Inhibition of orthotopic secondary hepatic carcinoma in mice by doxorubicin-loaded electrospun polylactide nanofibers†
Shi
Liu
ab,
Guangyuan
Zhou
*a,
Daxin
Liu
ab,
Zhigang
Xie
a,
Yubin
Huang
a,
Xue
Wang
c,
Wenbin
Wu
d and
Xiabin
Jing
*a
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, People's Republic of China. E-mail: gyzhou@ciac.jl.cn; xbjing@ciac.jl.cn; Fax: +86-431-85262775; Tel: +86-431-85262775
bGraduate School of Chinese Academy of Sciences, Beijing 100049, People's Republic of China
cChina-Japan Union Hospital of Jilin University, Changchun 130033, People's Republic of China
dThe Second Hospital of Jilin University, Changchun 130041, People's Republic of China
First published on 31st October 2012
Abstract
For the treatment of unresectable liver cancer or for the prevention of post-surgery tumor recurrence, local chemotherapy is probably a good choice. A pilot study was carried out to examine the efficacy of doxorubicin-loaded polylactide electrospun nanofibers (Dox fibers) as a local chemotherapy system against secondary hepatic carcinoma (SHCC). Two orthotopic SHCC models were prepared by injecting murine mammary carcinoma EMT6 cells into the left hepatic lobe and into the portal vein of Balb/c mice, resulting in nodular and diffuse SHCC (NSHCC and DSHCC), respectively. By covering the surface of the NSHCC tumor and by wrapping the whole liver bearing DSHCC with the Dox fiber-mat after explorative laparotomy, the growth of NSHCC was significantly inhibited and median survival time of the mice bearing DSHCC was increased from 14 days to 38 days. Safety study suggested that both blank polylactide fibers (PLLA fibers) and Dox fibers ignited typical inflammatory response in the liver tissue of healthy mice. Acute, reversible impairment on fiber-mat-covered area of hepatic parenchyma was induced by Dox fibers. But neither injury to neighboring liver tissue nor systemic adverse reactions were observed during the experimental period of 42 days. In order to explain the efficacy and safety of Dox fiber treatment, in vivo release and biodistribution of Dox from the fiber-mat was investigated. Dox was rapidly released from the fiber-mat in the first 24 h, preferably localized in fiber-mat-covered area of liver tissue. In conclusion, Dox-loaded polylactide fibers might be used for local chemotherapy of liver cancers.
Introduction
Both hepatocellular carcinoma (HCC) and liver metastases (secondary hepatic carcinoma, SHCC) represent a major clinical problem owing to the poor prognosis.1 Furthermore, recurrence of HCC or SHCC following resection is a common occurrence, with 50–80% of patients experiencing recurrence by 5 years after resection, partly resulting from invisible intrahepatic metastases during resection.2For the treatment of unresectable liver cancer or for the prevention of post-surgery tumor recurrence, local treatment is a good choice rather than systemic chemotherapy.3 The protocols presently employed in the treatment include thermal ablative techniques (RFA and microwave), ethanol injection, directed radiotherapy (RT), transarterial chemoembolization (TACE), and hepatic arterial infusion chemotherapy (HAIC). The extensive application of these percutaneous or transarterial locoregional interventions, however, is more or less limited due to some factors, such as restrictive selection criteria applied currently in patients (usually ≤3 in tumor lesions, <5 cm in tumor diameter and without medical contraindications), institutional-dependent equipment availability, technical complexity involved in operation, and cost effectiveness.4–6
Recently, localized and controlled delivery of antitumor drugs by using biodegradable polymer electrospun nanofibers as drug carriers seems to be a promising method for the following reasons: biodegradable carriers capable of undergoing gradual decomposition into carbon dioxide, water, and finally disappearing in vivo; maximization of local drug concentration in the immediate tumor environment and minimization of non-target organ toxicity; delayed release of the drugs at cytotoxic concentrations that are sustained for longer periods at much lower total doses than can be achieved with typical systemic chemotherapeutic regimes; increase in the duration of tumor exposure to the drug; reduction of the frequency of drug administration; and convenience of being cut to almost any size and fabricated into other shapes using different target geometries for clinical applications.7–9 When applied in the treatment of liver cancer, its theoretical potential – killing diffuse, small and even invisible multinodules; not being subject to the limitation of the anatomic constraints and size of tumor, lesion number as well some contraindications; and avoiding physical injury to normal hepatic parenchyma and blood vessels caused by percutaneous or transarterial interventions – seems especially appealing, as well the most distinct advantages over many presently employed regional therapies – much less cost and easy operation (as shown in Fig. 1, the therapeutic process of using nanofibers in the treatment of animals even can be finished by people of non-medical professions). Furthermore, nanofibers themselves may also offer a function of hemostasis due to their large surface area to volume ratios and porosity.10 So it is reasonable to believe that nanofibers may play a role in adjuvant chemotherapy after complete resection or palliative resection of HCC or SHCC.
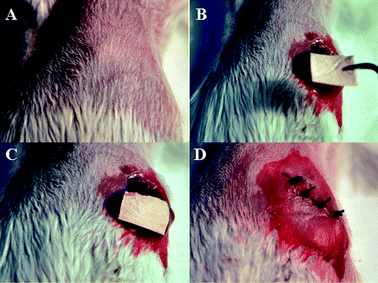 | ||
| Fig. 1 Therapeutic process of nanofibers in the treatment of mice: (A) Anesthetization of mice. (B) Exposure of the liver after laparotomy. (C) Direct placement of drug-loaded nanofibers on the tumor site. (D) Abdominal closure. | ||
Until now, there have been no reports on in situ treatment of liver cancer with drug-loaded electrospun nanofibers (exclusive of subcutaneously implanted tumor models). The purpose of our study is to seek the possibility and feasibility of nanofiber-based local drug delivery system as a new, reasonable, easy-to-use, and low-cost therapeutic option supplementary to currently limited clinical protocols for patients with HCC or SHCC.
As a model antitumor drug, doxorubicin hydrochloride (Dox) was chosen in the present study because of its broad spectrum antitumor activity and fluorescent property suitable for in vivo tracing. For the sake of simplicity and practicality, pure poly(L-lactide) (PLLA) was selected as drug carrier.
Experimental section
Materials
Dox (>99.0%) was purchased from Hisun Pharmaceutical Co. Ltd. (Zhejiang, China). PLLA (prepared from lactide) was synthesized in our laboratory. Its molecular weight (Mn) and polydispersity (PD) determined by GPC were 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 and 3.41, respectively.
800 and 3.41, respectively.
Preparation and characterization of PLLA fibers and Dox fibers
Dox-containing PLLA fibers were obtained by electrospinning of PLLA–Dox mixed solutions (3% and 6% Dox in weight percent of PLLA used) in chloroform–CH3OH–DMSO (80/14/6, v/v/v) at a polymer concentration of 6 wt%. Electrospinning parameters were as follows: electric field strength: 1.8–2.0 kV cm−1; air gap distance: 24 cm; inner diameter of spinneret: 0.4 mm; flow rate of solution: 1–2 ml h−1 and all the experiments were conducted at room temperature in air. In order to remove the residual solvent, the fibers collected were placed under vacuum for about 24 h at 25 °C. As a control, unloaded PLLA fibers were prepared.11An environmental scanning electron microscope (ESEM, Model XL 30 ESEM FEG from Micro FEI Philips) was used to observe the morphology of 6% Dox fibers.
Dox fibers were analyzed by fluorescent imaging (CRI Maestro 500FL) in order to demonstrate the presence of Dox in the fibers. PLLA fibers were also observed by fluorescence imaging for comparison.
The crystalline states of PLLA fibers and DOX fibers were analyzed by wide angle X-ray diffraction (WAXD, Bruker D8 Advance). The samples were scanned from 5° to 40° at a scanning rate of 3° min−1.
The in vitro drug release profile of Dox fibers was studied. The Dox fibers (144–250 mm2, 0.12–0.21 mg of Dox) were incubated at 37 °C in 25 ml of phosphate buffered saline (PBS, pH 7.4) in a thermostatic shaker. At the required incubation time, the sample was transferred to 25 ml of fresh buffer solution, and the released Dox in the original buffer solution was determined. The released Dox was quantified by High Performance Liquid Chromatography (HPLC) Instrument (Shimadzu CBM-20A). Detection was taken on a RF-20A fluorescence detector with excitation and emission wavelengths set at 480 and 580 nm, respectively. Chromatographic separation was performed on a reversed phase C18 column. And the column temperature was kept at 30 °C. The mobile phase consisted of 15 mM NaH2PO4–acetonitrile (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at a flow rate of 1 ml min−1.12 The total content of Dox in 3% and 6% Dox fibers was calculated from the average of the three accurately weighed fiber-mats, which were dissolved in CHCl3. After the organic phase was evaporated to dryness, the residue was resuspended in acetonitrile–CH3OH (2/1, v/v). After sonicated for 30 min, vortexed for 30 min, and centrifuged for 8 min at 10
1, v/v) at a flow rate of 1 ml min−1.12 The total content of Dox in 3% and 6% Dox fibers was calculated from the average of the three accurately weighed fiber-mats, which were dissolved in CHCl3. After the organic phase was evaporated to dryness, the residue was resuspended in acetonitrile–CH3OH (2/1, v/v). After sonicated for 30 min, vortexed for 30 min, and centrifuged for 8 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g, the resulting supernatant was collected, and analyzed by HPLC.
000 g, the resulting supernatant was collected, and analyzed by HPLC.
Experimental model of SHCC
Murine mammary carcinoma EMT6 cells were kindly supplied by the Medical Department of Jilin University in China. Balb/c mice (female, 8–12 weeks old) were used to prepare the models of SHCC. Two experimental models were established. For the first one (NSHCC), EMT6 cells (2 × 106 ml−1) in PBS (50 μl) were injected into the left lower hepatic lobe in situ. For the second one (DSHCC), an injection of EMT6 cells (1 × 106 ml−1) in PBS (100 μl) was given into the portal vein. The study protocol was approved by the local institution review board and performed according to the Guidelines of the Committee on Animal Use and Care of Chinese Academy of Sciences.Seven days after cell injection, NSHCC and DSHCC were obtained and the success rates for these two models were 92 and 100%, respectively. Obviously, they were murine mammary carcinoma liver metastases in nature.
In vivo Dox fibers treatment and efficacy evaluation
For the NSHCC model, 7 days after tumor inoculation, 6% Dox fibers mat (6 wt% of Dox with respect to PLLA used) of required sizes (144–250 mm2, 0.12–0.21 mg of Dox), flexibly resized according to the diameter of tumor nodules, were directly pasted on the surface of the tumor after definite diagnosis of the tumor during laparotomy (n = 6). The remaining mice were left without any treatment as a control (n = 5). On the 30th day after tumor inoculation, all animals were sacrificed and the excised livers were immediately weighed, macroscopically observed and histologically analyzed. Meanwhile, serum samples were taken for liver function evaluation.DSHCC model was used for survival observation. Seven days after tumor inoculation, experimental animals were randomly selected for fiber placement (n = 8). Each hepatic lobe (exclusive of the caudate lobe due to its anatomical position), both dorsal aspect and ventral aspect, were wrapped with 3% Dox fibers mat (3 wt% of Dox with respect to PLLA used) after exploratory laparotomy, covering the whole surface of the liver as much as possible. Approximately 700 mm2 Dox fibers (Dox content ≈ 0.3 mg) was used for each mouse. The rest of mice were left without any treatment as a control (n = 6). All animals were allowed to live until they succumbed to the tumor and survival days were recorded for each mouse.
Safety studies
Thirty-eight healthy KM mice were randomly divided into 3 groups: group 1 (n = 16) for PLLA fibers placement (150–200 mm2), group 2 (n = 16) for 6% Dox fibers placement (150–200 mm2 and 0.12–0.17 mg of Dox), and group 3 (n = 6) as control. The body weight of each mouse was determined every four days. Two animals separately from group 1 and group 2 were sacrificed on the 1st, 3rd, 7th, 14th, 21st, 28th, 35th, and 42nd day after fiber-mat implantation. Fiber-mat and liver tissues were harvested and macroscopically observed. Paraffin sectioning, hematoxylin and eosin (H&E) staining and semi-quantitative histological assessment were carried out according to the standard histological technique. The experiment was ended on day 42 and all animals left were sacrificed. Blood samples were taken for the evaluation of liver, heart, renal function and routine hematology. Meanwhile, the tissues such as heart, spleen, lung and kidneys were harvested for conventional histological analysis.Biodistribution and in vivo drug release
Thirty-three healthy female KM mice in a weight range of 20–25 g (8–12 weeks old) were used for the study of drug release profile of 6% Dox fibers in vivo and the biodistribution of Dox. After surgical laparotomy and exposure of the liver, accurately weighed 6% Dox fibers mat (150–200 mm2 and 0.12–0.17 mg of Dox) was directly pasted on the ventral aspect of the liver. The animals were sacrificed at 20 min, 1 h, 6 h, and 1, 3, 7, 14, 21, 28, 35, 42 days after fiber-mat implantation and samples of liver, kidneys, spleen, lungs, and heart were harvested. After the removal of the residual fibers from the liver, the excised organs were imaged by fluorescent imaging system (CRI Maestro 500FL) to follow the release of Dox from the fiber-mat and its biodistribution in mice. Fixed exposure time was adopted at all time points. Semi-quantitative comparison between different organs and different time intervals was also made by means of commercial software (MaestroTM 2.4).The residual Dox content in fiber-mat taken from the mice were analyzed by High Performance Liquid Chromatography (HPLC) and the released amount of Dox in vivo was calculated.
Statistical analysis
All data are expressed as mean ± SE. ANOVA with post-hoc analysis (Tukey's method) and pair-wise comparisons (Student's t-test), or nonparametric (Mantel–Cox) tests were applied where appropriate.Results
Characterization of electrospun nanofibers
ESEM micrographs of 6% Dox fibers revealed conglutinated nanofibers (Fig. 2A). The diameter range of the fibers was 0.4–1.3 μm. Their surface was smooth and no drug crystals were detected (Fig. 2B). Fluorescent images of PLLA and Dox fibers are shown in Fig. 2C. As expected, in the case of PLLA fibers no fluorescence was detected, while both 3% and 6% Dox fibers showed red fluorescence, and there was proportional relationship between Dox content and the fluorescence intensity. To further demonstrate the physical state of Dox in the fibers, PLLA and Dox fibers were characterized by wide angle X-ray diffraction (WAXD), showing that all fibers were amorphous and the crystalline Dox was not detected in all Dox fibers (Fig. 2D). Meanwhile, the release profile of Dox in vitro from 3% and 6% Dox fibers was studied, respectively (Fig. 2E). The drug release behaviors were similar, i.e., after a relatively fast release in 10 h (near 50%), the release curve of Dox leveled off.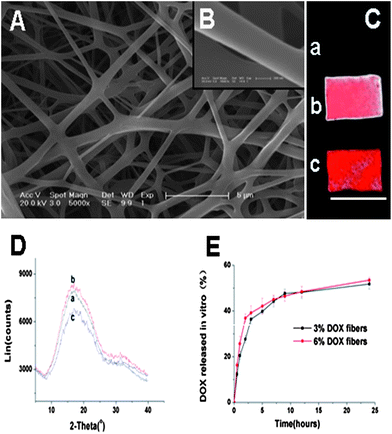 | ||
| Fig. 2 (A) ESEM images of 6% Dox fibers (bar = 5 μm). (B) ESEM images of 6% Dox fibers (bar = 200 nm). (C) Fluorescence images of PLLA fibers (a), 3% Dox fibers (b) and 6% Dox fibers (c). Bar = 10 mm. (D) WAXD patterns of PLLA fibers (a), 3% Dox fibers (b) and 6% Dox fibers (c). (E) The release profiles of Dox from 3% and 6% Dox fibers in PBS at 37 °C. | ||
In vivo anti-tumor efficacy of Dox fibers
The in vivo anti-tumor efficacy of Dox fibers was studied in Balb/c mice bearing NSHCC. The nodular tumor model was prepared by injecting 1 × 105 EMT6 cells into the left liver lobe. Seven days after the cell injection, whitish and irregular tumor nodules were observed in the liver lobe during explorative laparotomy of the mice (Fig. 3A and Table S1, ESI†).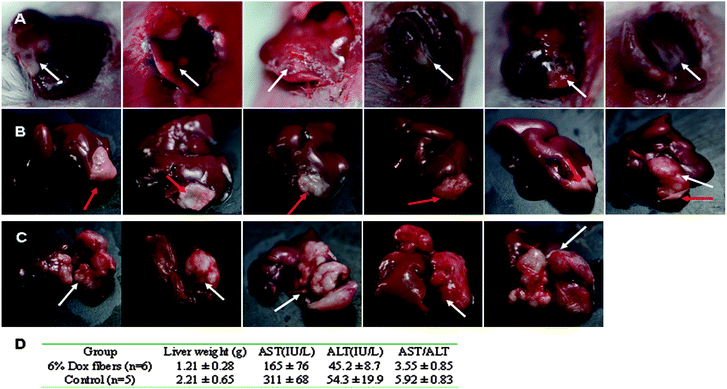 | ||
| Fig. 3 6% Dox fibers inhibited NSHCC in Balb/c mice. Photographs of NSHCC before fiber-mat placement (A), 23 days after 6% Dox fibers placement (B), and 30 days without any treatment (control) (C). The white arrow indicates the site of tumor and the red arrow indicates the site of Dox fiber-mat. (D) Anti-tumor effects of 6% Dox fibers in mice bearing NSHCC. | ||
After the identification of NSHCC, a 6% Dox fiber-mat of required size and shape was placed on the surface of the tumor nodule (Table S1, ESI†). Although the animals underwent the second surgery within a week, they soon returned to their preoperative physical state. On day 30, all animals remained alive. Then they were sacrificed and their livers were harvested. Macroscopic observation showed that most of the livers (5/6) in Dox fibers group displayed the appearance of normal liver tissue except the tumor site covered by the fiber-mat (Fig. 3B), whereas the tumor nodule was big and took a quite fraction of the liver in the control group (Fig. 3C).
Pasting of 6% Dox fibers did cause a statistically marked reduction in tumor burden in the liver (p < 0.01), the ratio of aspartate aminotransferase over alanine transarninase in serum (AST/ALT, a clinical indicator used in differentiating between causes of liver damage, p < 0.01), and the AST activities (p < 0.05) compared with those of the control group (Fig. 3D). The AST values were much less than those of the control group animals and close to those of normal mice (131 ± 30, see Table 2).
To comprehensively and objectively evaluate the inhibitory effect of Dox fibers, a series of micrographs of H&E-stained liver sections were merged together to produce a panoramic scene. As shown in Fig. 4A, the majority of the tumor tissue was in the state of degeneration or necrosis, between the necrotic (degenerated) tumor tissue and the normal liver tissue there was a thin layer of viable tumor remnants. Interestingly, a layer of normal hepatic parenchyma was observed between the fiber-mat and the necrotic tumor tissue (Fig. 4A and C). In control group, despite of widespread spontaneous necrosis and degeneration, large amounts of viable tumor cells still occupied the liver lobe (Fig. 4B).
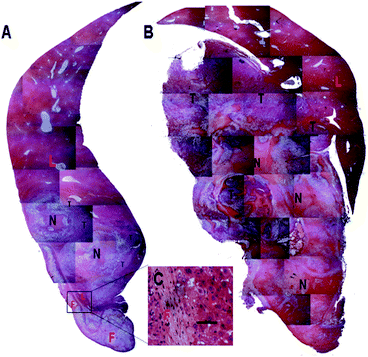 | ||
| Fig. 4 Histopathological observation of liver samples in 6% Dox fibers group (A) and control group (B) on the 30th day after tumor inoculation. F = Dox fibers; L = liver; N = necrosis or degeneration; T = viable tumor tissue. The selected area in A is shown in images C in a high magnification (bar = 50 μm). | ||
Further anti-tumor efficacy was performed on the DSHCC model, which was established by injecting 1 × 105 EMT6 cells through the portal vein. In 7 days, multiple interfused granules or nodules were observed in the liver in all of the 8 mice (100%) during the exploratory laparotomy (Fig. 5A). Autopsy results of the animals in the control group showed that tumor nodules spread over the entire liver (Fig. 5B), further confirming successful preparation of DSHCC models.
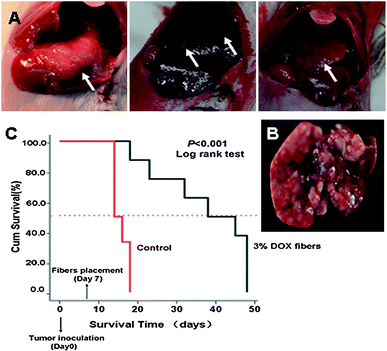 | ||
| Fig. 5 Inhibition of DSHCC in balb/c mice by 3% Dox fibers. (A) Photographs of DSHCC on the 7th day after EMT6 inoculation through portal vein. The white arrow indicates the site of tumor. (B) A liver taken from a dead mouse in control group on the 14th day after tumor inoculation. (C) Survival curves of the mice bearing DSHCC in 3% Dox fibers group (n = 8) and control group (n = 6). | ||
Due to the pervasive and multicentric occurrence of DSHCC, nearly every hepatic lobe was wrapped with 3% Dox fiber-mat.
Because covering the whole liver needed quite an area of fiber-mat, 3% Dox content was used to lower the total Dox dosage and to reduce possible side effects. Survival curves of DSHCC-bearing mice after 3% Dox fibers treatment were presented in Fig. 5C as a Kaplane Meier plot. 100% animal death occurred 18 days after the tumor cell injection and the median survival time was 14 days in the control group. In contrast, the median survival time of fiber-mat-treated animals was 38 days from the day of cell injection. Three of the eight mice remained alive until the end of the test (48 days). The difference between the test and control group was extremely significant (p < 0.001).
Macroscopic and microscopic observation of local liver tissue responses
6% Dox fibers adhered on the liver surface of healthy KM mice were taken out on the 1st, 3rd, 7th, 14th, 21st, 28th, 35th, and 42nd day post-surgery, and analyzed by macroscopic observation (Fig. 6A). Up to 7 days post-surgery, both Dox fibers and PLLA fibers, without obvious fibrous encapsulation, were easily separated from liver surface and kept their original shape. With the passing of time, the fiber-mat became tightly combined with the underlying liver tissue and increasingly difficult to separate from the liver. On day 14, fibrous encapsulation of the fiber-mat was visible to the naked eye and the extent of fibrosis gradually increased afterwards until the end of the 6 week period. The fiber-mat on day 42 looked to be covered by a layer of transparent tissues and much thicker than the original.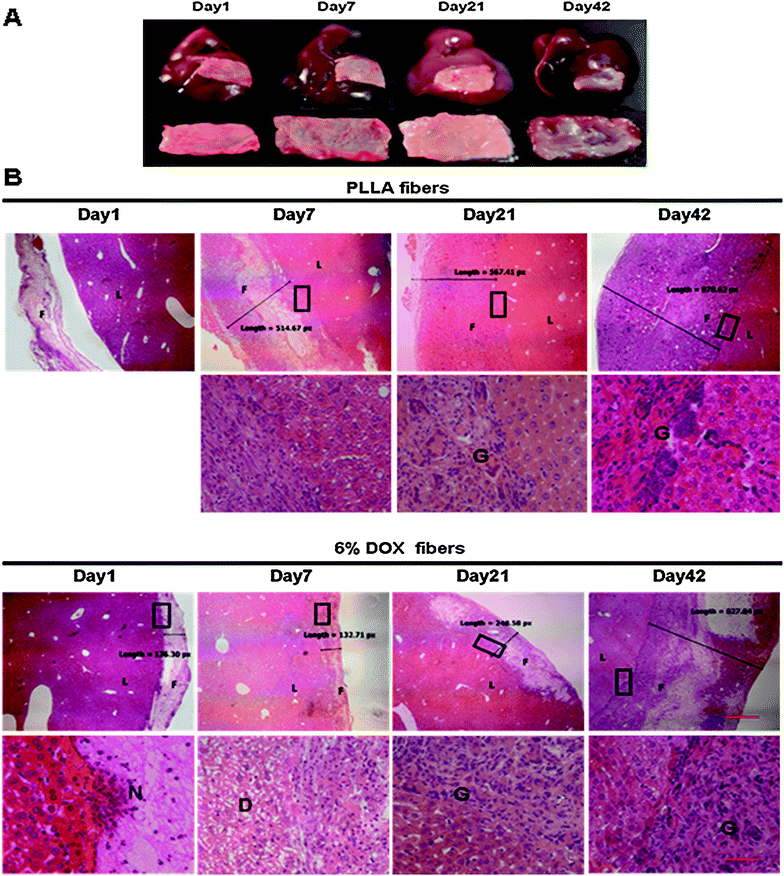 | ||
| Fig. 6 Local liver tissue response to 6% Dox fibers. (A) Photographs of the excised livers with adhered 6% Dox fibers (the upper panel) and the fiber-mats separated from the liver (the lower panel). (B) Micrographs of the borderline between fiber-mat and liver tissue. F = fibers; L = liver; N = neutrophil; G = giant cells; D = degeneration; the bar is 500 μm in the upper panel and 50 μm in the lower panel. The boundary areas in the rectangles are enlarged to form the lower panel images. | ||
Fig. 6B and Table 1 showed microscopic observation of liver tissue response to PLLA fibers and 6% Dox fibers at different time points. On day 1, PLLA fibers were detached from the liver tissue during the process of paraffin sectioning because the material had not tightly combined with the liver tissue. Then typical acute and chronic inflammatory response was observed, including the infiltration of inflammatory cells and fibroblasts, resulting in the gradual increase in the thickness of fiber-mat from less than 200 px on day 1 to more than 800 px on day 42 as seen in the micrographs.
| Items | PLLA fibers | 6% Dox fibers | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1b | 3 | 7 | 14 | 21 | 28 | 35 | 42 | 1 | 3 | 7 | 14 | 21 | 28 | 35 | 42 | |
| a Grading: 0, nil; 1, occasional; 2, mild; 3, marked. Phagocytic cells include monocyte-derived macrophages and monocytes; non-phagocytic cells include lymphocytes, plasma cells and mast cells. b Fiber-mat detached. | ||||||||||||||||
| Neutrophil | b | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| Phagocytic cells | b | 1 | 3 | 3 | 3 | 3 | 3 | 2 | 1 | 1 | 2 | 3 | 3 | 3 | 3 | 3 |
| Non-phagocytic cells | b | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Giant cells | b | 0 | 0 | 2 | 3 | 3 | 3 | 3 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 2 |
| Degeneration | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 1 | 0 | 0 | 0 | 0 |
| Necrosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eosinophil | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Congestion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liver fibrosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Proliferation in bile duct | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
In the case of 6% Dox fibers, in addition to typical foreign body-induced immune response, Dox released from nanofibers caused an acute and reversible toxicity. On day 3, extensive degeneration in the liver tissue underneath the fiber-mat was detected, and continued worsening until the 7th day. On day 14, the structure of hepatic sinusoid and cord recovered basically. Damages to the liver tissues outside of the fiber-mat were not observed (micrographs not shown). The extent and speed of giant cell infiltration induced by Dox fibers was much less than that by PLLA fibers all along. But the apparent thickening was similar, from 140 px on day 1 to 830 px on day 42. Nevertheless, other pathological changes in the liver, such as congestion, liver fibrosis, proliferation in bile ducts and so on, were not observed during the whole experimental period of 42 days after PLLA or Dox fibers placement.
Systemic effects
Throughout the study period, all animals were in very good health status, with smooth and shiny hair, robust appetite, appearance and behaviors of healthy mice. Relative body weight (Wt/Wo) over 42 days after 6% Dox fibers placement were used as an indication of safety. As illustrated in Fig. S1(A),† the body weights of mice treated with both Dox fibers and PLLA fibers rapidly recovered after laparotomy and kept steadily growing throughout the entire experiment.A full pathological examination was also performed. On day 42, heart, kidneys, lung, and spleen were normal with no microscopic changes for 6% Dox fibers-placed animals (Fig. S1(B), ESI†).
To reveal any potential toxic effect of Dox fibers on the treated mice, blood biochemical and hematological analyses were carried out. Different biochemical parameters were tested, including liver function markers (AST, ALT), heart function signals (creatine kinase, CK; lactate dehydrogenase, LDH) and kidney function indicators (blood urea nitrogen, BUN; creatinine). For the hematological assessment, white blood cells (WBC), red blood cells (RBC) and platelets were counted. As shown in Table 2, the fiber-treated animals did not exhibit statistically significant differences in all of the above parameters in comparison with the control groups.
| PLLA fibers | 6% Dox fibers | Healthy mice | |
|---|---|---|---|
| ALT (IU L−1) | 47.3 ± 9.9 | 62.3 ± 24.1 | 49 ± 13.4 |
| AST (IU L−1) | 145 ± 58 | 138 ± 26 | 131 ± 30 |
| AST/ALT | 3.08 | 2.22 | 2.67 |
| BUN (mmol L−1) | 6.73 ± 0.81 | 6.60 ± 0.53 | 9.93 ± 3.11 |
| CREA (μmol L−1) | 20 ± 5.7 | 18.6 ± 4.4 | 15.3 ± 3.8 |
| CK (IU L−1) | 1510 ± 466 | 1230 ± 229 | 1340 ± 749 |
| LDH (IU L−1) | 1592 ± 372 | 1182 ± 515 | 1363 ± 266 |
| WBC (109 L−1) | 5.70 ± 0.57 | 5.63 ± 0.75 | 5.52 ± 0.95 |
| RBC (1012 L−1) | 8.58 ± 0.16 | 9.46 ± 0.63 | 9.36 ± 0.21 |
| PLT (109 L−1) | 880 ± 48 | 763 ± 96 | 748 ± 169 |
In vivo release and biodistribution
To illustrate the above anti-tumor and safety results, in vivo Dox release from the Dox fibers and biodistribution of the released Dox were studied. By virtue of the red fluorescence of Dox itself, its biodistribution was visualized. As shown in Fig. 7A, intense fluorescence was observed in Dox fibers-covered region after removal of the mat within 24 h, clearly showing the contour of the fiber-mat. Semi-quantitative determination of the fluorescence intensities showed that Dox were preferably distributed in the liver rather than in the other four organs, i.e., liver ⋙ kidneys > spleen ≫ lung > heart within 24 h, whereas the average fluorescence intensity of Dox on the whole liver surface markedly declined in 3 days to about 12% of the maximum at 6 h, even less than that of the spleen and kidneys (Fig. 7B). The average fluorescence signal of the fiber-mat-covered area was 6.36 fold more than that of the normal area without fiber-mat coverage at 1 h, then the ratio gradually dropped to 1.05 on day 7 (Fig. 7C). The release curve of Dox from the fiber-mats was determined by measuring the Dox content in the Dox fibers detached from the liver surface. As shown in Fig. 7D, a rapid release of Dox was observed, i.e., about 97% of total Dox was released within 24 h.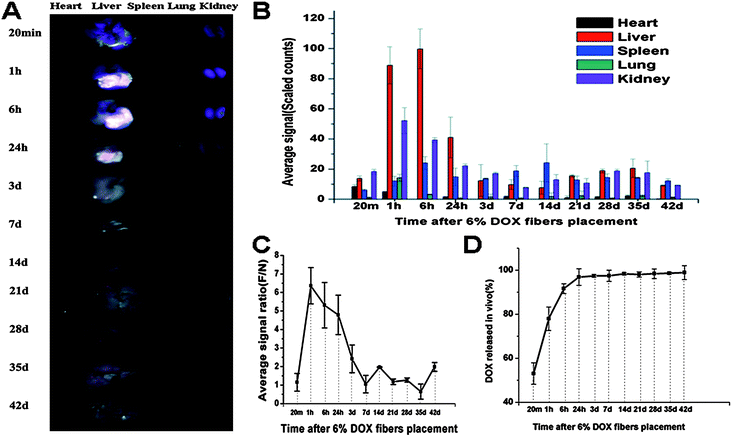 | ||
| Fig. 7 Biodistribution and in vivo release of 6% Dox fibers. (A) Typical ex vivo images of the excised organs examined by CRI Maestro 500FL 20 min, 1 h, 6 h, 1 d, 3 d, 7 d, 14 d, 21 d, 28 d, 35 d, and 42 d after 6% Dox fibers placement on the liver of healthy KM mice. (B) Semi-quantitative fluorescence intensities of various organs determined at different time points. (C) The fluorescence intensity ratio between Dox fibers-covered area and normal liver tissue area (F/N) as a function of time. (D) The cumulative release profile of Dox from 6% Dox fibers in vivo. The results mentioned above were given as mean value ± SD, over three mice in a group. | ||
Discussion
Like other regional chemotherapy of the liver such as HAIC, the nanofiber-based local drug delivery system is expected to deliver a much higher concentration of chemotherapeutic drugs to the tumor site while keeping the normal hepatic parenchyma intact, and to achieve improved efficacy and reduced systemic toxicity.To evaluate the efficacy of Dox fibers in the treatment of SHCC, two different orthotopic tumor models of SHCC in Balb/c mice were prepared to mimic the two common types of liver cancer, nodular tumors and diffuse tumors. For the NSHCC model, 6% Dox fibers markedly inhibited the growth of nodular tumor and the liver functions indicated by AST and ALT were almost recovered to the normal status (Fig. 3) in 30 days, mostly due to the increase in local concentration of Dox in the tumor site, the duration of tumor exposure to the drug and reduced systemic toxicity. For DSHCC model, wrapping of 3% Dox fibers around each liver lobe led to prolongation of the median survival time from 14 days to 38 days counted from the day of cell injection (Fig. 5C). Such survival time prolongation implies the effectiveness of the drug-loaded polylactide nanofibers in suppressing the unresectable tumors or in killing the remaining cancer cells after the resection operation.
As a new therapy that is markedly different from currently employed protocol against liver cancer, the safety of nanofibers-based drug delivery system is a primary concern. As expected, low extent of side effects of Dox fibers and PLLA fibers were observed. The damage of Dox fiber-mat to liver tissues was limited to the fiber-mat-covered region after Dox fibers placement and the mice kept normal growing. Biochemical or homological analyses did not reveal dysfunction of the liver, spleen, kidneys, heart, or blood (Table 2). Meanwhile, despite of the occurrence of typical foreign body responses induced by PLLA or Dox fibers which is consistent with the well-known fact that foreign-body-induced inflammation is quite generic when biomaterials are implanted into the peritoneal cavity, all animals were in good condition, showing no signs of obvious discomfort.13–15 Therefore, the Dox-loaded fibers were safe enough as a local chemotherapy system. On the other hand, it was observed in our experiments that the presence of nanofibers in peritoneal cavity for a long period often caused adhesion between liver lobes or between liver and peritoneum. This is probably the biggest obstacle to public acceptance of nanofibers. So the nanofibers possessing both anti-cancer and anti-adhesion functions would be the most desirable.
To illustrate the above results of significant anti-tumor effect and high security of Dox fibers, in vivo Dox release from the Dox fibers adhered on the liver and biodistribution of the released Dox were studied. The results of biodistribution study suggest that the released Dox molecules mainly entered the liver underneath the fiber-mat so that the Dox fluorescence in this part was 6 fold more intense than that in the naked part of the liver at 1 h, and the Dox fluorescence from the liver was much more intense than that from all other organs examined. At around 6 h, the pasted part of the liver got its maximal Dox concentration. The local distribution within the liver lasted seven days.
As shown in Fig. 7A and B, Dox fluorescence from the liver markedly decayed after 24 h, becoming comparable with other organs after 3 days. Of these organs, spleen and kidneys displayed appreciable Dox fluorescence. Furthermore, the difference between the covered area and the naked area of the liver disappeared after 7 days, indicating the presence of Dox molecules in the healthy part of the liver. Obviously, these Dox molecules in healthy part of the liver or in other organs came from the systemic circulation, and the Dox entered the systemic circulation in two ways: (1) the Dox was released from the fiber-mat into the liver underneath it and entered the blood vessels in the liver; (2) the Dox was released outwards into the abdominal cavity and entered the blood vessels there. Obviously, the part of Dox that has entered the systemic circulation and entered other organs or the healthy part of the liver was the origin of the side effects of the drug. Nevertheless, as shown in Fig. 7, the great majority of Dox was concentrated underneath the fiber-mat within the first 24 h, and afterwards the Dox concentration in other organs or in the healthy part of the liver was quite low and not enough to induce adverse effects. Therefore, this kind of biodistribution caused by fiber-mat pasting, i.e., highly local distribution at the tumor site and very low concentration outside the tumor, especially in the first 24 h, was responsible for the inhibitory effect of Dox fibers on SHCC and the safety of Dox fiber-mat pasting.
It is noticed that the in vitro and in vivo release curves of Dox fiber-mat gave the same result (Fig. 2E and 7D): Dox loaded in the fiber-mat was released at a high rate within 24 hours. Rapid release of Dox is ascribed to its fast diffusion rate resulting from high hydrophilicity of Dox·HCl and huge specific surface area of the fiber-mat, and to the enzyme-catalyzed hydrolysis and degradation of PLLA in vivo.16–18 On the one hand, rapid release of Dox provided high enough local Dox concentration in the tumor site, possibly achieving efficient and effective inhibition of tumor within a short time period; on the other hand, due to rapid consumption of Dox and limited loading capacity (6% in the present study), the Dox fiber-mat cannot provide sustained Dox supply for a long enough time period to prevent the recurrence of the tumor. Therefore, technical measures should be taken to enhance the drug content and to adjust the release kinetics to ensure release of enough amount of the drug in a mode of initial rapid release plus subsequent sustained release. Related investigations are now under way.
Conclusion
In summary, significant anti-tumor efficacy was observed by directly placing Dox fiber-mats on the liver surface of the mice bearing orthotopic NSHCC or DSHCC. The majority of the loaded Dox in the fiber-mat was released and diffused into the tumor site underneath the fiber-mat, leading to a great inhibitory effect on tumor growth and little damage to other organs. These results provide an encouraging prospect of using drug-loaded electrospun nanofibers in local chemotherapy against HCC, SHCC, or other tumors, alone or combined with presently employed treatment protocols, especially for those patients receiving complete tumor resection or cytoreductive surgery. But further improvements are needed to suppress the inflammatory responses, to increase the drug content in the fibers, and to improve the release kinetics of the drug from the fibers.Acknowledgements
Financial support was provided by the National Natural Science Foundation of China (Project no. 21004062, 51103148), and by the Ministry of Science and Technology of China (“973 Project”, no. 2009CB930102).Notes and references
- R. Lencioni, Hepatology, 2010, 52, 762 CrossRef CAS.
- C. H. Cha, M. W. Saif, B. H. Yamane and S. M. Weber, Curr. Probl. Surg., 2010, 47, 10 CrossRef.
- O. Dudeck and J. Ricke, Expert Opin. Drug Delivery, 2011, 8, 1057 CrossRef CAS.
- H. E. Blum, Best Pract. Res. Clin. Gastroenterol., 2005, 19, 129 CrossRef CAS.
- J. J. Skitzki and A. E. Chang, Surg. Oncol., 2002, 11, 123 CrossRef.
- T. C. Chua, A. Saxena, W. Liauw, F. Chu and D. L. Morris, Eur. J. Cancer, 2011, 47, 2282 CrossRef.
- R. Toshkovaa, N. Manolovab, E. Gardevaa, M. Ignatovab, L. Yossifovaa, I. Rashkovb and M. Alexandrov, Int. J. Pharm., 2010, 400, 221 CrossRef.
- W. L. Hunter, H. M. Burt and L. Machan, Adv. Drug Delivery Rev., 1997, 26, 199 CrossRef CAS.
- P. Chen, Q. S. Wu and Y. P. Ding, Eur. J. Pharm. Biopharm., 2010, 76, 413 CrossRef CAS.
- H. J. Jin, S. Fridrikh, G. C. Rutledge and D. Kalan, Biomacromolecules, 2002, 3, 1233 CrossRef CAS.
- D. X. Liu, S. Liu, X. B. Jing, X. Y. Li, W. L. Li and Y. B. Huang, Biomaterials, 2012, 43, 4362 CrossRef.
- D. L. Gustafson and M. E. Long, Chem.-Biol. Interact., 2001, 138, 43 CrossRef CAS.
- R. D. Souza, P. Zahedi, C. J. Allen and M. M. Piquette, Biomaterials, 2009, 30, 3818 CrossRef.
- D. S. Kohane and R. Langer, Chem. Sci., 2010, 1, 441 RSC.
- J. B. Mendes, P. P. Campos, M. Ferreira, Y. S. Bakhle and S. P. Andrade, J. Biomed. Mater. Res., Part B, 2007, 83, 408 CrossRef.
- X. L. Xu, X. S. Chen, Z. F. Wang and X. B. Jing, Eur. J. Pharm. Biopharm., 2009, 72, 18 CrossRef CAS.
- X. L. Xu, X. S. Chen, P. A. Ma, X. R. Wang and X. B. Jing, Eur. J. Pharm. Biopharm., 2008, 70, 165 CrossRef CAS.
- D. Ishii, T. H. Ying, A. Mahara, S. Murakami, T. Yamaoka, W. K. Lee and T. Iwata, Biomacromolecules, 2009, 10, 237 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2tb00121g |
| This journal is © The Royal Society of Chemistry 2013 |
