The synthesis of a novel magnetic demulsifier and its application for the demulsification of oil-charged industrial wastewaters
Shuqiang
Li†
,
Naixu
Li†
,
Shanbo
Yang
,
Fangyuan
Liu
and
Jiancheng
Zhou
*
School of Chemistry and Chemical Engineering, Southeast University, Nanjing, 211189, China. E-mail: jczhou@seu.edu.cn
First published on 11th October 2013
Abstract
Water produced from alkaline–surfactant–polymer flooding technology is more difficult to treat than that from water flooding. In this study, a novel magnetic demulsifier, called M-5010, was synthesized through the reaction of 5010, a type of demulsifier currently used in oilfields, and epoxy group functionalized Fe3O4@SiO2 microspheres. The oil removal rates and effect of the demulsifier dosage as well as the settling time were investigated. It was found that the oil removal rate of M-5010 was higher than that of 5010 under the same conditions. In addition, M-5010 can be recycled and reused by using an external magnetic field that acts as a material phase separator. Accordingly, we found that M-5010 is still effective after being reused for 5 cycles. Our results indicate that the magnetic demulsifier we fabricated can be used as an effective and recyclable material for the removal of oil from oil contaminated wastewater.
1 Introduction
At present, in China, alkaline–surfactant–polymer flooding technology is widely used for oil extractions in order to improve the recovery of oil, therefore more and more wastewater has been generated.1 The water produced from this technology contains residual chemicals and is more difficult to treat than that from water flooding.2 Therefore, it is necessary to purify the produced water so that it can be reused in order to protect the environment. If the type of produced water has a high oil content and rather small oil droplet size it is termed an oil-in-water (O/W) emulsion.3 O/W emulsions, which are comprised of oil droplets, surfactants and co-surfactants, are highly stable because the surfactants reduce the oil–water interfacial tension and zeta potential at the surface of the oil droplets.4 Polymers can increase the viscosity of the aqueous phase,5 the produced water forms a complex and stable O/W emulsion system and an electrical double layer may exist at the surface of this O/W emulsion.6 Thus, it is more difficult to dispose of the produced water. As a result, this brings about a lot of environmental problems. Considerable effort has been made to develop effective treatment techniques such as flotation,7 chemical coagulation coupled with flotation,8 chemical and electrochemical techniques,9 chemical demulsification,10 membrane separation11 and biotechnology.12 Although these methods have been proven to be effective techniques, their use can not fully meet the requirements for this type of produced water. As a consequence, it is necessary to find a suitable method with properties including a high oil removal rate and fast oil–water separation.Since magnetic materials possess special magnetic properties that allow them to be conveniently separated from complex multiphase systems, these materials have attracted a significant amount of interest during the past decade. Magnetic materials have been widely used in a variety of fields, such as separation and purification,13 catalysis,14 drug delivery,15 and contrast enhancement in magnetic resonance imaging.16 Among these materials, magnetite (Fe3O4) is considered to be an ideal candidate because of its low cytotoxicity and good biocompatibility.17 Y. F. Shen et al.18 added Fe3O4 nanoparticles into wastewater contaminated with metal ions and found that the contaminants could be effectively and conveniently removed. However, during the process of application, the chemical reactivity of the magnetite particles is not satisfactory. From this point of view, it is necessary to develop efficient strategies in order to improve the chemical reactivity of magnetite particles. As previously reported,19 a common protection strategy is the formation of a core–shell structure, i.e., the magnetite particles form a core that is coated by a shell. The shell can be divided into two types: organic shell coatings, including surfactants and polymers, or inorganic coating components, including silica, carbon and precious metals. Moreover, a protective shell not only protects the magnetite particles against oxidation, but can also be used for further functionalization of the particles with functional groups, such as amines, carboxyl groups and so on. Silica coated core–shell magnetite nanoparticles, i.e., Fe3O4@SiO2, are usually prepared because the SiO2 shell has abundant surface hydroxyl groups, which allow easy subsequent functionalization of the magnetite nanoparticles.20 Recently, the introduction of magnetic properties into functional materials has attracted researchers' attention. For example, Z. N. Liu et al.21 synthesized Fe3O4 nanoparticles coated with dense nonporous silica as an inter layer and mesoporous silica as an outer layer and then functionalized the Fe3O4 nanoparticles with poly methyl acrylate through a plasma polymerization technique. These nanoparticles could remove most of the pollutants from oilfield wastewater. In contrast to this, J. X. Peng et al.4 synthesized a novel magnetic demulsifier by grafting ethyl cellulose onto the surface of amino-functionalized Fe3O4 nanoparticles coated with silica in order to remove water from a diluted bitumen emulsion. P. Calcagnile et al.22 prepared a novel composite material based on polyurethane foam functionalized with colloidal magnetic iron oxide nanoparticles, which efficiently separated oil from water. However, the above materials can not effectively treat oilfield O/W wastewater.
In this study, Fe3O4 microspheres were first coated with a thin shell of amorphous silica. These Fe3O4@SiO2 microspheres were then modified using the silane coupling agent, (3-glycidoxypropyl)trimethoxysilane. Subsequently, the epoxy group modified Fe3O4@SiO2 microspheres were reacted with a demulsifier known as 5010, which is used in oilfields and is a type of polyether polyol. The prepared novel magnetic demulsifier was applied to O/W wastewater and the oil removal rate was higher than that of the 5010 demulsifier used in oilfields. Moreover, this novel magnetic demulsifier could be rapidly separated in the presence of an external magnetic field and then reused, which means that the dosage of demulsifier used to treat the oilfield wastewater could be reduced. To the best of our knowledge, there are no reports concerning the treatment of O/W wastewater from oilfields with this kind of magnetic demulsifier. This is the first report on the synthesis of a magnetically responsive demulsifier applied to O/W emulsions produced from oilfields.
2 Experimental methods
2.1 Chemicals
Anhydrous FeCl3, trisodium citrate, 4-dimethylaminopyridine (DMAP), tetraethyl orthosilicate (TEOS), polyacrylic acid, ethylene glycol, (3-glycidoxypropyl)trimethoxysilane (GPTMS), sodium acetate, sodium dodecyl sulfate (SDS), ammonia (28 wt%), polyacrylamide, ethanol and toluene. All of the chemicals were purchased from the Shanghai Chemical Reagent Company in analytical grade, and were used as received without further purification.2.2 Procedure for the synthesis of the novel magnetic demulsifier
The epoxy group-functionalized Fe3O4@SiO2 microspheres were prepared by surface functionalization of the Fe3O4@SiO2 microspheres using (3-glycidoxypropyl)trimethoxysilane (GPTMS) as a silane coupling agent. Fe3O4@SiO2 microspheres (0.6 g) and toluene (120 mL) were added to a four-neck round-bottom flask and then ultrasonically dispersed for 30 min. GPTMS (1.5 mL) was then added into the flask, and the mixture was refluxed at 80 °C with continuous mechanical stirring for 8 h under a flow of nitrogen. The functionalized Fe3O4@SiO2 microspheres were collected with a magnet then washed with ethanol several times and vacuum dried at 60 °C for 12 h. The obtained product is hereafter referred to as Fe3O4@SiO2-epoxy.
2.3 Preparation of the O/W emulsion
The O/W emulsion was prepared in the laboratory, using a commercial oil (ExxonMobil Co. USA). Oil (0.5 g), SDS (0.001 g), polyacrylamide (0.05 g) and 500 mL H2O were added into a beaker. The mixture was stirred using a homogenizer operated at 1500 rpm for 30 min, which caused disruption of the droplets by a combination of turbulence and intense shear flow. In this process, SDS, which has hydrophilic and hydrophobic groups, was absorbed at oil–water interface forming a stable layer on the surfaces of the droplets. By adsorbing at the interface, SDS can reduce the free energy of the system and the interfacial tension.23 Therefore, the obtained emulsion can be kept stable. The resulting emulsion is hereafter referred to as the O/W emulsion and the oil content in the O/W emulsion is about 1000 mg L−1.2.4 Demulsification tests
Typically, different quantities of the M-5010 and 5010 demulsifiers were added to 25 mL of the O/W emulsion in a colorimeter tube and the mixture was shaken 200 times by hand. The mixture was then placed in a water bath and heated at 60 °C for a period of time. Subsequently, the solution at the bottom of colorimeter tube was removed and the oil content in the solution was measured using a spectrophotometric technique. Each sample was repeated 3 times and the oil content reported is the average of the three repetitions. The demulsification performance is derived from the oil removal rate, which can be calculated from the equation:where R (%) is the oil removal rate, C0 (mg L−1) is the initial oil content, and C (mg L−1) represents the oil content after the demulsifier was added.
2.5 Recycling tests
In order to evaluate the reusability of M-5010, the used M-5010 was separated from the solution using a magnet after the demulsification test and thoroughly washed several times with both petroleum ether and ethanol. The washed M-5010 was vacuum dried at 50 °C for 8 h for use in the next cycle. Similar to the above the demulsification test, the recycled M-5010 was added to the O/W emulsion. In our study, 5 recycles were performed in order to test the reusability of this type of novel magnetic demulsifier.2.6 Characterization
The morphology of the samples was characterized by transmission electron microscopy (TEM) with a Hitachi H-600 microscope operating at 120 kV. X-ray diffraction (XRD) patterns were recorded using a Rigaku D/MAX-R diffractometer with a copper target at 40 kV and 30 mA. Fourier transform infrared (FT-IR) spectra were obtained with a Bruker Tensor 27 FT-IR spectrometer at room temperature using KBr pellets. Thermal analysis experiments were collected using a TA Q-600 TGA apparatus operated at a heating rate of 10 K min−1 under a nitrogen atmosphere. Magnetic hysteresis loops were measured using a vibrating sample magnetometer (VSM, Lakeshore 7407) with an applied field between −20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe and 20
000 Oe and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe at 300 K.
000 Oe at 300 K.
3 Results and discussion
The morphology of the magnetite particles is shown in Fig. 1a. As revealed by TEM, the magnetite particles are uniform and nearly monodisperse spherical particles with a mean diameter of about 420 nm. On the other hand, it is clear from Fig. 1b that the microspheres have well defined core–shell structures. In this case, it should be noted that the uniform silica layer was formed on the individual magnetite particle through a sol–gel process involving the hydrolysis and condensation of tetraethyl orthosilicate (TEOS) in a mixture of deionized water, ethanol and ammonia.24In order to investigate the crystal structures of Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2-epoxy, XRD experiments were carried out. As shown in Fig. 2a, diffraction peaks at 2θ values of 30.4°, 35.5°, 43.3°, 57.4°and 62.8° were observed as for Fe3O4. Obviously, these diffraction peaks could be easily indexed to the reflections of standard Fe3O4, which confirmed that the magnetite microspheres were successfully synthesized. In contrast, similar diffraction peaks are also found for the samples of Fe3O4@SiO2 and Fe3O4@SiO2-epoxy, as shown in Fig. 2b and c, suggesting that the Fe3O4 microspheres were well retained in the silica layer. No diffraction peaks corresponding to SiO2 were observed because silica is amorphous.
FTIR spectra of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2-epoxy, 5010 and M-5010 are shown in Fig. 3. Typical bands associated with Fe–O stretching are visible at around 585 and 443 cm−1. For Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2-epoxy, the bands observed at around 1635 and 1384 cm−1 correspond to the carboxylate group of trisodium citrate. Due to the citrate groups, the Fe3O4 microspheres have excellent dispersibility in polar solvents, which is beneficial for the SiO2 coating and modification processes. In addition, as shown in Fig. 3a and b, a new strong band appears around 1093 cm−1 and can be ascribed to Si–O–Si vibrations, indicating that there is a coating of silica on the magnetite surface. Compared with Fig. 3b, Fig. 3cshows new peaks at 2923 and 2856 cm−1, corresponding to the –CH2– groups of GPTMS, which can provide epoxy functionalities and exhibits many advantageous properties such as hydrophilicity, specific chemical reaction behaviour and high reactivity. Due to its three-membered ring, an epoxy group can react with different functional groups such as amines or hydroxyl groups. Moreover, the peak at 885 cm−1 can be assigned to the epoxy groups.25 In Fig. 3d, a broad band around 3400 cm−1 is observed that is caused by O–H stretching vibrations, reflecting the fact that 5010 contains abundant hydroxyl groups. This is because 5010 was synthesized using an initial self-made agent in order to enable copolymerization with ethylene oxide and propylene oxide. Therefore, the novel magnetic demulsifier can be prepared through the chemical reaction between the epoxy functionalities of the microspheres and the hydroxyl groups of 5010. Fig. 3e also indicates that the reaction happens.
The amount of the organic compound on the surface of the samples was determined using thermogravimetric analysis (TGA) under a nitrogen atmosphere. It is known that the decomposition of an organic compound can occur when the temperature is gradually increased. Therefore, the weight loss of the samples can be determined. Fig. 4 shows the variation of the weight of the sample versus temperature for Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2-epoxy and M-5010. With regards to the Fe3O4 and Fe3O4@SiO2 samples, a weight loss (4.58 wt%) is observed at temperatures below 300 °C and this phenomenon may be attributed to the desorption of water. TGA analysis of Fe3O4@SiO2-epoxy shows a weight loss (11.69 wt%) from 0 to 800 °C, which may indicate decomposition of the hydration water and epoxy groups. In addition, a significant weight loss (48.64 wt%) is observed for M-5010, which could mainly be attributed to the decomposition of 5010, which is an organic compound. These results clearly imply that 5010 has been successfully grafted onto the surface of Fe3O4@SiO2-epoxy.
The vibrating sample magnetization (VSM) curves for Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2-epoxy and M-5010 are shown in Fig. 5. It can be seen that Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2-epoxy and M-5010 all exhibit ferromagnetic behavior at 300 K. In these circumstances, the saturation magnetization (Ms) values of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2-epoxy and M-5010 are 49.3 emu g−1, 42.3 emu g−1, 40.4 emu g−1, 35.1 emu g−1, respectively. Compared with the bare Fe3O4 microspheres, the other three sample exhibited lower saturation magnetization values. It is likely that a silica coating formed on the Fe3O4 microspheres. Although the saturation magnetization of M-5010 is decreased, M-5010 still remains magnetically responsive. Therefore, M-5010 can be completely separated from an O/W emulsion with the help of a magnet, as shown in the photograph in Fig. 6. M-5010 was separately dispersed in deionized water and an O/W emulsion before the demulsification test, as shown in Fig. 6a and b, respectively. Fig. 6c shows a prepared O/W emulsion. Fig. 6d reveals the solution after the demulsification test was carried out using a magnet, which confirms that M-5010 can be separated from the solution using an external magnetic field.
Fig. 7 shows the effect of the demulsifier dosage on the oil removal rate. To this end, the demulsifier dosage with respect to the total volume of the O/W emulsion was varied from 72 mg L−1 to 198 mg L−1. In addition, the settling time was kept constant at 3 h together with the temperature (60 °C). It can be seen that the oil removal rate increases with the increasing demulsifier dosage. However, the oil removal rate does not obviously change if the demulsifier dosage continuously increases. The maximum oil removal rate is achieved when the dosage of demulsifier is about 198 mg L−1, at which point some oil droplets are observed suspended in the water. The maximum oil removal rate of M-5010 (which is as high as 97.3%) can be observed in Fig. 7. Obviously, M-5010 has a higher oil removal rate than 5010 under the same conditions. This is probably because the electrical double layer can be compressed further when M-5010 is added.
The relationship between the settling time and oil removal rate was also studied. Different settling times, i.e., 0.5 h, 1 h, 1.5 h, 2 h, 2.5 h and 3 h, were tested while the amount of demulsifier (162 mg L−1) and the temperature (60 °C) were kept constant. As exhibited in Fig. 8, the oil removal rate increases with the increase in settling time. After 3 h settling time, the oil–water separation was more efficient when M-5010 was used rather than 5010. The oil removal rate of M-5010 was 94.9%, showing its better performance in oil–water separation.
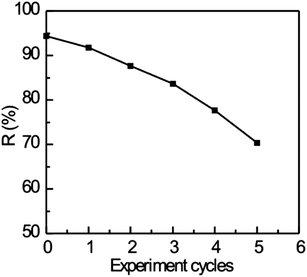
In contrast to the conventional demulsifier 5010, M-5010 can be recycled by using a magnet and can be reused after demulsification, which is a unique feature of this magnetic demulsifier. The recycled M-5010 was washed with petroleum ether and ethanol several times in order to remove any residue of the emulsion and then used for a subsequent demulsification test. In the recycle test, the dosage of recycled M-5010 was 162 mg L−1, the temperature was 60 °C and the settling time was 3 h. The demulsification test procedure described above was repeated up to 5 cycles. The oil removal rate of the recycled M-5010 was studied during each cycle, as shown in Fig. 9. Although the oil removal rate gradually decreases for each recycle, the oil removal rate of the last cycle is still above 70.4%. Our study indicates that M-5010 may potentially be an excellent demulsifier for produced wastewater containing O/W emulsions.
4 Conclusions
In summary, a novel magnetic demulsifier M-5010 was synthesized using the reaction between 5010 and epoxy group functionalized Fe3O4@SiO2 microspheres. The obtained M-5010 and 5010 were applied to O/W emulsions and the influence of varying the dosage of demulsifier and the settling time were studied. Furthermore, the oil removal rates of M-5010 and 5010 were compared. The results show that the oil removal rate increases with increasing dosage and settling time within a specific range. In addition, the maximum oil removal rate of M-5010 is 97.3% when the dosage is 198 mg L−1 and settling time is 3 h, which shows a better performance in oil–water separation than 5010, which is used in oilfields, under the same conditions. Furthermore, the demulsifier M-5010 can be recycled and reused with the help of a magnet because of its high saturation magnetization (35.1 emu g−1) and the oil removal rate remains above 70.4% after recycling 5 times. This excellent performance allows us to draw the primary conclusion that this novel magnetic demulsifier may hold great promise for the treatment of O/W wastewater from oilfields.Acknowledgements
This work is funded by the National Natural Science Foundation of China (31070517).References
- K. Panthi and K. K. Mohanty, Energy Fuels, 2013, 27, 764 CrossRef CAS.
- W. K. Qi, Z. C. Yu, Y. Y. Liu and Y. Y. Li, Chem. Eng. Sci., 2013, 91, 122 CrossRef CAS PubMed.
- G. Q. Jiang, L. Wei and C. Y. Li, Res. J. Appl. Sci., Eng. Technol., 2012, 4, 4444 Search PubMed.
- J. X. Peng, Q. X. Liu, Z. H. Xu and J. Masliyah, Energy Fuels, 2012, 26, 2705 CrossRef CAS.
- S. B. Deng, R. B. Bai, J. P. Chen, G. Yu, Z. P. Jiang and F. S. Zhou, Colloids Surf., A, 2002, 211, 275 CrossRef CAS.
- M. Karhu, V. Kuokkanen, T. Kuokkanen and J. Ramo, Sep. Purif. Technol., 2012, 96, 296 CrossRef CAS PubMed.
- H. Maruyama, H. Seki and Y. Satoh, Water Res., 2012, 46, 3094 CrossRef CAS PubMed.
- C. E. Santo, V. J. P. Vilar, C. M. S. Botelho, A. Bhatnagar, E. Kumar and R. A. R. Boaventura, Chem. Eng. J., 2012, 183, 117 CrossRef CAS PubMed.
- H. Z. Ma and B. Wang, J. Hazard. Mater., 2006, 132, 237 CrossRef CAS PubMed.
- A. A. Hafiz, H. M. El-Din and A. M. Badawi, J. Colloid Interface Sci., 2005, 284, 167 CrossRef CAS PubMed.
- B. P. Tripathi, N. C. Dubey, S. Choudhury and M. Stamm, J. Mater. Chem., 2012, 22, 19981 RSC.
- X. F. Huang, J. Liu, L. J. Lu, Y. Wen, J. C. Xu, D. H. Yang and Q. Zhou, Bioresour. Technol., 2009, 100, 1358 CrossRef CAS PubMed.
- M. Kokate, K. Garadkar and A. Gole, J. Mater. Chem. A, 2013, 1, 2022 CAS.
- H. Zhang, G. Y. Zhang, X. Bi and X. T. Chen, J. Mater. Chem. A, 2013, 1, 5934 CAS.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995 CrossRef CAS PubMed.
- S. A. G. Lopera, J. L. Arias, V. Gallardo and A. V. Delgado, Langmuir, 2006, 22, 2816 CrossRef PubMed.
- J. Liu, Z. K. Sun, Y. H. Deng, Y. Zou, C. Y. Li, X. H. Guo, L. Q. Xiong, Y. Gao, F. Y. Li and D. Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 5875 CrossRef CAS PubMed.
- Y. F. Shen, J. Tang, Z. H. Nie, Y. D. Wang, Y. Ren and L. Zuo, Sep. Purif. Technol., 2009, 68, 312 CrossRef CAS PubMed.
- A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS PubMed.
- J. H. Wang, S. R. Zheng, Y. Shao, J. L. Liu, Z. Y. Xu and D. Q. Zhu, J. Colloid Interface Sci., 2010, 349, 293 CrossRef CAS PubMed.
- Z. N. Liu, H. H. Yang, H. Zhang, C. J. Huang and L. F. Li, Cryogenics, 2012, 52, 699 CrossRef CAS PubMed.
- P. Calcagnile, D. Fragouli, I. S. Bayer, G. C. Anyfantis, L. Martiradonna, P. D. Cozzoli, R. Cingolani and A. Athanassiou, ACS Nano, 2012, 6, 5413 CrossRef CAS PubMed.
- H. Singh, A. Q. Ye and D. Horne, Prog. Lipid Res., 2009, 48, 93 CrossRef PubMed.
- Y. H. Deng, Y. Cai, Z. K. Sun, J. Liu, C. Liu, J. Wei, W. Li, C. Liu, Y. Wang and D. Y. Zhao, J. Am. Chem. Soc., 2010, 132, 8468 Search PubMed.
- Y. Y. Chen, J. S. Qiu, X. K. Wang and J. H. Xiu, J. Catal., 2006, 242, 229 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |