphiphilic carbosilane-thioether dendrimers by thiol–ene chemistry and their application in the in situ formation of silver nanoparticles (AgNPs) with unusual versatility in approach. In the presence of the dendrimers, AgNPs can be prepared in either water or organic solvents, and by simply using UV irradiation or heat in the reduction step without the need for a chemical reducing agent. Monodisperse size-tunable AgNPs possessing a simple cubic crystal structure were characterized by TEM, SAED, XRD, and other techniques. Susceptibility examination of E. coli indicated strong antibacterial activity of the AgNPs in aqueous solution.
Introduction
Due to quantum size effects, metal nanoparticles exhibit novel physical, chemical, and biological properties compared to the corresponding bulk materials, resulting in their extensive applications in areas such as catalysis, biology, optoelectronic devices, and chemical sensors.1–5 In particular, silver nanoparticles (AgNPs) are promising candidates for use in catalysis and even as antibacterial agents.6,7 However, bare AgNPs are not stable and tend to form larger structures through aggregation. As a result, capping agents of varying molecular size have been used to stabilize AgNPs, including citrate,8 thiol-containing compounds,9–11 surfactants,12–16 and polymers.17–19 Dendrimers, a unique class of monodisperse polymers with intrinsically well-defined globular structures and highly modifiable surface groups, are a new type of template for the formation of inorganic nanoparticles or nanoclusters.20–23 With increasing demand for nanoparticles in biological and nanostructural applications, the ability to prepare and stabilize nanoparticles in both aqueous and organic solvents is growing in importance.24,25 To date, it is possible to prepare AgNPs dispersed in water,26 and water-soluble dendrimers have also been successfully used to control the size, stability, and solubility of various other metal nanoparticles.23,27–30 However, the preparation of dendrimer–Ag (or Au) nanoparticles in polar aprotic solvents has rarely been reported.31 Most hydrophobic nanoparticles are prepared by chemical reduction of the metal cations in a suitable organic solvent mixture.32–36 However, very few publications have reported the use of stabilizers that are effective in both organic solvents and water.37–40In general, dendrimer-encapsulated metal nanoparticles are prepared in a two-step process. First, metal ions are sequestered within the dendrimers and then the ions are chemically reduced. Although the reducing agents are generally mild, additional steps are necessary to purify the nanoparticles from excess reagents and any byproducts. One interesting approach to generate metal nanoparticles is to use reagents which combine reducing and stabilizing properties.20,21 For example, amino-containing small molecules and polymers have been utilized in this regard in Au or Ag nanoparticle synthesis.41–45 Amino-terminated poly(amidoamine) (PAMAM) dendrimers or amino-terminated poly(propyleneimine) (PPI) dendrimers were used as templates to generate and stabilize AgNPs in water,46,47 but their toxicity and nonspecific cell membrane binding limited their application in biological systems. Organosilicon polymers have long attracted scientific and industrial attention as a consequence of their outstanding heat stability, excellent mechanical performance, and biocompatibility.48 Several linear polysilanes with heteroatom substituents were reported to act as reducing agents in the formation of metal nanoparticles.49–51 However, to our knowledge the application of organosilicon dendrimers to stabilize metal nanoparticles has been extremely limited.52,53
In view of the above considerations, we present a simple one-pot and one-step in situ method for the preparation of stable Ag nanoparticles in either organic solvents or water without the need for a chemical reducing agent. This method is the first example that relies on carbosilane-thioether dendrimers as both reducing and capping agents that control the growth of the particles and stabilize them in different solvents. One aim of this work was to exploit the thioether metal binding properties of the dendrimers to stabilize the formation of nanoparticles or nanoclusters. The thioether groups can entrap Ag+ ions within the dendrimer and the presence of silicon can increase the stability of the materials as well as their biocompatibility. Another strategy of this work was to attach hydrophilic sodium sulfonate groups to the exterior of the hydrophobic dendrimer core, to provide dendrimers that could control the formation and the stabilization of AgNPs in different media.
Experimental section
Materials
Unless otherwise noted, all starting materials were obtained commercially and used as received. Ultraviolet radiation was provided by a General Electric (GE) 275 W Sunlamp bulb with a total light output of 9.28 W and a maximum emission at 365 nm.Characterization
1H NMR data were obtained on a JEOL ECA-500 NMR spectrometer. UV-visible spectral data were collected using an Ocean Optics USB-ISS-UV/VIS spectrometer or an Optizen 2120 UV-visible spectrophotometer (Mecasys, Daejeon, Korea) with a 1 cm quartz cell. Dynamic light scattering (DLS) was conducted with a Malvern Zetasizer Nano-ZS equipped with a 4 mW, 633 nm He–Ne laser, and an Avalanche photodiode detector at an angle of 173°. Scanning electron microscope (SEM) observation was obtained using a Hitachi S-4800 scanning electron microscope. The elemental composition of dendrimer-encapsulated Ag nanoparticles was determined by an energy dispersive X-ray detector (EDX, QUANTA 200). Transmission electron microscopy (TEM) was performed using a JEOL JEM-2010 transmission electron microscope. The sample was placed on a copper grid that was left to dry before transferring into the TEM sample chamber. Power X-ray diffraction (XRD) patterns were recorded on a Rigaku D/Max 2550 X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å) operated at 200 mA and 40 kV. A laboratory wild type strain E. coli was used to investigate the anti-bacterium properties of silver nanoparticles. Cultures were grown at 37 °C in LB (LB media: 1% tryptone, 0.5% yeast extract, and 0.5% NaCl). For preparation of agar plates, 1.5% agar was added to the media as appropriate.Preparation of carbosilane-thioether dendrimers
The hydrophobic vinyl-terminated dendrimers (named G1-Vi to G3-Vi, where G# corresponds to the generation number) were synthesized according to our previously reported methods.54Functionalization of G2-Vi with 3-mercapto-1-propanesulfonic acid sodium salt (G2-S)
G2-Vi (0.50 g, 0.162 mmol), 3-mercapto-1-propanesulfonic acid sodium salt (1.195 g, 6.71 mmol) and benzophenone (0.01 g, 0.06 mmol) were added to a 50 mL single-necked round-bottomed flask equipped with a stir bar and condenser. A 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol–H2O solution (20 mL) was added and the reaction mixture was irradiated for 4 h, during which time small aliquots of water were added to dissolve the precipitate that formed (< 10 mL total). Most of the volatiles were removed via rotary evaporation and the residue was poured into 2-propanol (300 mL) to precipitate the product. The supernatant was decanted and the product was dried under vacuum with heating to afford G2-S as a light yellow solid (1.139 g, 74%). The 1H NMR spectrum of G2-S is provided in the ESI (Fig. S1†).
1 methanol–H2O solution (20 mL) was added and the reaction mixture was irradiated for 4 h, during which time small aliquots of water were added to dissolve the precipitate that formed (< 10 mL total). Most of the volatiles were removed via rotary evaporation and the residue was poured into 2-propanol (300 mL) to precipitate the product. The supernatant was decanted and the product was dried under vacuum with heating to afford G2-S as a light yellow solid (1.139 g, 74%). The 1H NMR spectrum of G2-S is provided in the ESI (Fig. S1†).
Functionalization of G3-Vi with 3-mercapto-1-propanesulfonic acid sodium salt (G3-S)
Functionalization of G3-Vi was carried out according to a similar procedure with G2-S, with the following amounts of reagents: G3-Vi (1.42 g, 0.15 mmol), 3-mercapto-1-propanesulfonic acid sodium salt (3.31 g, 19 mmol) and benzophenone (0.3 g, 1.6 mmol). The mixture was dissolved in 80 mL of methanol and 13 mL of water, and irradiated for six hours. During the irradiation period, water was occasionally added to dissolve the precipitate that formed. After precipitation into 2-propanol, G3-S was obtained as a yellow powder (4.12 g, 97%).Preparation of silver nanoparticles
The water-soluble silver nanoparticles used in this work were synthesized by three different methods. In the UV irradiation or heating approach, a typical procedure was as follows. G3-S (1.5 mg, 5.180 × 10−5 mmol, the number of sulfur atoms in one molecule of dendrimer m = 160) was dissolved in 15 g H2O or a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O–methanol solution (10 mL H2O and 5 mL MeOH). A KOH aqueous solution (0.2 M) was used to adjust the pH to 8. AgNO3 solution (1.41 mg in 1 mL H2O, dendrimer
1 H2O–methanol solution (10 mL H2O and 5 mL MeOH). A KOH aqueous solution (0.2 M) was used to adjust the pH to 8. AgNO3 solution (1.41 mg in 1 mL H2O, dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 1
Ag = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 based on the number of sulfur atoms in one molecule of dendrimer) was added and the reaction mixture was stirred under UV irradiation with a 275 W sunlamp or by heating at boiling point. The reduction process was allowed to proceed overnight, during which time the color of the solution changed from yellow to brown gradually. UV-visible spectral data were monitored during this procedure. For the chemical reduction process, after the AgNO3 solution was added and the reaction mixture was stirred for 30 minutes, sodium borohydride (NaBH4) (4.70 mg, 15 equiv. with respect to Ag) was added with stirring, at which point the color of the solution immediately changed from pale yellow to brown. The reduction process was allowed to proceed overnight.
1 based on the number of sulfur atoms in one molecule of dendrimer) was added and the reaction mixture was stirred under UV irradiation with a 275 W sunlamp or by heating at boiling point. The reduction process was allowed to proceed overnight, during which time the color of the solution changed from yellow to brown gradually. UV-visible spectral data were monitored during this procedure. For the chemical reduction process, after the AgNO3 solution was added and the reaction mixture was stirred for 30 minutes, sodium borohydride (NaBH4) (4.70 mg, 15 equiv. with respect to Ag) was added with stirring, at which point the color of the solution immediately changed from pale yellow to brown. The reduction process was allowed to proceed overnight.
Organic-soluble G3-S–Ag nanoparticles were prepared as follows
G3-S (1.5 mg, 5.180 × 10−5 mmol) was dissolved in DMSO and CF3SO3Ag (1.065 mg in 0.1 mL DMSO, dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 2
Ag = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was introduced by slow dropwise addition. Sodium borohydride (NaBH4) (7.838 mg in 0.1 mL DI water, 25 equiv., NaBH4
1) was introduced by slow dropwise addition. Sodium borohydride (NaBH4) (7.838 mg in 0.1 mL DI water, 25 equiv., NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 25
Ag = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution was added with stirring and the reduction process was allowed to proceed for 4 h.
1) solution was added with stirring and the reduction process was allowed to proceed for 4 h.
Organic-soluble G3-Vi–Ag nanoparticles were prepared as follows
G3-Vi (1.6 mg, 1.645 × 10−4 mmol) was dissolved in THF and CF3SO3Ag (1.099 mg in 0.1 mL THF, dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 2
Ag = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol) was introduced by slow dropwise addition. Sodium borohydride (NaBH4) (8.094 mg in 0.1 mL DI water, 25 equiv., NaBH4
1 mol/mol) was introduced by slow dropwise addition. Sodium borohydride (NaBH4) (8.094 mg in 0.1 mL DI water, 25 equiv., NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 25
Ag = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution was added with stirring and the reduction process was allowed to proceed for 4 h.
1) solution was added with stirring and the reduction process was allowed to proceed for 4 h.
Organism preparation
A laboratory wild type strain E. coli was grown overnight in LB at 37 °C. Washed cells were re-suspended in LB, and the optical density (OD) was adjusted to 0.01, corresponding to 107 CFU per mL at 600 nm.Bacterial susceptibility to nanosilver
To examine the susceptibility of E. coli to different silver nanoparticles, nutrient agar plates from a solution of agar were prepared. A 100 μL sample of bacterial suspension cultured in NB (with a concentration of 107 CFU per mL of E. coli) was plated on a nutrient agar plate. The plates were then supplemented with different amounts of nanosized silver particles (1 to 100 μg), and the plates were incubated further at 37 °C. The numbers of resultant colonies were counted after 24 h of incubation. Silver-free plates incubated under the same conditions were used as controls. The counts from three independent experiments corresponding to a particular sample were averaged.Results and discussion
The hydrophobic vinyl-terminated dendrimers, G1-Vi to G3-Vi, were synthesized according to our previous work.54 Amphiphilic SO3Na-terminated dendrimers (G2-S (or G3-S)) were easily prepared by functionalization of G2-Vi (or G3-Vi) with 3-mercapto-1-propanesulfonic acid sodium salt using a thiol–ene click reaction. The general synthesis of the sulfonate-terminated dendrimers is shown in Scheme 1.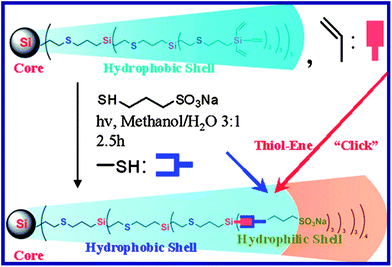 | ||
| Scheme 1 Synthesis of G3-S. | ||
The water-soluble AgNPs used in this work were synthesized by three different methods: a two-step chemical reduction method, a one-step UV irradiation reduction, and a heating reduction approach (Scheme 2). In this case, dendrimers can provide template sites so that Ag particles can be entrapped by the dendrimer branches, which allows the formation of dendrimer-encapsulated NPs (DENPs) under UV irradiation, using a reducing agent, or heating. Also, some Ag nanoparticles may be stabilized at the dendrimer periphery, resulting in dendrimer-stabilized NPs (DSNPs).55
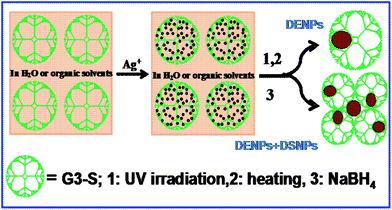 | ||
| Scheme 2 Schematic representation of the dendrimer-assisted formation of NPs. | ||
First, for comparison purposes, dendrimer–Ag nanoparticles were prepared by a conventional two-step chemical reduction method. In a typical experiment, G2-S or G3-S dendrimer and silver nitrate (dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 2
Ag = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by moles based on the number of sulfur atoms in one molecule of dendrimer) were dissolved in H2O (15 mL) and the pH was adjusted to 6–8. Sodium borohydride (NaBH4
1 by moles based on the number of sulfur atoms in one molecule of dendrimer) were dissolved in H2O (15 mL) and the pH was adjusted to 6–8. Sodium borohydride (NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 25
Ag = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by moles) was added to the solution, at which point the color of the solution immediately changed from pale yellow to brown. At the same time two characteristic absorption bands at 230 and 430 nm appeared in the UV-vis spectra (Fig. 1, Curve a). The band at 430 nm is attributed to the plasmon absorption band of silver nanoparticles and the absorption at 230 nm may be attributed to ligand-to-metal charge transfer (LMCT).56,57 After addition of NaBH4, the appearance of the 430 nm peak showed little change after overnight stirring (Fig. 2). In the preparation of G2-S–Ag, the average particle size of the as-prepared Ag nanoparticles was 56.5 nm as determined by dynamic light scattering (DLS) characterization (Fig. 1, inset a). The addition of small amounts of methanol or ethanol to improve the solubility of the dendrimer in water caused the peak at 430 nm to undergo a slight blue shift and narrow in width (Fig. 1, curve b), as well as a significant increase of absorbance intensity, indicating the formation of smaller and more uniformly distributed Ag nanoparticles, with an average particle size of 31.8 nm (Fig. 1, inset b). A slight red shift was also observed at 230 nm, which may be attributed to the effect of the polar solvent on the spectrum.
1 by moles) was added to the solution, at which point the color of the solution immediately changed from pale yellow to brown. At the same time two characteristic absorption bands at 230 and 430 nm appeared in the UV-vis spectra (Fig. 1, Curve a). The band at 430 nm is attributed to the plasmon absorption band of silver nanoparticles and the absorption at 230 nm may be attributed to ligand-to-metal charge transfer (LMCT).56,57 After addition of NaBH4, the appearance of the 430 nm peak showed little change after overnight stirring (Fig. 2). In the preparation of G2-S–Ag, the average particle size of the as-prepared Ag nanoparticles was 56.5 nm as determined by dynamic light scattering (DLS) characterization (Fig. 1, inset a). The addition of small amounts of methanol or ethanol to improve the solubility of the dendrimer in water caused the peak at 430 nm to undergo a slight blue shift and narrow in width (Fig. 1, curve b), as well as a significant increase of absorbance intensity, indicating the formation of smaller and more uniformly distributed Ag nanoparticles, with an average particle size of 31.8 nm (Fig. 1, inset b). A slight red shift was also observed at 230 nm, which may be attributed to the effect of the polar solvent on the spectrum.
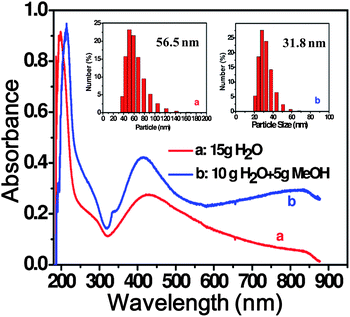 | ||
Fig. 1 UV-visible spectrum of AgNPs formed by chemical reduction. Insets: DLS profiles of G2-S–Ag (pH = 6, dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 2 Ag = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NaBH4 1, NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 25 Ag = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
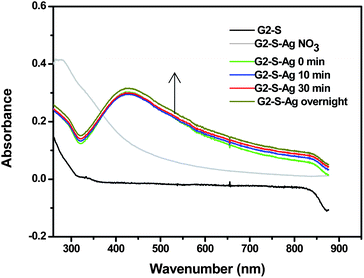 | ||
Fig. 2 UV-visible spectra of G2-S–Ag at different reaction times (pH = 6, 15 g H2O, NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 25 Ag = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dendrimer 1, dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 2 Ag = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
We varied the ratio of silver to dendrimer, the dendrimer concentration in solution, the pH of the solution, and the dendrimer generation in order to examine the effects of these variables on the nanoparticle size. Initially, the dendrimer–silver ratio was adjusted to prevent the precipitation of silver. We observed that the maximum amount of silver that could be added to the solution without precipitation corresponded to dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silver ratios up to 1
silver ratios up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (ratio of sulfur atoms in the dendrimer to silver). Fig. S2 (ESI†) shows that the characteristic band position blue shifts for the dendrimer stabilized AgNPs with a 1
1.5 (ratio of sulfur atoms in the dendrimer to silver). Fig. S2 (ESI†) shows that the characteristic band position blue shifts for the dendrimer stabilized AgNPs with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal dendrimer ratio. The pH of the nanoparticle solution was another optimized parameter in the experiment. We observed that the AgNPs could be stabilized in a pH range of 4 to 8. When the pH of the solution was higher than 9, silver ions precipitated out of solution as silver hydroxide or oxide particles. On the other hand, transparent Ag+ solutions were obtained at pH values lower than 4. The maximum absorption peak was blue shifted at higher pH values accompanied by a corresponding color change in the stabilized pH range (4–8), indicating the formation of silver nanoparticles of smaller size (Fig. S3 in the ESI†).
1 metal dendrimer ratio. The pH of the nanoparticle solution was another optimized parameter in the experiment. We observed that the AgNPs could be stabilized in a pH range of 4 to 8. When the pH of the solution was higher than 9, silver ions precipitated out of solution as silver hydroxide or oxide particles. On the other hand, transparent Ag+ solutions were obtained at pH values lower than 4. The maximum absorption peak was blue shifted at higher pH values accompanied by a corresponding color change in the stabilized pH range (4–8), indicating the formation of silver nanoparticles of smaller size (Fig. S3 in the ESI†).
Under the influence of UV irradiation, silver nanoparticles were formed in the presence of amine-containing dendrimers such as PAMAM.55 We were pleased to discover that AgNPs could also be formed using simple UV irradiation in the presence of the carbosilane-thioether dendrimers. In these experiments, the dendrimer–silver salt solution was irradiated with a 275 W sunlamp and placed in an ice water bath to avoid the effect of heat on the reduction reaction. The absorption spectra of the irradiated G3-S–Ag aqueous solution (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio of sulfur atoms in the dendrimer to AgNO3) are shown in Fig. 3(a). The ligand-to-metal charge transfer (LMCT) band of Ag ions clearly shifted from about 295 nm to 270 nm and a new absorption band corresponding to colloidal Ag appeared at around 500 nm after irradiation for 40 min, and its intensity increased and blue shifted to 410 nm gradually with an increase of irradiation time. During the irradiation, as seen in Fig. 3(b) from left to right, the color gradually turned darker with an increase of irradiation time and eventually changed into a bright brown after two hours of irradiation. DLS shows that the average particle size decreased from 104.8 nm after irradiation for 10 min to about 4.3 nm after irradiation for 240 min (Fig. 3(c)–(f)).
1 mole ratio of sulfur atoms in the dendrimer to AgNO3) are shown in Fig. 3(a). The ligand-to-metal charge transfer (LMCT) band of Ag ions clearly shifted from about 295 nm to 270 nm and a new absorption band corresponding to colloidal Ag appeared at around 500 nm after irradiation for 40 min, and its intensity increased and blue shifted to 410 nm gradually with an increase of irradiation time. During the irradiation, as seen in Fig. 3(b) from left to right, the color gradually turned darker with an increase of irradiation time and eventually changed into a bright brown after two hours of irradiation. DLS shows that the average particle size decreased from 104.8 nm after irradiation for 10 min to about 4.3 nm after irradiation for 240 min (Fig. 3(c)–(f)).
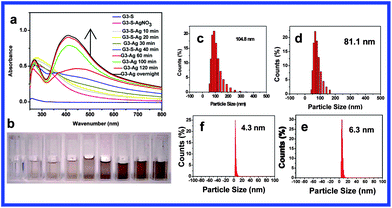 | ||
Fig. 3 (a) UV-visible spectra of AgNP solution with increasing UV irradiation time; (b) photos of gradual formation of G3-S–Ag with UV irradiation from 0 to 240 min (pH = 8, 10 mL H2O mixed with 5 mL MeOH, dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 2 Ag = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); (c)–(f) DLS profiles of G3-S–Ag after UV irradiation (clockwise from upper left): 10 min, 20 min, 60 min, and 240 min. 1); (c)–(f) DLS profiles of G3-S–Ag after UV irradiation (clockwise from upper left): 10 min, 20 min, 60 min, and 240 min. | ||
The dendrimer generation had a significant influence on the size of the AgNPs using UV irradiation. It was observed from UV-vis spectra (Fig. 4) that the plasmon band of AgNPs stabilized by the G3 dendrimer (G3-S–Ag) in the visible region was blue shifted and the half-peak-width narrowed compared with that of G2-S–Ag, indicating the formation of smaller silver nanoparticles. The greater number of stabilizing thioether groups in G3 allows the formation of smaller particles and also reduces the likelihood of particle aggregation. Consequently, AgNPs stabilized by a higher generation dendrimer are well dispersed. In order to prove the necessity of having the dendrimer in the solution, we used 3-mercapto-1-propanesulfonic acid sodium salt as a stabilizing agent under the same experimental conditions as a comparison. No coloration was observed after irradiation of this solution (G0-S–Ag), and no new absorption band appeared between 400 and 500 nm (Fig. 4).
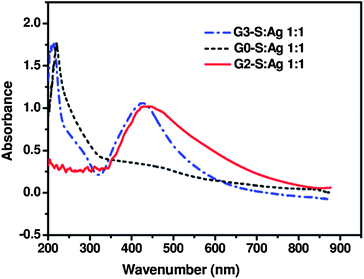 | ||
Fig. 4 Effect of dendrimer generation on AgNP formation using UV irradiation (pH = 8, 10 mL H2O mixed with 5 mL MeOH, dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 1 Ag = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 h UV irradiation). 1, 2 h UV irradiation). | ||
Organic-soluble AgNPs were prepared using two kinds of dendrimers, hydrophobic vinyl-terminated dendrimer G3-Vi (G3-Vi–Ag) or the amphiphilic sulfonate-terminated dendrimer G3-S (G3-S–Ag) as a stabilizer. The synthetic preparation of the AgNPs was similar to that of the water-soluble AgNPs described previously, except silver triflate (AgOTf) was used instead of AgNO3 and tetrahydrofuran (THF) or dimethylsulfoxide (DMSO) was used as a solvent. Fig. 5 shows the UV-visible spectra of the AgNPs prepared in the different solvents. The different characteristic band positions and half-peak widths as well as the color differences suggest different particle sizes and distributions in the different solvents. These AgNP solutions are stable and no significant changes in absorbance were observed for at least 6 months.
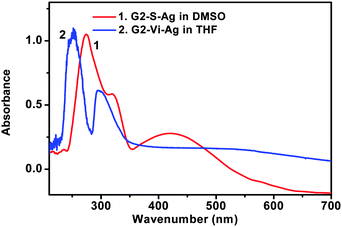 | ||
Fig. 5 UV-visible spectra of AgNPs formed in organic solvents (dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 2 Ag = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NaBH4 1, NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 25 Ag = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
AgNP formation was confirmed by SEM and HRTEM analyses. G3-S–Ag is stable and well-dispersed in water, with no precipitation observed for at least six months. SEM micrographs of G3-S–Ag prepared by the two-step chemical reduction method (Fig. 6(a)) and the one-step UV irradiation reduction approach (Fig. 6(c)) were obtained. Careful comparison of the SEM images clearly demonstrates that spherical type G3-S–Ag nanoparticles with uniform size (mean particle size of 4.295 nm in Fig. 6(d)) were generated under UV irradiation conditions, and G3-S–Ag nanoparticles with a larger average particle size of about 12.87 nm (Fig. 6(b)) were formed from chemical reduction.
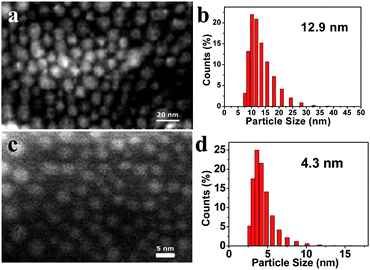 | ||
| Fig. 6 (a) SEM micrograph and (b) DLS profile of G3-S–Ag prepared by two-step chemical reduction method; (c) SEM micrograph and (d) DLS profile of G3-S–Ag prepared by UV-irradiation. | ||
Selected area electron diffraction and X-ray diffraction (XRD) data of the AgNPs were obtained to further verify their formation. Fig. 7(a) shows the electron diffraction of the crystal. The data from five Debye rings show that the silver nanoparticles are spathic structures. XRD measurements (Fig. 7(b)) show a remarkably intense diffraction peak at 38.04 degrees, characteristic of face-centered cubic (fcc) silver, unequivocally demonstrating that the particles are composed of silver and possess a simple cubic crystal structure.24 HRTEM measurements performed on G3-S–Ag prepared using UV irradiation (Fig. 7(c)) confirmed the size and shape of the AgNPs. Furthermore, the lattice fringes of the particles are visible, suggesting that the silver nanoparticles possess a clear crystalline order. The lattice space of the AgNPs measured from the HRTEM images is 2.35 Å, which is assigned to the d spacing of the crystal plane of face centered cubic (FCC) Ag(111).24
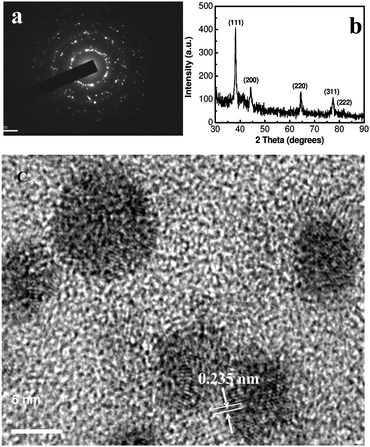 | ||
Fig. 7 (a) selected area electron diffraction pattern of G3-S–Ag. (b) XRD patterns and (c) HRTEM micrograph of G3-S–Ag (pH = 8, 10 mL H2O mixed with 5 mL MeOH, dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 1 Ag = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, UV irradiation). 1, UV irradiation). | ||
Energy-dispersive X-ray (EDX) spectroscopy studies were carried out to verify the elemental composition of the dendrimer-stabilized AgNPs (G3-S–Ag). The resulting EDX spectrum (Fig. 8) shows a strong silver peak as expected, revealing that the particles are mainly composed of silver. The carbon peak likely arises from both the dendrimer component and the conductive adhesive on the sample holder. Additional peaks corresponding to oxygen, sodium, sulfur, and silicon in the spectrum are likely due to the carbosilane-thioether dendrimer component in the G3-S–Ag sample, providing further evidence for the formation of dendrimer-stabilized Ag nanoparticles.
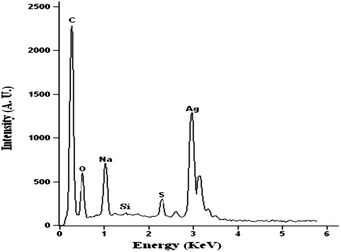 | ||
Fig. 8 EDX spectrum of G3-S–Ag (pH = 8, 10 mL H2O mixed with 5 mL MeOH, dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 1 Ag = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Another method developed for the preparation of AgNPs was simply stirring the dendrimer–silver salt solutions at the boiling point in the dark. Stable and monodisperse AgNPs were fabricated under these conditions. UV-visible measurements (Fig. S4†) indicated that the AgNPs formed under heating conditions were similar to those formed under UV irradiation. The characteristic absorption band corresponding to colloidal Ag stabilized at around 410 nm after boiling for 70 min. We also observed that nanoparticle formation was faster with heat than with UV-irradiation.
As an example of AgNP application, the antibacterial activity of the as-prepared AgNPs was examined. Laboratory wild type strain E. coli was grown overnight in lipid bodies at 37 °C and nutrient agar plates from a solution of agar were prepared. Fig. 9 reveals that the presence of AgNPs at a certain level inhibited bacterial growth. Silver concentrations above 10 μg per 100 μL sample of bacterial suspension reduced the number of bacterial colonies significantly, while a total silver concentration of 100 μg per 100 μL sample of bacterial suspension almost completely prevented bacterial growth. It is speculated that the interaction between the AgNPs and the bacterial membranes caused structural changes, finally leading to cell death.58 The relationship between dendrimer generation, pH, the size of AgNPs and antimicrobial activity is under investigation and will be reported elsewhere.
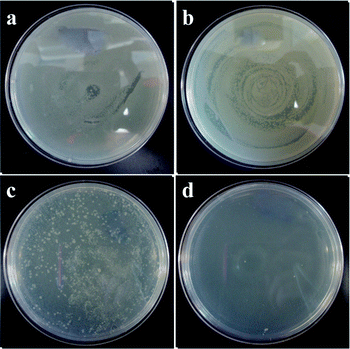 | ||
Fig. 9 Petri dishes initially supplemented with 107 CFU per mL of E. coli and incubated with G2-S–AgNPs at (a) control, (b) 1, (c) 30, and (d) 100 μg (pH = 8, dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 2 Ag = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in 15 g H2O, UV irradiation). 1, in 15 g H2O, UV irradiation). | ||
Conclusions
In conclusion, modified carbosilane-thioether dendrimers were synthesized and used for the in situ formation of AgNPs using either UV light or heating. With this approach, the use of chemical reducing agents is avoided. Moreover, the amphiphilic nature of the dendrimer structure allows the AgNPs to be stable in water and organic solvents. We have also demonstrated that the as-formed AgNPs display strong antibacterial activity in aqueous solution. We believe that these multilayered shell amphiphilic architectures have outstanding potential not only in biological applications, but also in plasmonics, molecular electronics and catalytic applications. These studies are currently in progress.Notes and references
- L. N. Lewis, Chem. Rev., 1993, 93, 2693 CrossRef CAS.
- J. Wang, M. Sui and W. Fan, Curr. Drug Metab., 2010, 11, 129 CrossRef CAS.
- M. De, P. S. Ghosh and V. M. Rotello, Adv. Mater., 2008, 20, 4225 CrossRef CAS.
- Y. Shan, T. Luo, C. Peng, R. Sheng, A. Cao, X. Cao, M. Shen, R. Guo, H. Tomás and X. Shi, Biomaterials, 2012, 33, 3025 CrossRef CAS.
- Y. An, X. Jiang, W. Bi, H. Chen, L. Jin, S. Zhang, C. Wang and W. Zhang, Biosens. Bioelectron., 2012, 32, 224 CrossRef CAS.
- S. Pal, Y. K. Tak and J. M. Song, Appl. Environ. Microbiol., 2007, 73, 1712 CrossRef CAS.
- E. Murugan and G. Vimala, J. Colloid Interface Sci., 2011, 357, 354 CrossRef CAS.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55 RSC.
- A. C. Templeton, M. P. Wuelfing and R. W. Murray, Acc. Chem. Res., 2000, 33, 27 CrossRef CAS.
- K. Aslan and V. H. Perez-Luna, Langmuir, 2002, 18, 6059 CrossRef CAS.
- S. Y. Lin, Y. T. Tsai, C. C. Chen, C. M. Lin and C. H. Chen, J. Phys. Chem. B, 2004, 108, 2134 CrossRef CAS.
- K. G. Thomas, J. Zajicek and P. V. Kamat, Langmuir, 2002, 18, 3722 CrossRef CAS.
- W. L. Cheng, S. J. Dong and E. K. Wang, Langmuir, 2003, 19, 9434 CrossRef CAS.
- H. Choo, S. R. Isaacs, A. Small, S. Parmley and Y. S. Shon, J. Colloid Interface Sci., 2007, 316, 66 CrossRef CAS.
- S. R. Isaacs, E. C. Cutler, J. S. Park, T. R. Lee and Y. S. Shon, Langmuir, 2005, 21, 5689 CrossRef CAS.
- H. F. Zhu, C. Tao, S. P. Zheng, S. K. Wu and J. B. Li, Colloids Surf., A, 2005, 256, 17 CrossRef CAS.
- Q. Tang, F. Cheng, X. L. Lou, H. J. Liu and Y. Chen, J. Colloid Interface Sci., 2009, 337, 485 CrossRef CAS.
- J. Shan and H. Tenhu, Chem. Commun., 2007, 4580 RSC.
- D. G. Shchukin, I. L. Radtchenko and G. B. Sukhorukov, ChemPhysChem, 2003, 4, 1101 CrossRef CAS.
- T. Vassilieff, A. Sutton and A. K. Kakkar, J. Mater. Chem., 2008, 18, 4031 RSC.
- M. Kavitha, M. R. Parida, E. Prasad, C. Vijayan and P. C. Deshmukh, Macromol. Chem. Phys., 2009, 210, 1310 CrossRef CAS.
- M. Q. Zhao, L. Sun and R. M. Crooks, J. Am. Chem. Soc., 1998, 120, 4877–4878 CrossRef CAS.
- L. Balogh and D. A. Tomalia, J. Am. Chem. Soc., 1998, 120, 7355 CrossRef CAS.
- C. J. Kiely, J. Fink, J. G. Zheng, M. Brust, D. Bethell and D. J. Schiffrin, Adv. Mater., 2000, 12, 640 CrossRef CAS.
- K. S. Mayya and F. Caruso, Langmuir, 2003, 19, 6987 CrossRef CAS.
- J. Kimling, M. Maier, B. Okenve, V. Kotaidis, H. Ballot and A. Plech, J. Phys. Chem. B, 2006, 110, 15700 CrossRef CAS.
- L. Balogh, D. R. Swanson, D. A. Tomalia, G. L. Hagnauer and A. T. McManus, Nano Lett., 2001, 1, 18 CrossRef CAS.
- R. M. Crooks, M. Q. Zhao, L. Sun, V. Chechik and L. K. Yeung, Acc. Chem. Res., 2001, 34, 181 CrossRef CAS.
- A. Taubert, U. M. Wiesler and K. Muellen, J. Mater. Chem., 2003, 13, 1090 RSC.
- X. Luo, Colloid J., 2009, 71, 281 CrossRef CAS.
- M. Stemmler, F. D. Stefani, S. Bernhardt, R. E. Bauer, M. Kreiter, K. Mullen and W. Knoll, Langmuir, 2009, 25, 12425 CrossRef CAS.
- M. J. Hostetler, J. E. Wingate, C. J. Zhong, J. E. Harris, R. W. Vachet, M. R. Clark, J. D. Londono, S. J. Green, J. J. Stokes, G. D. Wignall, G. L. Glish, M. D. Porter, N. D. Evans and R. W. Murray, Langmuir, 1998, 14, 17 CrossRef CAS.
- W. P. Wuelfing, S. M. Gross, D. T. Miles and R. W. Murray, J. Am. Chem. Soc., 1998, 120, 12696 CrossRef CAS.
- M. Bartz, J. Kuther, G. Nelles, N. Weber, R. Seshadri and W. Tremel, J. Mater. Chem., 1999, 9, 1121 RSC.
- A. P. Alivisatos, K. P. Johnsson, X. G. Peng, T. E. Wilson, C. J. Loweth, M. P. Bruchez and P. G. Schultz, Nature, 1996, 382, 609 CrossRef CAS.
- A. G. Kanaras, F. S. Kamounah, K. Schaumburg, C. J. Kiely and M. Brust, Chem. Commun., 2002, 2294 RSC.
- J. Keilitz, M. R. Radowski, J. D. Marty, R. Haag, F. Gauffre and C. Mingotaud, Chem. Mater., 2008, 20, 2423 CrossRef CAS.
- S. Minko, D. Usov, E. Goreshnik and M. Stamm, Macromol. Rapid Commun., 2001, 22, 206 CrossRef CAS.
- B. L. V. Prasad, S. K. Arumugarn, T. Bala and M. Sastry, Langmuir, 2005, 21, 822 CrossRef CAS.
- S. Minko, S. Patil, V. Datsyuk, F. Simon, K. J. Eichhorn, M. Motornov, D. Usov, I. Tokarev and M. Stamm, Langmuir, 2002, 18, 289 CrossRef CAS.
- J. D. S. Newman and G. J. Blanchard, Langmuir, 2006, 22, 5882 CrossRef CAS.
- C. Subramaniam, R. T. Tom and T. Pradeep, J. Nanopart. Res., 2005, 7, 209 CrossRef CAS.
- S. K. Bhargava, J. M. Booth, S. Agrawal, P. Coloe and G. Kar, Langmuir, 2005, 21, 5949 CrossRef CAS.
- M. Aslam, L. Fu, M. Su, K. Vijayamohanan and V. P. Dravid, J. Mater. Chem., 2004, 14, 1795 RSC.
- X. P. Sun, X. Jiang, S. J. Dong and E. K. Wang, Macromol. Rapid Commun., 2003, 24, 1024 CrossRef CAS.
- K. Esumi, A. Suzuki, N. Aihara, K. Usui and K. Torigoe, Langmuir, 1998, 14, 3157 CrossRef CAS.
- S. Kéki, J. Török, G. Deák, L. Daróczi and M. Zsuga, J. Colloid Interface Sci., 2000, 229, 550 CrossRef.
- R. Shankar, V. Shahi and U. Sahoo, Chem. Mater., 2010, 22, 1367 CrossRef CAS.
- Y. H. Chang, H. W. Wang, C. W. Chiu, D. S. Cheng, M. Y. Yen and H. T. Chiu, Chem. Mater., 2002, 14, 4334 CrossRef CAS.
- R. Shankar and V. J. Shahi, J. Organomet. Chem., 2008, 693, 307 CrossRef CAS.
- R. Shankar and V. Shahi, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7816 CrossRef CAS.
- C. Li, D. Li, Z. Sh. Zhao, X. M. Duan and W. Hou, Colloids Surf., A, 2010, 366, 45 CrossRef CAS.
- F. G. Rivera, L. I. Rodríguez, O. Rossell, M. Seco, N. J. Divins, I. Casanova and J. Llorca, J. Organomet. Chem., 2011, 696, 2287 CrossRef.
- C. Rissing and D. Y. Son, Organometallics, 2009, 28, 3167 CrossRef CAS.
- A. Castonguay and A. K. Kakkar, Adv. Colloid Interface Sci., 2010, 160, 76–87 CrossRef CAS.
- F. Groehn, B. J. Bauer, Y. A. Akpalu, C. L. Jackson and E. J. Amis, Macromolecules, 2000, 33, 6042 CrossRef CAS.
- A. L. Rogach, G. P. Shevchenko, Z. M. Afanaseva and V. V. Sviridov, J. Phys. Chem. B, 1997, 101, 8129 CrossRef CAS.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177 CrossRef CAS.
Footnote |
† Electronic supplementary information (ESI) available: 1H NMR of G2-S in D2O, UV-visible spectra and DLS data of G3-S–Ag prepared with different dendrimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag ratios, UV-visible spectra and photographic data of G2-S–Ag prepared at different pH values, as well as UV-visible spectra of G3-S–Ag prepared with heating. See DOI: 10.1039/c2tb00279e Ag ratios, UV-visible spectra and photographic data of G2-S–Ag prepared at different pH values, as well as UV-visible spectra of G3-S–Ag prepared with heating. See DOI: 10.1039/c2tb00279e |
| This journal is © The Royal Society of Chemistry 2013 |
