Design and synthesis of ice-templated PSA cryogels for water purification: towards tailored morphology and properties†
Siew-Leng Looab, William B. Krantzac, Teik-Thye Limab, Anthony G. Fane*ab and Xiao Hu*ad
aSingapore Membrane Technology Centre, Nanyang Technological University, 1 Cleantech Loop, CleanTech One, #05-05, Singapore 637141, Singapore. E-mail: a.fane@unsw.edu.au; Fax: +61 2 9385 5966; Tel: +61 2 9385 4315
bSchool of Civil and Environmental Engineering, Nanyang Technological University, Block N1, 50 Nanyang Avenue, Singapore 639798, Singapore
cDepartment of Chemical and Biological Engineering, University of Colorado, Boulder, Colorado 80309-0424, USA
dSchool of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore. E-mail: ASXHU@ntu.edu.sg; Fax: +65 68909081; Tel: +65 67904610
First published on 16th October 2012
Abstract
Hydrogels are capable of absorbing water several times their dry mass that subsequently can be released by the application of pressure, temperature change, or other external stimuli. As such, they offer promise for providing potable water in disaster relief applications. However, the swelling and mechanical properties of hydrogels need to be improved. The objectives of this study were (i) to demonstrate that the properties of poly(sodium acrylate) (PSA) cryogels can be tuned by modulating synthesis conditions such as freezing temperature, initial monomer and initiator concentrations, and crosslinker ratio, and (ii) to investigate the potential of PSA cryogels as an integral membrane for water purification in emergencies. PSA cryogels with a superfast swelling rate and a high degree of swelling that can withstand large compression strains were synthesized by conducting copolymerization reactions between N,N′-methylenebis(acrylamide) and sodium acrylate under subzero temperature conditions. The pore morphology was characterized using confocal laser scanning microscopy and scanning electron microscopy. It was shown that a lower freezing temperature and reduced initial monomer concentrations formed PSA cryogels with smaller more interconnected pores, while a higher initiator concentration in the “freezing before gelation” mode resulted in smaller pores. PSA cryogels with open interconnected pores had both a higher rate and degree of swelling, and high elasticity in response to compression. The separation efficiency of PSA cryogels was evaluated by determining turbidity removal over five operational cycles. The turbidity removal efficiency of the PSA cryogel having the highest swelling degree increased to 90% towards the fifth cycle. The water recovery during the five operational cycles ranged from 71 to 77% under a vacuum suction of 70 kPa (absolute pressure) for one minute. PSA cryogels having smaller average pore sizes were found to have higher turbidity removal efficiencies.
Introduction
Hydrogels are three-dimensional (3D) polymer networks that are capable of absorbing a large amount of water. Hence, they provide a means for absorbing water that subsequently can be released via compression, temperature change, or other external stimuli. However, the absorption and mechanical properties of hydrogels need to be improved for a variety of applications for which these materials are being considered. These applications include direct use of hydrogels for water treatment1–3 as well as hybrid processes that employ the properties of hydrogels to absorb water that can be recovered via application of external stimuli.It is thought that porous hydrogels can improve the swelling and mechanical properties of conventional hydrogels. Conventional hydrogels swell slowly because the water influx is limited by diffusion through the nonporous gel matrix. The presence of a large number of pores effectively increases the surface/volume ratio and could allow faster water absorption and desorption by osmotically driven bulk flow, which is a much faster process than diffusion.4,5 Various methods such as gas foaming,6 micro-emulsification,7 phase separation,8 freeze-drying,9 and porogen leaching10 have been used to produce macroporous hydrogels. Although these methods improved the swelling properties of hydrogels, the mechanical properties were not significantly enhanced.
Cryogelation is a unique technique used to synthesize macroporous hydrogels having tissue-like elasticity that can withstand extensive deformation without mechanical degradation.11 This technique is based on the formation of a polymeric network in a semi-frozen system in which solvent crystals act as porogens.12–14 Most previous cryogel syntheses used non-ionic monomers such as acrylamide15–23 and n-isopropylamide.24–26 In this study ionic sodium acrylate was selected as the monomer because of its high degree of water absorption arising from the chemical potential of the mobile counterions.
This paper reports the synthesis and characterization of ionic poly(sodium acrylate) (PSA) cryogels synthesized via copolymerization reactions between sodium acrylate and N,N′-methylenebis(acrylamide) at subzero temperatures. These PSA cryogels are being considered for water treatment applications, which require the cryogels to exhibit the following properties: (i) fast swelling, (ii) significant swelling, (iii) high reusability, (iv) high water recovery, and (v) high retention towards particulates. To meet the aforementioned criteria a systematic study of the effects of synthesis conditions on the properties of PSA cryogels is needed. The present study aimed to understand the effects of the preparation temperature, initial monomer and initiator concentrations, and crosslinker ratio on PSA cryogel formation, and to establish a correlation between the morphology of PSA cryogels and their properties to develop a design strategy. We believe that PSA cryogels provide a simple approach for water treatment in acute emergencies due to their portability and negligible energy operation in comparison to the existing emergency water technologies.27
Note that for the following sections we have reserved the term “cryogel” for porous PSA gels that were synthesized at a preparation temperature of −9 °C and below, while the term “hydrogel” is used for non-porous PSA gels synthesized at a temperature of −6 °C and above. This distinction is based on the morphological differences observed for PSA gels synthesized at different preparation temperatures in the present study. The term “gel” is used as a general term that includes both hydrogels and cryogels.
Experimental
Chemicals and reagents
Monomer, sodium acrylate (SA, 97%), crosslinker, N,N′-methylenebis(acrylamide) (MBA, 99%), initiator, ammonium persulfate (APS, 98%), activator, N,N,N′,N′-tetramethylethylenediamine (TEMED, ≥ 99%), fluorescein isothiocyanate isomer I (FITC, 90%), and bentonite were purchased from Sigma-Aldrich and used as received. Other chemicals used were of analytical grade. Milli-Q water (18.2 MΩ cm at 25 °C) was used throughout the study unless otherwise stated. Degassed Milli-Q water was used for preparing the synthesis solutions.Preparation of PSA cryogels
The reaction mixture of SA and MBA was degassed with oxygen-free nitrogen for 20 minutes and chilled in an ice bath for another 20 minutes. Then APS and TEMED were added and mixed for 10–15 s. 3 mL of the reaction mixture was transferred into a poly(propylene) syringe (3 mL and 9 mm ID); the syringe was placed into the liquid cooling chamber of a chiller (Polyscience 9012) for 24 h. To ensure the reproducibility of the freezing patterns, reaction mixtures of the same volume and syringes of the same dimensions were used for every synthesis. The resultant PSA gels were thoroughly washed with Milli-Q water and were dehydrated in a series of aqueous ethanol solutions (25–99.5%) followed by drying in a freeze-dryer (Alpha 1-4LD) before they were fractured into smaller disk samples. Table 1 summarizes the detailed synthesis conditions employed in this study. The yield of the syntheses was calculated by taking the ratio of the mass of dried gel to the mass of monomers used for the synthesis.| Experimental variables | Gel yield (%) | Mechanical properties | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Preparation temperature (°C) | Initial concentration | Cross-linker ratio (mol mol−1) | Young's modulus (kPa) | Failure stress (kPa) | Failure strain (%) | ||||
| TEMED (% v/v) | APS (mM) | Monomer (%) | |||||||
| a Note: N.D. = not determinable because the reaction mixture did not gel or the as-synthesized gel was too weak for further characterization, X = the gel was unable to complete the twenty cycles of swelling–deswelling, and O = the gel was able to complete twenty cycles of swelling–deswelling. Standard deviations are shown in parentheses.b No failure was observed at the end of the compression test.c Cryogel A.d Cryogel B.e Cryogel C.f Cryogel D.g Cryogel E. | |||||||||
| 25 | 0.125 | 1.75 | 8 | 0.15 | 90 | 24.6 (1.4) | 2.12 | 18 | X |
| 0 | 0.125 | 1.75 | 8 | 0.15 | 0 | N.D. | N.D. | N.D. | N.D. |
| −6 | 0.125 | 1.75 | 8 | 0.15 | 37 | 35.6 (1.9) | 6.7 | 29.2 (1.2) | X |
| −9 | 0.125 | 1.75 | 8 | 0.15 | 80 | 12.2 (1.9) | 8.4 (1.4) | 65.9 (1.2) | X |
| −12 | 0.125 | 1.75 | 8 | 0.15 | 85 | 4.7 (0.25) | 10.8 (1.7) | 79.0 (5.9) | Oc |
| −15 | 0.125 | 1.75 | 8 | 0.15 | 84 | 5.25 (0.25) | b | b | Od |
| −20 | 0.125 | 1.75 | 8 | 0.15 | 85 | 6.25 (0.25) | b | b | Oe |
| −20 | 0.0625 | 0.875 | 8 | 0.15 | 0 | N.D. | N.D. | N.D. | N.D. |
| −20 | 0.125 | 1.75 | 8 | 0.15 | 85.0 | 6.25 (0.25) | b | b | Oe |
| −20 | 0.25 | 3.50 | 8 | 0.15 | 85.4 | 13.7 (5.6) | b | b | Og |
| −20 | 0.50 | 7.00 | 8 | 0.15 | 81.6 | 106.3 | 15.4 | 25.5 | X |
| −20 | 0.125 | 1.75 | 1 | 0.05 | 0 | N.D. | N.D. | N.D. | N.D. |
| −20 | 0.125 | 1.75 | 3 | 0.05 | 54.1 | N.D. | N.D. | N.D. | N.D. |
| −20 | 0.125 | 1.75 | 5 | 0.05 | 52.2 | N.D. | N.D. | N.D. | N.D. |
| −20 | 0.125 | 1.75 | 8 | 0.05 | 95.3 | 2.2 (0.1) | b | b | Of |
| −20 | 0.125 | 1.75 | 10 | 0.05 | 92.9 | 4.5 (0.5) | b | b | O |
| −20 | 0.125 | 1.75 | 12 | 0.05 | 81.7 | 8.0 | 12.9 (1.6) | 95 | O |
| −20 | 0.125 | 1.75 | 15 | 0.05 | 78.7 | 10 | 18.2 (0.1) | 86.7 (1.7) | X |
| −20 | 0.125 | 1.75 | 20 | 0.05 | 40.5 | N.D. | N.D. | N.D. | N.D. |
| −20 | 0.125 | 1.75 | 8 | 0.0125 | 89.0 | 3.2 (0.5) | b | b | O |
| −20 | 0.125 | 1.75 | 8 | 0.05 | 95.3 | 2.2 (0.1) | b | b | Of |
| −20 | 0.125 | 1.75 | 8 | 0.10 | 83.5 | 3.3 (0.6) | b | b | O |
| −20 | 0.125 | 1.75 | 8 | 0.15 | 85.0 | 6.25 (0.25) | b | b | O |
Characterization of pore structure
The morphology of dried gels was characterized with a Scanning Electron Microscope (SEM) (Zeiss EVO50) at an accelerating voltage of 10 kV. The used PSA cryogel samples were fixed in 2% glutaraldehyde solution for 3 h and washed with 0.10 M cacodylate buffer before they were dried according to the aforementioned procedure. The dried gel samples were coated with Pt for 30 s (20 mA) using an auto-fine coater (JEOL JFC-1600). SEM micrographs were analyzed using image analysis software (ImageJ) to determine the pore-size distribution of the PSA cryogels. At least 5 images taken at different regions of the fractured gel cross-section were used for the pore-size determination.A confocal laser scanning microscope (CLSM) (Nikon A1R) was used to obtain images of the swollen gels. The gel samples (∼1 mm thickness) were stained by incubation in 1 mL of 0.1 M carbonate buffer (pH 9.45) and 40 μL of FITC (fluorescein isothiocyanate, 1 mg mL−1 in DMSO). After 24 h of incubation the gels were washed with carbonate buffer and Milli-Q water. They were then examined with CLSM at excitation and emission wavelengths of 488 and 530 nm, respectively. The 3D images were reconstructed from 2D images obtained by performing optical sectioning in the lateral-planes along the vertical axis.
The specific pore volume of the dried gels was estimated by the uptake of cyclohexane after 3 h of immersion.28 The specific pore volume, Vp (cm3 g−1) was calculated as follows:
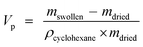 | (1) |
The water states in the swollen gel samples were characterized according to the method described by Plieva et al.19,29 with slight modifications to obtain more reproducible readings. The polymer-bound water was quantified by water-vapor adsorption in which the dried gel was placed in a chamber saturated with water vapor (but not in contact with liquid water) until its mass became constant. The mass increase due to water vapor adsorption is the mass of polymer-bound water. The amount of free water in the swollen gel was estimated by the mass decrease in the swollen gel after being subjected to vacuum suction on a microfilter unit at an absolute pressure of 70 kPa (differential pressure ∼30 kPa) for 15 min. A microfiltration membrane (Advantec, 0.45 μm) was used to prevent weak gel samples from being destroyed by the suction. The mass of capillary water was determined by taking the difference between the mass of the deswollen gel after vacuum suction and the mass of the gel at the end of the water-vapor adsorption experiment. The determination of specific pore volume and characterization of the water states were conducted in triplicate.
Uniaxial compression measurements
Fully swollen gel samples of 10 mm thickness were tested under an unconfined compression condition using a custom computer-controlled mechanical testing system (Instron 5567). A 5 kN load cell at a ramp rate of 10 mm min−1 was used and the sample was compressed up to 95% strain of its initial total length at room temperature. The experiments were conducted in triplicate. The Young's modulus, E, was determined from the initial linear slopes of the stress–strain curves plotted according to the following equation: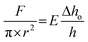 | (2) |
Determination of swelling and deswelling properties
The swelling properties were determined by gravimetric analysis. For the determination of the swelling profile, a dried gel sample with 5 mm diameter and a 5 mm height was allowed to swell in an excess of Milli-Q water. Water uptake by the gel sample was determined by measuring the cumulative mass increase at a pre-determined time interval. Excess surface water was gently wiped off using a damp paper towel before measuring the mass of the swollen gel. The degree of swelling at time t was calculated as follows: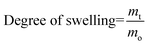 | (3) |
 | (4) |
Assessment of PSA cryogels for removing particulates
In natural waters, most pathogens are attached to particulates in water. Hence, the potential use of the PSA cryogels for water treatment was evaluated based on the efficiency of turbidity removal over five operational cycles. Turbidity was measured using a turbidimeter (Hach 2100N). The water recovery for each cycle was also determined. Contaminated water was obtained from a freshwater pond on the campus of Nanyang Technological University to which bentonite was added to increase its turbidity. Dried cryogels were allowed to fully swell in the manually stirred contaminated water (∼2 min) after which they were gently rinsed in Milli-Q water for 10 s to remove superficial contaminants (Fig. 1). The absorbed water was recovered by vacuum suction using a microfilter unit for 1 min (without using a membrane support) (Fig. 1). The turbidity removal efficiency was determined by comparing the turbidity of the raw and treated water samples. The experiments were conducted in triplicate.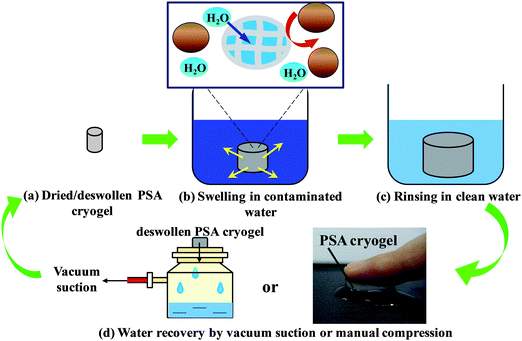 | ||
| Fig. 1 Conceptual diagram depicting the use of a PSA cryogel as an integral membrane for water purification in emergencies. | ||
Results and discussion
Syntheses and gel yields of PSA cryogels
PSA cryogels were synthesized by conducting crosslinking reactions at subzero temperatures in a semi-frozen state. The semi-frozen system was heterogenous and contained an unfrozen liquid microphase (UFLP) along with ice crystals.12–14 When the reaction mixture containing SA, MBA, TEMED, and APS was cooled to a temperature below the nominal freezing temperature, the majority of water formed ice crystals whereas bound water and soluble substances accumulated in the UFLP as a result of the freezing-point depression.30 This phenomenon is commonly referred to as cryo-concentration. In the UFLP TEMED catalyzes the decomposition of the persulfate ions to form sulfate free radicals that initiate the copolymerization reaction between SA and MBA under subzero temperature conditions.The gel yields for most of the PSA cryogels synthesized below Tprep of −9 °C were 80–95% (Table 1). The relatively high gel yield was attributed to the cryo-concentration phenomenon that effectively increased the reaction mixture concentration. A portion (or all) of the reaction mixture cooled to −6 and 0 °C was neither crosslinked nor frozen during the course of reaction. This could be due to a decrease in the reaction rate constant (at low temperature reaction) that was not accompanied by an increase in the reaction mixture concentration in the absence of cryo-concentration as the Tprep was thought to be higher than the initial ice crystallization temperature of the system.
The concentration of the initiator pair used also has a significant influence on the gel conversion. As shown in Table 1, when the concentrations of APS and TEMED were lower than 1.75 mM and 0.125%, respectively, no gelation was observed because the concentrations were too low such that the number of free radicals generated was insufficient to initiate the crosslinking reaction of SA and MBA.
The gel yield was found to increase with an increase in monomer concentration from 1 to 10% due to an increased polymerization rate (Table 1). However, a further increase in the initial monomer concentration resulted in a lower gel yield (Table 1). This may be the result of the increased viscosity of the UFLP that affected the reaction between the free radical and monomer owing to a corresponding decrease in their diffusivities. Note that the concentration of the reaction mixture can be significantly increased due to the cryo-concentration phenomenon. Kirsebom et al.31 have shown that the concentration of solute in the UFLP increased from an initial 6% monomer solution of dimethylacrylamide and poly(ethylene glycol) diacrylate to 33% and 46% at Tprep of −10 and −20 °C, respectively.
Morphological characterization of PSA gels
The study of pore structures in gel samples presents a major challenge since there is no standard method for pore-structure determination of soft matter. Pore-structure characterization techniques such as mercury intrusion porosimetry and gas adsorption–desorption among others are not suitable due to the compressibility of gels under high pressure. Microscopic techniques that allow examination of swollen gels can be used to reveal their actual structure (prior to drying). Examples of such techniques are optical microscopy, environmental scanning electron microscopy (ESEM), and confocal laser scanning microscopy (CLSM).CLSM was used to obtain 3D images of swollen PSA gels. Fig. S1† shows a comparison of the 3D reconstructed images of PSA gels synthesized at 25 and −15 °C that were previously stained with a fluorescent probe. This figure shows that a highly porous network can be obtained when the polymerization was carried out at subzero temperatures in comparison to the nonporous network obtained at ambient temperature (Fig. S1†). Although CLSM can be used to visualize the 3D pore structure of swollen gels, this technique did not have sufficient resolution to reveal the fine structure of cryogels.
SEM (Scanning Electron Microscopy) is a suitable technique to characterize the fine structure of porous materials. However, this technique requires drying of the sample that might result in structural changes. Nevertheless, cryogel scientists believe that the SEM sample preparation procedure does not result in alteration of the pore structures due to the formation of dense pore walls during cryo-concentration.15,31,32
Fig. 2a and S2† show the SEM images of PSA cryogels synthesized at different Tprep. A highly porous network was obtained when Tprep was below −9 °C while those synthesized above −6 °C were essentially nonporous (Fig. 2a and S2†). The SEM images of PSA hydrogels showed some crack development because they could not withstand the internal volume changes during the solvent-exchange process used to prepare the samples for analysis (Fig. 2a). The pore interconnectivity and porosity increased while the pore-size distribution became narrower as Tprep decreased from −9 to −20 °C (Fig. 2a, S2 and S3†). Indeed, the specific pore volume was found to increase from 2.43 to 2.92 cm3 g−1 as Tprep decreases. Similar observations were made by Hwang et al.33 who observed that poly(ethylene glycol) cryogels prepared at −14 and −20 °C had a closed and an interconnected structure, respectively. Furthermore, the average pore size decreased from 25 to 11 μm as Tprep decreased from −9 to −20 °C (Fig. S3†). Note that a lower Tprep corresponds to a faster freezing rate. Hence, this is consistent with the thermodynamics of ice nucleation that predicts the formation of a larger number of small ice crystals at a faster freezing rate.18,34,35
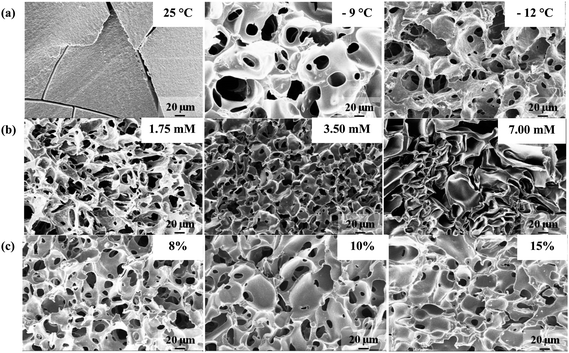 | ||
| Fig. 2 Selected SEM images of PSA gel cross-sections synthesized at various (a) preparation temperatures, (b) APS concentrations (at constant TEMED to APS ratio), and (c) initial monomer concentrations. Unless otherwise stated, the above PSA cryogels were synthesized at −20 °C for an 8% monomer concentration; the APS and TEMED concentrations used were 1.75 mM and 0.125%, respectively. The crosslinker ratio used for cryogels shown in (a) was 0.15 mol MBA/mol SA while those in rows (b) and (c) employed a crosslinker ratio of 0.05 mol MBA/mol SA. | ||
The concentration of the APS/TEMED initiator pair controls the polymerization rate and also has a significant effect on the gel morphology as shown in Fig. 2b. In order to discuss the effects of the initiator content on the gel morphology, two modes of gelation need to be distinguished, namely: (i) freezing before gelation, and (ii) freezing after gelation.21 It was observed that cryogelation using an APS concentration between 1.75 and 3.50 mM for an 8% monomer concentration followed the first mode of gelation and gave rise to a well-interconnected structure (Fig. 2b). The specific pore volume decreased slightly from 2.92 to 2.70 cm3 g−1 on doubling the APS concentration of 1.75 mM (at a constant APS/TEMED ratio). The average pore size decreased as the initiator concentration increased from 1.75 to 3.50 mM. This may be due to a faster polymerization rate that leads to smaller ice crystals (Fig. S3†).
A further increase in the APS concentration resulted in PSA cryogels with a larger average pore size. A mixture of open and closed pores was formed indicating that the gelation mode was “freezing after gelation” (Fig. 2b). The specific pore volume was significantly reduced to 0.19 cm3 g−1. This can be attributed to the presence of numerous closed pores that could have prevented the absorption of cyclohexane. Closed rather than interconnected pores were formed because it was not possible for the ice crystals to merge in a reaction mixture that was already crosslinked. In addition, it is thought that larger pores are formed because the water in large voids is preferentially frozen relative to that in small capillaries due to a smaller freezing-point depression.21 The smaller pores observed in the SEM image of the PSA cryogel obtained by the “freezing after gelation” mode were probably the result of collapsed pores due to weak pore walls that were not formed by cryo-concentration (Fig. 2b).
Fig. 2c shows that increasing the initial monomer concentration resulted in lower porosity, less pore interconnectivity, and thicker pore walls. In addition, the SEM images revealed that there were numerous large closed pores that were formed as the monomer concentration was increased (Fig. 2c). This resulted in a larger average pore size as the monomer concentration increased (Fig. S3†). The formation of large closed pores could be the result of (i) an increased viscosity of the UFLP that reduced the rate of ice crystallization and (ii) an enhanced rate of polymerization due to the higher monomer concentration. This could have caused gelation to take place faster than freezing, thereby leading to the formation of large closed pores. Ozmen and Okay36 also found that increasing the 2-acrylamido-2-methylpropane sulfonic acid (monomer) concentration resulted in the formation of larger pores with thicker pore walls. They believed that the larger pore size resulted from a higher charge density in the network chain that increased the ice volume fraction.37 However, other researchers have reported that an increase in the initial monomer concentration led to cryogels with thicker pore walls but a smaller pore size due to a higher amount of UFLP.19,23 These conflicting findings might be attributed to the difference in the nature of the monomers (i.e., ionic or non-ionic) used.
The crosslinker ratio (mol MBA/mol SA) was found to have little effect on the PSA cryogel morphology (Fig. S4†); the specific pore volume ranged from 2.92 to 3.39 cm3 g−1. PSA cryogels synthesized using crosslinking ratios of 0.0125 to 0.1500 (mol/mol) (for an 8% monomer solution at −20 °C, 0.125% TEMED, and 1.75 mM APS) all formed highly interconnected pores. However, it was noted that on increasing the crosslinker ratio, the average pore size decreased slightly (Fig. S3†). This could be attributed to the decrease of network charge density with increasing crosslinker ratio (i.e., lower SA content) leading to a smaller pore size.
Mechanical properties of PSA gels
The stress–strain curve shown in Fig. 3a shows the typical elastic behavior of a PSA cryogel in contrast to a weak and fragile hydrogel. Digital photographs of a PSA hydrogel and cryogel during compression tests provide a visual comparison of their mechanical properties (Fig. 3a, inset). The PSA hydrogel was completely broken when compressed to 18% strain (Fig. 3a, inset: i–iii). In contrast, the PSA cryogel did not show any crack development after being compressed to 95% strain (accompanied by loss of water or deswelling) and re-swelled to its original shape after relieving the pressure (Fig. 3a, inset: iv–vii). Most PSA cryogels synthesized in this study did not break when compressed to 80% strain (Table 1). However, the PSA cryogel obtained by “freezing after gelation” had a very fragile network (Table 1). This may be attributed to the formation of ice crystals in the gelled reaction mixture that damaged the network because it could not withstand the stress exerted by the growing ice crystals.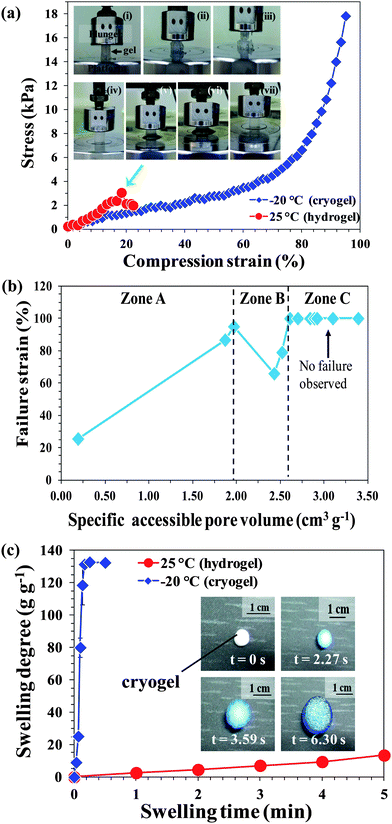 | ||
| Fig. 3 (a) Comparison of the mechanical properties of PSA hydrogel (inset: i–iii) and cryogel (inset: iv–vii). Note that the green arrow shows the point at which hydrogel started to fail/disintegrate. (b) Relationship between the mechanical properties (failure strain) of all the PSA cryogels synthesized in this study with their corresponding pore volumes. (c) Dynamic swelling profiles of PSA hydrogel and cryogel. The inset shows the time-dependent swelling of a typical PSA cryogel. | ||
In order to apply PSA cryogels for water purification, it is important that the cryogels have high elasticity to undergo oscillatory swelling–deswelling cycles while maintaining the network integrity. In addition, the network should not be too rigid so that water recovery can be conducted using mild pressure. The stiffness of a conventional hydrogel mainly depends on the network structure such as the crosslinking density of the material. In contrast, for porous materials such as cryogels, the stiffness is a function of the porosity.38 In addition, the swelling degree could have an effect on the cryogel's stiffness since the compression of a cryogel involves the release of absorbed water.
To gain insights into morphologies that improved the elasticity of PSA cryogels, the failure strains of all the cryogels synthesized were plotted against their corresponding pore volumes (Fig. 3b). Interestingly, three distinct zones could be identified with a common morphology. Zone A represents PSA cryogels with a mixture of closed and open pores while Zone B classifies cryogels with very large open pores (average pore size >20 μm). The PSA cryogels in Zone C have open interconnected pores with sufficiently small pores (average pore size: 10–14 μm) such that they have desirable mechanical properties and did not fail at the end of the compression tests. Overall, the correlation indicated that the elasticity of cryogels improved with increasing pore volume (Fig. 3b). However, two anomalous points were observed in Zone B. The unusually low failure strains for the cryogels in Zone B could be attributed to the very large pore size that weakened the cryogel network, and the thick pore wall that reduced the stretchability of the cryogel network. Hence, the PSA cryogels in Zone C have the best mechanical properties among the gels synthesized in this work.
Swelling behavior of PSA gels
From the viewpoint of application, gels that can swell quickly are desirable since this reduces the treatment time. In this respect decreasing the Tprep drastically improved the swelling rate of the PSA gels (Fig. 3c and S5†). PSA cryogels reached their equilibrium degree of swelling within 15 s while the PSA hydrogels took about 30–90 min (Fig. S5†). The swelling profiles of PSA cryogels indicate that those with large and open interconnected pores had a faster swelling rate than those having small or closed pores.Another property of interest is the equilibrium degree of swelling. A higher degree of swelling is desired because a greater amount of water can be treated per unit dry mass of cryogel. This would reduce the freight requirement when deployed for applications such as disaster relief. Fig. 4a–d show the effects of the synthesis parameters on the degree of swelling. It was found that decreasing the Tprep resulted in a substantial increase in the degree of swelling (Fig. 4a). In contrast, the degree of swelling decreased with an increase in initiator content and initial monomer concentration (Fig. 4b and d).
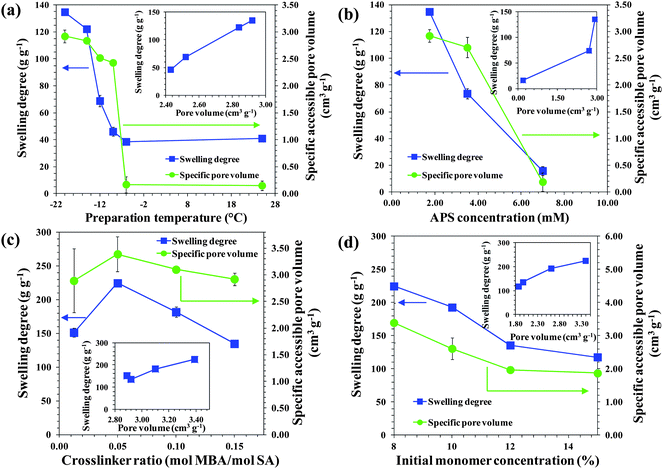 | ||
| Fig. 4 Effects of (a) preparation temperature, (b) APS concentration, (c) crosslinker ratio, and (d) initial monomer concentration on the equilibrium swelling degree and specific pore volume of PSA gels. The insets show the relationship between the swelling degree and pore volume. | ||
Overall, a higher degree of swelling was observed for PSA cryogels having a larger pore volume although the correlation is not necessarily linear (Fig. 4a–d, inset). It is postulated that PSA cryogels with a higher pore volume could absorb more water due to the additional volume present in the cryogel matrix. This hypothesis is supported by the findings from the characterization of the water states in swollen cryogels, which indicated that the increase in the degree of swelling was due to a greater amount of free water in the pores (Fig. S6†). The specific pore volumes of dried PSA cryogels synthesized in this study ranged from 1.9 to 3.4 cm3 g−1. The relatively small difference in the specific pore volume among the dried PSA cryogels cannot explain the large increase in the degree of swelling. However, it is likely that the network expansion as the cryogel swells led to an enlarged pore volume that allowed even more absorption of water. Indeed, the CLSM image of a swollen PSA cryogel showed an expanded network with a larger pore size in comparison to a dried PSA cryogel (Fig. S1†).
In addition to the pore volume, the network flexibility could also affect the swelling degree. It was found that the swelling degree increased with decreasing crosslinker ratio and reached a maximum at a crosslinker ratio of 0.05 (mol MBA/mol SA) (Fig. 4c). A lower crosslinking density would facilitate network expansion due to flexible polymer chains. However, a further decrease of the crosslinker ratio resulted in a lower degree of swelling (Fig. 4d). It is thought that at a low crosslinking density there could be a tradeoff between chain flexibility and the ability of the polymer chains to withstand elongation. It is believed that the latter factor predominated in this case causing less network expansion that resulted in a lower degree of swelling.
Oscillatory swelling–deswelling behavior of PSA cryogels
Another remarkable feature of cryogels is their ability to reversibly swell and deswell without losing mechanical integrity.39 As shown in Fig. 5a and S7,† most of the PSA cryogels exhibited a superfast and stable oscillatory swelling–deswelling up to 20 cycles without any significant loss in the degree of swelling and recovery of water. In addition, the deswollen PSA cryogels re-swell instantaneously once in contact with water. The stable, rapid, and large oscillations of PSA cryogels indicate that they have the robustness to be reusable in emergency situations. Digital photographs of a typical PSA cryogel that underwent 20 cycles of swelling–deswelling while maintaining its network integrity are shown in Fig. S8a.†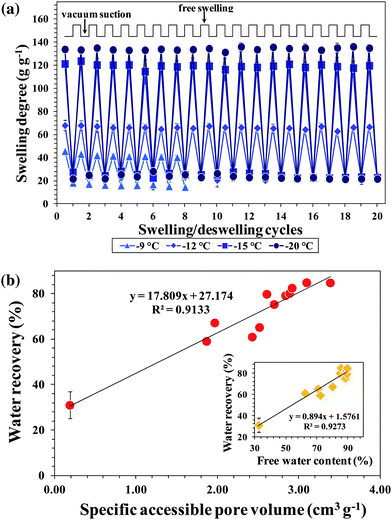 | ||
| Fig. 5 (a) Effect of preparation temperature on the oscillatory swelling–unswelling behavior of PSA cryogels. (b) Correlation of water recoveries of PSA cryogels with their corresponding pore volumes. The inset shows the correlation between water recovery and free water content. | ||
Cryogels that lacked elasticity could not withstand the swelling–deswelling cycles developed cracks and broke before the completion of the 20 cycles. The cryogels that broke before the completion of the swelling–deswelling test are indicated with the symbol “X” in the “Remarks” column of Table 1. A cross examination of their morphologies indicates that PSA cryogels with closed and/or large pores (PSA cryogels in Zones A and B of Fig. 3b) could not survive the 20 swelling–deswelling cycles whereas those with small and open interconnected pores could survive the oscillatory swelling–deswelling without significant mechanical degradation. Fig. S8b and c† show digital photographs of the PSA cryogels that were damaged during the swelling–deswelling tests.
In addition to reversible behavior, a large difference in the swelling–deswelling magnitude is also desired since it permits higher water recovery. Also, the absorbed water should be recoverable using mild compression or vacuum suction because the use of high energy-consuming devices is usually not possible after a disaster due to power breakdown. Most of the water in swollen conventional hydrogels is polymer-bound and cannot be released easily. It has been reported that 3 MPa pressure could recover only 5% of the absorbed water in a PSA hydrogel.2 In contrast, there are three different water states in a swollen cryogel, namely (i) water that is hydrogen-bonded to the polymer (polymer-bound water), (ii) water that is weakly interacting with the polymer and is freezable (capillary water), and (iii) free water that does not interact with the polymer (free water).40 The majority of the water in a swollen cryogel exists as free water and can be recovered by low-pressure compression.12,18
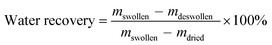
The water recoveries varied between 31 and 85% depending on the synthesis conditions of the PSA cryogels. It was found that the water recoveries were higher for cryogels synthesized with a lower Tprep, initiator and initial monomer concentration, but were not significantly affected by the crosslinker ratio. This is because these conditions allowed the formation of a swollen network in which the proportion of free water was higher. This is supported by the fact that water recovery has a good correlation with the dry pore volume and free water content of swollen PSA cryogels (Fig. 5b).
Potential of PSA cryogels for particulate removal
Fig. 1 illustrates the concept of using a PSA cryogel as an integral membrane for water purification in disaster relief. In order to be applied for water purification in emergency situations, PSA cryogels must exhibit the following properties: (i) fast swelling, (ii) significant swelling, (iii) high reusability, (iv) high water recovery, and (v) high retention towards particulates. For example, the PSA cryogel with the highest degree of swelling could swell up to 224.6 ± 2.6 g g−1 of which 84.8 ± 1.0% could be recovered. This means that about 190 mL of water can be treated by 1 g of the dried PSA cryogel in one cycle. In addition, the rapid swelling kinetics that allowed the PSA cryogel to reach equilibrium within 15 s and the relatively high water recovery using mild vacuum suction for a short duration (1 min) showed that the PSA cryogel has met the first four criteria required for emergency drinking water response applications. Note that the absorbed water can also be recovered via manual hand compression (Fig. 1). Hence, to evaluate the potential of PSA cryogels for water purification in emergencies, the separation efficiency of PSA cryogels needs to be investigated.The separation efficiency of the PSA cryogels was evaluated by measuring the turbidity removal. A contaminated water sample with a turbidity of about 650 NTU (Nephelometric Turbidity Unit) was used as the raw water. The PSA cryogel having the highest swelling degree was tested for its separation efficiency (Cryogel C, Table 1). It was found that the PSA cryogels took a longer time to reach their equilibrium swollen state in the contaminated water (2 min versus 15 s). In addition, the water recovery decreased by 5–10% relative to the tests conducted using Milli-Q water (Fig. 6a). This could be due to the partial clogging of the cryogel pores by the particulates present in the contaminated water. Fig. S9a and b† show the morphological differences between fresh and used cryogels. For the used PSA cryogel the SEM image shows a large number of foreign particles that were not present in the fresh PSA cryogel (Fig. S9a and b†). The particulates were distributed rather uniformly throughout the cross-section of the cryogel and were deposited mostly in the internal pore surface (Fig. S9b†).
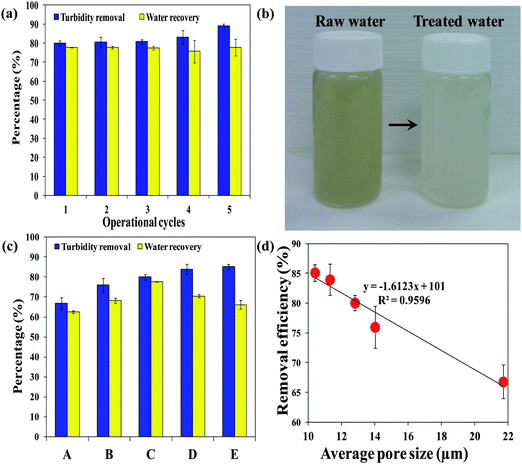 | ||
| Fig. 6 (a) Turbidity removal efficiencies and water recoveries of PSA cryogels with the highest swelling degree over five operational cycles, (b) digital photograph of the raw and treated water, (c) turbidity removal efficiencies and water recoveries of five PSA cryogels having different average pore sizes, and (d) the correlation between the turbidity removal efficiency and average pore size. Note: synthesis conditions for cryogels A, B, C, D, and E can be found in Table 1. | ||
Although the deposited particulates resulted in some reduction in the water recovery and a lower swelling rate, they enhanced the removal of turbidity (Fig. 6a). The PSA cryogel had an initial turbidity removal efficiency of 80% and increased to 90% towards the fifth cycle (Fig. 6a). This could be due to the decrease in the cryogel pore size as the particulates were deposited in the pores. Fig. 6b shows the visual improvement of the treated water samples. The turbidity removal efficiency and water recovery of a series of PSA cryogels having different average pore sizes (labeled A–E in the order of decreasing average pore size, Table 1) are shown in Fig. 6c. Turbidity removal efficiency was found to increase linearly with decreasing average pore size (Fig. 6d).
The results indicated that PSA cryogels have a relatively good turbidity removal. Nevertheless, the separation properties of PSA cryogels need to be improved in order to be used for drinking water production in emergencies. It is believed that the separation properties of PSA cryogels can be improved by the inclusion of a fast freezing step to produce smaller pores that can be achieved by flash freezing in liquid nitrogen.41,42 Alternatively, surface modifications could be employed to improve the separation efficiency of PSA cryogels. Also, antimicrobial agents such as silver nanoparticles can be incorporated into a PSA cryogel matrix to impart disinfection properties.43 We believe that the PSA cryogels synthesized in this work have the potential to be developed into portable water filters for applications in acute emergencies. A particular advantage offered by PSA cryogels is that they can be used to produce drinking water in the absence of conventional water sources. They can absorb water from mildew, humidity, or moisture that after treatment can be used for drinking purposes.
Design strategies of PSA cryogels
The findings in this study indicated that PSA cryogels with open interconnected pores and high porosity are desired for water treatment applications because they had a higher rate and degree of swelling, and could withstand large deformation. In addition, the pore size of the PSA cryogels should be sufficiently small in order to achieve satisfactory separation and mechanical properties although there could be a compromise in the swelling rate. Controlling the Tprep is instrumental in achieving the desired pore morphology since pore formation results from ice crystallization. However, it is also important to control the initial monomer and initiator concentrations in order to tune the polymerization rate such that ice crystallization occurs prior to gelation. The crosslinker ratio can be varied to tune the swelling degree of PSA cryogels although it did not have a substantive effect on the morphology. Table 2 summarizes the design strategy that can be employed to fabricate cryogels with tailored properties. This study found a range of conditions for synthesis of PSA cryogels with the desired morphologies and properties (Tprep of −15 to −20 °C; 8–10% monomer concentration; 0.125–0.250% APS and 1.75–3.50 mM TEMED; 0.0125–0.150 mol MBA/mol SA). Further optimization based on the range identified may further improve the properties of PSA cryogels.| Criteria | Desired morphology | Design strategy |
|---|---|---|
| Fast swelling | Large and open interconnected pores | Moderate freezing rate |
| Ensure “freezing before gelation” mode by minimizing monomer and initiator concentrations | ||
| Significant swelling | Open interconnected pores with high pore volume | Ensure “freezing before gelation” mode by minimizing monomer and initiator concentrations |
| Use a lower freezing temperature | ||
| Minimize crosslinker ratio to obtain a flexible network | ||
| High reusability/robust | Small and open interconnected pores with high pore volume | Ensure “freezing before gelation” mode by minimizing monomer and initiator concentrations |
| Use a lower freezing temperature and fast freezing rate | ||
| High water recovery | Open interconnected pores with high pore volume | Ensure “freezing before gelation” mode by minimizing monomer and initiator concentrations |
| Use a lower freezing temperature | ||
| Good separation | Small pores | Use a fast freezing rate |
| Use high initiator content | ||
| Minimize monomer concentration |
Conclusions
This study demonstrates that the pore structure and swelling properties of poly(sodium acrylate) (PSA) cryogels can be modulated by synthesis conditions such as the preparation temperature, initial monomer and initiator concentrations, and crosslinker ratio. By varying the synthesis conditions PSA cryogels were prepared that displayed a superfast swelling rate, high degree of swelling, and the ability to withstand large deformation. In addition, the predominant form of water in swollen PSA cryogels is free water that can be recovered easily by mild pressure. The use of PSA cryogels as integral membranes for turbidity removal was tested. A relatively high water recovery and particulate removal efficiency were achieved. In addition, turbidity removal efficiency was found to increase with operational cycles due to pore size reduction as the particulates in the raw water become deposited in the cryogel pores. These cryogels can also be used to absorb water for the exclusion of particulates in hybrid processes that employ other agents to remove the pathogens in contaminated sources. Hence, the PSA cryogels fabricated in this study have the potential to be developed into portable water filters for application in acute emergencies.Acknowledgements
This project was carried out in the Singapore Membrane Technology Centre at Nanyang Technological University (NTU) that is supported by the Economic Development Board of Singapore. S.-L. Loo acknowledges NTU for a PhD research scholarship award. The authors are grateful to Dr Yang Nan, Ms Ma Jizhen, and Ms Chen Xi from the Advanced Environmental Biotechnology Centre for their assistance with CLSM characterization.References
- D. Li, X. Zhang, J. Yao, Y. Zeng, G. P. Simon and H. Wang, Soft Matter, 2011, 7, 10048–10056 RSC.
- D. Li, X. Zhang, J. Yao, G. P. Simon and H. Wang, Chem. Commun., 2011, 47, 1710–1712 RSC.
- J. Höpfner, C. Klein and M. Wilhelm, Macromol. Rapid Commun., 2010, 31, 1337–1342 Search PubMed.
- C. Liu, N. Wei, S. Wang and Y. Xu, Carbohydr. Polym., 2009, 78, 1–4 Search PubMed.
- H. Omidian, J. G. Rocca and K. Park, J. Controlled Release, 2005, 102, 3–12 CrossRef CAS.
- K. Kabiri, H. Omidian, S. A. Hashemi and M. J. Zohuriaan-Mehr, Eur. Polym. J., 2003, 39, 1341–1348 Search PubMed.
- D. J. Bennett, R. P. Burford, T. P. Davis and H. J. Tilley, Polym. Int., 1995, 36, 219–226 CrossRef CAS.
- V. Gopishetty, I. Tokarev and S. Minko, J. Mater. Chem., 2012, 22, 19482–19487 RSC.
- S. Deng, R. Wang, H. Xu, X. Jiang and J. Yin, J. Mater. Chem., 2012, 22, 10055–10061 RSC.
- E. Kemal, K. O. Adesanya and S. Deb, J. Mater. Chem., 2011, 21, 2237–2245 RSC.
- F. M. Plieva, I. Y. Galaev, W. Noppe and B. Mattiasson, Trends Microbiol., 2008, 16, 543–551 CrossRef CAS.
- V. I. Lozinsky, Russ. Chem. Rev., 2002, 71, 489–511 RSC.
- V. Lozinsky, Russ. Chem. Bull., 2008, 57, 1015–1032 Search PubMed.
- V. I. Lozinsky, I. Y. Galaev, F. M. Plieva, I. N. Savina, H. Jungvid and B. Mattiasson, Trends Biotechnol., 2003, 21, 445–451 CrossRef CAS.
- H. Kirsebom, D. Topgaard, I. Y. Galaev and B. Mattiasson, Langmuir, 2010, 26, 16129–16133 CrossRef CAS.
- K. Yao, S. Shen, J. Yun, L. Wang, X. He and X. Yu, Chem. Eng. Sci., 2006, 61, 6701–6708 CrossRef CAS.
- X. He, K. Yao, S. Shen and J. Yun, Chem. Eng. Sci., 2007, 62, 1334–1342 Search PubMed.
- F. M. Plieva, I. N. Savina, S. Deraz, J. Andersson, I. Y. Galaev and B. Mattiasson, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2004, 807, 129–137 CrossRef CAS.
- F. M. Plieva, M. Karlsson, M.-R. Aguilar, D. Gomez, S. Mikhalovsky and I. Y. Galaev, Soft Matter, 2005, 1, 303–309 RSC.
- Q. Zhao, J. Sun, X. Wu and Y. Lin, Soft Matter, 2011, 7, 4284–4293 RSC.
- F. Plieva, X. Huiting, I. Y. Galaev, B. Bergenstahl and B. Mattiasson, J. Mater. Chem., 2006, 16, 4065–4073 RSC.
- M. M. Ozmen, M. V. Dinu, E. S. Dragan and O. Okay, J. Macromol. Sci., Part A: Pure Appl. Chem., 2007, 44, 1195–1202 Search PubMed.
- M. V. Dinu, M. M. Ozmen, E. S. Dragan and O. Okay, Polymer, 2007, 48, 195–204 CrossRef CAS.
- X.-Z. Zhang and C.-C. Chu, J. Mater. Chem., 2003, 13, 2457–2464 RSC.
- X.-Z. Zhang and C.-C. Chu, Chem. Commun., 2003, 1446–1447 RSC.
- A. Srivastava, E. Jain and A. Kumar, Mater. Sci. Eng., A, 2007, 464, 93–100 Search PubMed.
- S.-L. Loo, A. G. Fane, W. B. Krantz and T.-T. Lim, Water Res., 2012, 46, 3125–3151 Search PubMed.
- F. Topuz and O. Okay, React. Funct. Polym., 2009, 69, 273–280 Search PubMed.
- F. M. Plieva, P. Ekstrom, I. Y. Galaev and B. Mattiasson, Soft Matter, 2008, 4, 2418–2428 RSC.
- J. Wolfe, G. Bryant and K. L. Koster, CryoLetters, 2002, 23, 157–166 Search PubMed.
- H. Kirsebom, G. Rata, D. Topgaard, B. Mattiasson and I. Y. Galaev, Macromolecules, 2009, 42, 5208–5214 CrossRef CAS.
- P. Arvidsson, F. M. Plieva, I. N. Savina, V. I. Lozinsky, S. Fexby, L. Bülow, I. Y. Galaev and B. Mattiasson, J. Chromatogr., A, 2002, 977, 27–38 CrossRef CAS.
- Y. Hwang, C. Zhang and S. Varghese, J. Mater. Chem., 2010, 20, 345–351 RSC.
- H. Kirsebom and B. Mattiasson, Polym. Chem., 2011, 2, 1059–1062 RSC.
- V. Lozinsky, F. Plieva, I. Galaev and B. Mattiasson, Bioseparation, 2001, 10, 163–188 CrossRef CAS.
- M. M. Ozmen and O. Okay, J. Macromol. Sci., Part A: Pure Appl. Chem., 2006, 43, 1215–1225 Search PubMed.
- M. M. Ozmen and O. Okay, Polymer, 2005, 46, 8119–8127 CrossRef CAS.
- I. N. Savina, V. Cnudde, S. D'Hollander, L. Van Hoorebeke, B. Mattiasson, I. Y. Galaev and F. Du Prez, Soft Matter, 2007, 3, 1176–1184 RSC.
- S. V. Mikhalovsky, I. N. Savina, M. Dainiak, A. E. Ivanov and I. Y. Galaev, in Comprehensive Biotechnology, ed. M.-Y. Murray, Academic Press, Burlington, 2nd edn, 2011, pp. 11–22 Search PubMed.
- I. N. Savina, V. M. Gun'ko, V. V. Turov, M. Dainiak, G. J. Phillips, I. Y. Galaev and S. V. Mikhalovsky, Soft Matter, 2011, 7, 4276–4283 RSC.
- L. Qian, A. Ahmed, A. Foster, S. P. Rannard, A. I. Cooper and H. Zhang, J. Mater. Chem., 2009, 19, 5212–5219 RSC.
- J. Wu, Q. Zhao, J. Sun and Q. Zhou, Soft Matter, 2012, 8, 3620–3626 RSC.
- Q. Li, S. Mahendra, D. Y. Lyon, L. Brunet, M. V. Liga, D. Li and P. J. J. Alvarez, Water Res., 2008, 42, 4591–4602 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: CLSM and SEM images, pore-size distribution, swelling–deswelling profiles, and distribution of water states. See DOI: 10.1039/c2sm26859k |
| This journal is © The Royal Society of Chemistry 2013 |
