 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tuning colourimetric indicator displacement assays for naked-eye sensing of pyrophosphate in aqueous media†
Xuejian
Liu‡
,
Huy Tien
Ngo‡
,
Zijun
Ge
,
Stephen J.
Butler
and
Katrina A.
Jolliffe
*
School of Chemistry, The University of Sydney, 2006, NSW, Australia. E-mail: kate.jolliffe@sydney.edu.au; Fax: +61 2 9351 3329; Tel: +61 2 9351 2297
First published on 31st January 2013
Abstract
A library of anion receptors comprising cyclic peptide scaffolds bearing two (ZnII-DPA) anion binding sites have been synthesised and their anion binding abilities evaluated using colourimetric indicator displacement assays with three different indicators. The resulting chemosensing ensembles provided excellent discrimination between polyphosphate ions, with several of the receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) indicator combinations providing naked-eye sensing of pyrophosphate ions in aqueous solutions containing more than 100 fold excess of ATP.
indicator combinations providing naked-eye sensing of pyrophosphate ions in aqueous solutions containing more than 100 fold excess of ATP.
Introduction
The design of receptors capable of the selective recognition and sensing of biologically relevant anionic species is an area of intense recent interest.1–7 Anions such as pyrophosphate (P2O74−, PPi) and adenosine triphosphate (ATP) play important roles in metabolic and bioenergetic processes. For example, in the DNA polymerization reaction, the hydrolysis of ATP occurs with concomitant release of PPi, and thus sensors capable of discrimination between these two phosphate oxoanions have the potential for real-time monitoring of such a reaction.8 However, the selective recognition of phosphate oxoanions in aqueous media under physiological conditions remains a significant challenge. Although recent advances in receptor development have identified several sensors capable of selectively recognizing PPi in water, selectivity for the binding of PPi over other polyphosphate oxoanions such as ATP and ADP remains modest and in general is unsuitable for practical use.9–21 Furthermore, very few PPi sensors have been shown to operate in biologically relevant media, where high salt concentrations and the presence of a range of competing anions and cations are expected to affect both the binding affinity and selectivity.22Indicator displacement assays (IDAs) have been widely used for the sensing of anionic species.2,23 The ability to readily alter the indicator in such assays provides an additional means by which selectivity of a receptor can be increased.17,24,25 However, careful tuning of the interactions between receptor, indicator, target analyte and other interfering species in solution is required to achieve the desired selectivity and affinity. We recently reported the use of a family of receptors based on the bis(ZnII) complexes of backbone modified cyclic peptide scaffolds with pendant dipicolylamino (DPA) arms, capable of the selective recognition of PPi under physiological conditions using a fluorescent IDA.19,26 We have also demonstrated that these cyclic peptide based chemosensing ensembles exhibit enhanced selectivity for PPi over ATP in complex, biologically relevant media such as Krebs buffer (commonly used for cell and tissue culture and approximately isotonic with blood serum27).22 We envisaged that by screening libraries of both receptors and indicators, chemosensing ensembles with improved selectivity for PPi over other polyphosphates might be obtained. We report here our initial studies towards this goal using small libraries of both anion receptors and colourimetric indicators, and demonstrate that with careful selection of the indicator together with a suitable scaffold, near complete selectivity for PPi over other anions including ATP and ADP in both aqueous buffer and Krebs buffer can be achieved at biologically relevant concentrations. In addition, we have found that the stoichiometry of the receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) indicator complex is an additional factor that can be used to tune selectivity in IDAs.
indicator complex is an additional factor that can be used to tune selectivity in IDAs.
Results and discussion
Guided by our previous work which indicated that backbone modified cyclic peptide receptors bearing two (ZnII-DPA) functionalised sidechains provide good selectivity towards polyphosphate ions and that smaller ‘non-binding’ sidechains, e.g. alanine derivatives, favour binding of PPi over ATP and ADP,19,22,26 we prepared a new family of bis(ZnII-DPA) complexes 1·Zn2–8·Zn2 using our previously reported solid phase peptide synthesis approach26 with slight modifications (see ESI for details†). Compounds 4·Zn2–8·Zn2 contain shortened sidechains to alter the distance between the cyclic peptide scaffold and the anion binding sites since we have previously found that cyclic peptides of this type can contribute to anion binding through hydrogen bonding interactions with the amide protons and that reducing the distance between the peptidic scaffold and side chain binding sites results in increased anion binding affinity.28–30 For comparison we also prepared receptor 9·Zn231 which has previously been used in IDAs for sensing of PPi,16,32 but was not able to distinguish between PPi and ATP in IDAs with a pyrocatechol violet (PV) indicator.32Despite their lower sensitivity, an advantage of colourimetric over fluorescent IDAs is that they allow an assessment of the presence of target analytes by the naked eye, providing the ability to visually screen samples, while also allowing ratiometric assays to be developed using spectrometric techniques. We therefore visually screened a number of commercially available colourimetric indicators to identify those suitable for use in IDAs with the bis(ZnII-DPA) complexes. Of these, the three catechol-type indicators pyrocatechol violet (PV), bromopyrogallol red (BPR) and pyrogallol red (PR) (Fig. 1) were found to exhibit distinctive color changes upon mixing with 1 equiv. of receptors 1·Zn2–9·Zn2 in aqueous HEPES buffer (5 mM, pH 7.4, 145 mM NaCl) at room temperature (HEPES = 2-[4-(2-hydroxyethyl)-1-piperazinyl]-ethanesulfonic acid), attributable to binding of a bridging catecholate moiety to the two metal ions,33 and a reversal in this colour change was observed upon addition of less than five equivalents of PPi in all cases. Subsequently, UV-visible titration experiments were carried out to quantify the association constants between receptors 1·Zn2–9·Zn2 and the three indicators in HEPES buffer (5 mM, pH 7.4, 145 mM NaCl) at 25 °C. The absorption spectrum of PV (20 μM) changes upon incremental additions of each receptor. The absorption band at 640 nm increases with a concomitant decrease in the band at 444 nm (Fig. 2a). The change quickly reaches saturation as approx. 1 equiv. of each receptor is added, suggesting the formation of strong 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes. The apparent association constants (Table 1) were estimated via non-linear least squares fitting of absorption data to a 1
1 complexes. The apparent association constants (Table 1) were estimated via non-linear least squares fitting of absorption data to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model using the Hyperquad® package,34 and stoichiometry was confirmed by Job plots. The apparent association constant determined for the complex between 9·Zn2 and PV (log Ka = 8.8) is in agreement with previous reports32,35 after consideration of the higher ionic strength used in the present study. Similar titrations were carried out for BPR and PR. Surprisingly, despite their similar structures to PV, while both of these indicators were observed to bind strongly to all nine receptors, the binding data for PR to 1·Zn2–9·Zn2 could not be fit to a suitable binding model, and the data for BPR was best fitted to a stepwise 1
1 binding model using the Hyperquad® package,34 and stoichiometry was confirmed by Job plots. The apparent association constant determined for the complex between 9·Zn2 and PV (log Ka = 8.8) is in agreement with previous reports32,35 after consideration of the higher ionic strength used in the present study. Similar titrations were carried out for BPR and PR. Surprisingly, despite their similar structures to PV, while both of these indicators were observed to bind strongly to all nine receptors, the binding data for PR to 1·Zn2–9·Zn2 could not be fit to a suitable binding model, and the data for BPR was best fitted to a stepwise 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor
1 receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) indicator model, with 2
indicator model, with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry confirmed by Job plot analysis. Job plot analysis of the complexes of 1·Zn2–9·Zn2 with PR did not provide clear evidence for compound stoichiometry, suggesting the presence of multiple equilibria in solution.
1 stoichiometry confirmed by Job plot analysis. Job plot analysis of the complexes of 1·Zn2–9·Zn2 with PR did not provide clear evidence for compound stoichiometry, suggesting the presence of multiple equilibria in solution.
 | ||
| Fig. 1 Structures of receptors 1·Zn2–9·Zn2 and indicators PV, BPR and PR. | ||
![(a) Changes in absorbance of PV solution (20 μM) upon addition of 3·Zn222, [3·Zn222] = 0, 2, 4, 6, 8, 12, 16, 20, 24, 29, 39, 71, 102, 130 and 156 μM. (b) Changes in absorbance for 1 : 1 mixtures of receptor 3·Zn222 : PV (20 μM each) upon addition of PPi (sodium salt), [PPi] = 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 30, 40, 60, 79, 116, 152, 183 μM. All spectra were measured in aqueous HEPES buffer (5 mM, pH 7.4, 145 mM NaCl) at 25 °C. Insets: fitting curves at 640 nm.](/image/article/2013/SC/c3sc22233k/c3sc22233k-f2.gif) | ||
Fig. 2 (a) Changes in absorbance of PV solution (20 μM) upon addition of 3·Zn222, [3·Zn222] = 0, 2, 4, 6, 8, 12, 16, 20, 24, 29, 39, 71, 102, 130 and 156 μM. (b) Changes in absorbance for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of receptor 3·Zn222 1 mixtures of receptor 3·Zn222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PV (20 μM each) upon addition of PPi (sodium salt), [PPi] = 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 30, 40, 60, 79, 116, 152, 183 μM. All spectra were measured in aqueous HEPES buffer (5 mM, pH 7.4, 145 mM NaCl) at 25 °C. Insets: fitting curves at 640 nm. PV (20 μM each) upon addition of PPi (sodium salt), [PPi] = 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 30, 40, 60, 79, 116, 152, 183 μM. All spectra were measured in aqueous HEPES buffer (5 mM, pH 7.4, 145 mM NaCl) at 25 °C. Insets: fitting curves at 640 nm. | ||
| 1·Zn2222 | 2·Zn22222 | 3·Zn222 | 4·Zn22 | 5·Zn22 | 6·Zn222 | 7·Zn22 | 8·Zn2222 | 9·Zn2 | |
|---|---|---|---|---|---|---|---|---|---|
| a Titrations were performed at 25 °C in aqueous solutions buffered at pH 7.4 with 5 mM HEPES in the presence of 145 mM NaCl. [PV] and [receptor] were 20 μM each for competition assays. Estimated errors in log Ka < 0.2. b Changes too small to fit. | |||||||||
| PV | 8.4 | 9.0 | 8.8 | 8.5 | 9.0 | 8.9 | 7.4 | 6.6 | 8.8 |
| PPi | >9 | >9 | >9 | >9 | >9 | >9 | 8.4 | 7.1 | >9 |
| ATP | 7.2 | 7.9 | 7.2 | 8.8 | 6.7 | 4.5 | 5.4 | 4.7 | >9 |
| ADP | 7.4 | 7.0 | 4.6 | 7.2 | —b | —b | 4.6 | —b | >9 |
| Citrate | 5.9 | 6.2 | 4.4 | 6.0 | —b | —b | —b | —b | 5.4 |
Despite the differences observed in stoichiometry between complexes of 1·Zn2–9·Zn2 and the three indicators, we employed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor
1 receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) indicator mixtures in subsequent IDAs with a range of anions in HEPES buffer (5 mM, pH 7.4, 145 mM NaCl),23,36 to investigate whether we could obtain a chemosensor for PPi with high selectivity over other anions. We initially focussed on use of PV as an indicator because the 1
indicator mixtures in subsequent IDAs with a range of anions in HEPES buffer (5 mM, pH 7.4, 145 mM NaCl),23,36 to investigate whether we could obtain a chemosensor for PPi with high selectivity over other anions. We initially focussed on use of PV as an indicator because the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry observed above allowed ready quantification of the apparent stability constants of a range of anions with receptors 1·Zn2–9·Zn2 using the standard equilibria previously described for competition assays.37 Addition of five equivalents of a range of anions (nitrate, acetate, hydrogensulfate, iodide, phosphate, phenylphosphate, phosphotyrosine, phosphothreonine, phosphoserine, AMP, ADP, ATP, PPi and citrate) as their sodium salts to 1
1 binding stoichiometry observed above allowed ready quantification of the apparent stability constants of a range of anions with receptors 1·Zn2–9·Zn2 using the standard equilibria previously described for competition assays.37 Addition of five equivalents of a range of anions (nitrate, acetate, hydrogensulfate, iodide, phosphate, phenylphosphate, phosphotyrosine, phosphothreonine, phosphoserine, AMP, ADP, ATP, PPi and citrate) as their sodium salts to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of the receptors and PV (20 μM each) indicated that only PPi, ATP, ADP and, in some cases, citrate were able to displace PV from the cyclic peptide derived receptor
1 mixtures of the receptors and PV (20 μM each) indicated that only PPi, ATP, ADP and, in some cases, citrate were able to displace PV from the cyclic peptide derived receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) indicator complexes, as indicated by a colour change from blue to yellow or green (Fig. 3). Titrations of 1
indicator complexes, as indicated by a colour change from blue to yellow or green (Fig. 3). Titrations of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solutions of receptors 1·Zn2–9·Zn2 and PV with PPi, ATP, ADP and citrate under the same conditions and curve fitting of the absorption data based on the equilibria for competition assays37 using the Hyperquad® program afforded the apparent receptor–anion binding constants in Table 1 (see Fig. 4a for an example of titration data). Under these conditions all cyclic peptide receptors (1·Zn2–8·Zn2) showed modest selectivity for PPi against ATP and ADP, while the simple m-xylene-based receptor (9·Zn2) did not discriminate between these anions,38 indicating that selectivity for PPi is enhanced when using cyclic peptide derived chemosensing ensembles. In three cases (receptors 5·Zn22, 6·Zn222 and 8·Zn2222), only ATP and PPi displaced the indicator to any extent indicating that shortening the side chains between the DPA groups and the cyclic peptide improves the ability of these receptors to discriminate between polyphosphate anions and suggesting that the optimum scaffolds for PPi selectivity are those derived from the larger tetraoxazole rather than trioxazole scaffolds.
1 solutions of receptors 1·Zn2–9·Zn2 and PV with PPi, ATP, ADP and citrate under the same conditions and curve fitting of the absorption data based on the equilibria for competition assays37 using the Hyperquad® program afforded the apparent receptor–anion binding constants in Table 1 (see Fig. 4a for an example of titration data). Under these conditions all cyclic peptide receptors (1·Zn2–8·Zn2) showed modest selectivity for PPi against ATP and ADP, while the simple m-xylene-based receptor (9·Zn2) did not discriminate between these anions,38 indicating that selectivity for PPi is enhanced when using cyclic peptide derived chemosensing ensembles. In three cases (receptors 5·Zn22, 6·Zn222 and 8·Zn2222), only ATP and PPi displaced the indicator to any extent indicating that shortening the side chains between the DPA groups and the cyclic peptide improves the ability of these receptors to discriminate between polyphosphate anions and suggesting that the optimum scaffolds for PPi selectivity are those derived from the larger tetraoxazole rather than trioxazole scaffolds.
 | ||
Fig. 3 The colours of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of 2·Zn22222 1 mixtures of 2·Zn22222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PV (20 μM each) with and without anions (sodium salts) from left to right: no anion, PPi, ATP, ADP, AMP, c-AMP, phosphothreonine, phosphoserine, phosphotyrosine, HPO42− and citrate (5 equiv. each). PV (20 μM each) with and without anions (sodium salts) from left to right: no anion, PPi, ATP, ADP, AMP, c-AMP, phosphothreonine, phosphoserine, phosphotyrosine, HPO42− and citrate (5 equiv. each). | ||
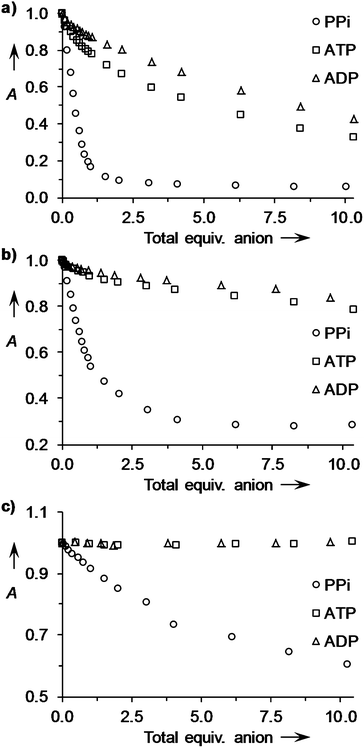 | ||
Fig. 4 Changes in absorbance for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of (a) 3·Zn222 1 mixtures of (a) 3·Zn222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PV (20 μM each) at 640 nm; (b) 3·Zn222 PV (20 μM each) at 640 nm; (b) 3·Zn222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PR (20 μM each) at 600 nm and (c) 3·Zn222 PR (20 μM each) at 600 nm and (c) 3·Zn222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BPR (10 μM each) at 610 nm; upon addition of up to 10 equiv. of anions (sodium salts) in aqueous solutions of HEPES buffer (5 mM, pH 7.4, 145 mM NaCl) at 25 °C. A = relative absorbance (arbitrary units). BPR (10 μM each) at 610 nm; upon addition of up to 10 equiv. of anions (sodium salts) in aqueous solutions of HEPES buffer (5 mM, pH 7.4, 145 mM NaCl) at 25 °C. A = relative absorbance (arbitrary units). | ||
We next screened receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) indicator complexes using PR and BPR as the indicators to investigate whether a change of indicator could be used to enhance selectivity for PPi over ATP, ADP and citrate. While it has previously been established that optimum selectivity in IDAs is obtained when Kguest ≫ Kind ≫ Kother,24,39 in the current case the differences in indicator
indicator complexes using PR and BPR as the indicators to investigate whether a change of indicator could be used to enhance selectivity for PPi over ATP, ADP and citrate. While it has previously been established that optimum selectivity in IDAs is obtained when Kguest ≫ Kind ≫ Kother,24,39 in the current case the differences in indicator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) receptor stoichiometry (and in the case of PR the ill-defined nature of this complex) make it difficult to predict how selectivity will be affected upon changing the indicator. Gratifyingly, in many cases use of PR and BPR indicators resulted in an improvement in the ability of the chemosensing ensembles to discriminate between polyphosphate and polycarboxylate ions. Using PR as the indicator, receptors 1·Zn2222, 2·Zn22222, 7·Zn22 and 8·Zn2222 exhibited excellent selectivity for PPi with no indicator displacement by ATP, ADP or citrate to any significant extent (<15% change in absorbance at 600 nm after addition of 10 equiv. of anion). Of the cyclic peptide derivatives examined with PR as the indicator, 3·Zn222 showed the lowest level of discrimination between PPi, ATP and ADP (Fig. 4b). This was significantly improved upon use of BPR as the indicator (Fig. 4c). In this case addition of at least 10 equiv. of ATP or ADP did not result in displacement of the indicator from any of the receptors examined (1·Zn222–3·Zn222 and 9·Zn2), notably including the xylene derivative 9·Zn2, which has previously shown poor discrimination between polyphosphate ions in IDAs with PV.32 These results indicate that the selectivity of IDAs can be tuned through choice of indicator, in agreement with previous observations17,24 and suggests that stoichiometries of >1
receptor stoichiometry (and in the case of PR the ill-defined nature of this complex) make it difficult to predict how selectivity will be affected upon changing the indicator. Gratifyingly, in many cases use of PR and BPR indicators resulted in an improvement in the ability of the chemosensing ensembles to discriminate between polyphosphate and polycarboxylate ions. Using PR as the indicator, receptors 1·Zn2222, 2·Zn22222, 7·Zn22 and 8·Zn2222 exhibited excellent selectivity for PPi with no indicator displacement by ATP, ADP or citrate to any significant extent (<15% change in absorbance at 600 nm after addition of 10 equiv. of anion). Of the cyclic peptide derivatives examined with PR as the indicator, 3·Zn222 showed the lowest level of discrimination between PPi, ATP and ADP (Fig. 4b). This was significantly improved upon use of BPR as the indicator (Fig. 4c). In this case addition of at least 10 equiv. of ATP or ADP did not result in displacement of the indicator from any of the receptors examined (1·Zn222–3·Zn222 and 9·Zn2), notably including the xylene derivative 9·Zn2, which has previously shown poor discrimination between polyphosphate ions in IDAs with PV.32 These results indicate that the selectivity of IDAs can be tuned through choice of indicator, in agreement with previous observations17,24 and suggests that stoichiometries of >1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the receptor
1 for the receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) indicator complex provide improved selectivity for these chemosensing ensembles.
indicator complex provide improved selectivity for these chemosensing ensembles.
Given that our previous studies have indicated that in a fluorescent IDA employing receptor 3·Zn222, selectivity for PPi over ATP and ADP improved in Krebs saline (composition: 137 mM NaCl, 5.4 mM KCl, 2.8 mM CaCl2, 1.2 mM MgSO4, 0.4 mM KH2PO4, 0.3 mM NaH2PO4, 10 mM glucose, 10 mM Tris-base buffered at pH 7.4), compared to 145 mM aq. sodium chloride solution, we were intrigued to see how chemosensing ensembles comprising 1·Zn2–9·Zn2 and a colourimetric indicator function in media with compositions matching those in biological systems such as extracellular fluid or urine.22 This was of particular interest since the effect of relatively high Mg2+ and Ca2+ concentrations on the colourimetric indicators used in the current assays has not previously been established and might be expected to be significant given the use of these compounds as complexometric indicators.40 IDAs were carried out with receptors 1·Zn22–7·Zn22 and 9·Zn2 in Krebs buffer using indicator PV to allow quantification of the apparent stability constants between the receptors and PPi, ATP, ADP and citrate for direct comparison with those obtained in saline solution (Table 2). In all cases, the colour of a PV solution changed from yellow to blue on addition of the receptors, indicating that the other anions and cations present in Krebs buffer did not interfere substantially with receptor–indicator binding.
| 1·Zn2222 | 2·Zn22222 | 3·Zn222 | 4·Zn22 | 5·Zn22 | 6·Zn222 | 7·Zn22 | 9·Zn2 | |
|---|---|---|---|---|---|---|---|---|
| a Titrations were performed at 25 °C in Krebs saline solutions buffered at pH 7.4. [PV] and [receptor] were 20 μM each for competition assays. Estimated errors in log Ka < 0.2. b Changes too small to fit. | ||||||||
| PV | 6.1 | 8.2 | 8.5 | 6.8 | 8.3 | 7.8 | 6.6 | 6.3 |
| PPi | 6.3 | 8.4 | 8.7 | 8.8 | >9 | 8.2 | 7.0 | 9.0 |
| ATP | 4.1 | —b | —b | 5.3 | —b | —b | —b | 6.8 |
| ADP | —b | —b | —b | —b | —b | —b | —b | 6.6 |
| Citrate | —b | —b | —b | —b | —b | —b | —b | 5.4 |
While the apparent stability constants of all receptors with PV were lower in Krebs saline in comparison to those observed in HEPES buffer, those for the smaller scaffolds (1·Zn2222, 4·Zn22, 7·Zn22 and 9·Zn2) were more strongly affected by the change in media composition (dropping by approximately two orders of magnitude) than those of the tetraoxazole based receptors. This is most likely a result of increased competition with phosphate ions, which have previously been shown to bind to 9·Zn2 (ref. 16 and 41). Notably, for the 3·Zn222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PV complex, there was only a very small change in the affinity constant on moving from HEPES buffer to Krebs saline. In all cases, selectivity for PPi over ATP, ADP and citrate was enhanced in Krebs saline as previously observed for fluorescent IDAs.22 While for 9·Zn2 and 4·Zn22, ATP and ADP still exhibited significant indicator displacement under these conditions, receptors 2·Zn22222, 3·Zn222, 5·Zn22, 6·Zn222 and 7·Zn22 exhibited almost complete selectivity for PPi. As illustrated in Fig. 5a, the binding of 4 equiv. of PPi to a 1
PV complex, there was only a very small change in the affinity constant on moving from HEPES buffer to Krebs saline. In all cases, selectivity for PPi over ATP, ADP and citrate was enhanced in Krebs saline as previously observed for fluorescent IDAs.22 While for 9·Zn2 and 4·Zn22, ATP and ADP still exhibited significant indicator displacement under these conditions, receptors 2·Zn22222, 3·Zn222, 5·Zn22, 6·Zn222 and 7·Zn22 exhibited almost complete selectivity for PPi. As illustrated in Fig. 5a, the binding of 4 equiv. of PPi to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 5·Zn22 and PV led to ∼90% restoration of PV's absorption, whereas 10 equiv. of either ATP or ADP only resulted in minor absorption changes (<20%). This level of selectivity represents a remarkable improvement compared to that observed in HEPES buffer (Fig. 4a). This can be attributed to two predominant factors: the numerous competitive equilibrium processes occurring in the complex Krebs mixture as a result of strong binding of Ca2+ and Mg2+ to PPi, ATP and ADP (log K′s ∼ 3–4);42–44 and the higher affinity for PPi by receptors 2·Zn22222, 3·Zn222, 5·Zn22, 6·Zn222 and 7·Zn22 compared to ATP or ADP that enables this ion to displace the strongly bound PV. Similarly, addition of 6 equiv. of PPi to a 1
1 mixture of 5·Zn22 and PV led to ∼90% restoration of PV's absorption, whereas 10 equiv. of either ATP or ADP only resulted in minor absorption changes (<20%). This level of selectivity represents a remarkable improvement compared to that observed in HEPES buffer (Fig. 4a). This can be attributed to two predominant factors: the numerous competitive equilibrium processes occurring in the complex Krebs mixture as a result of strong binding of Ca2+ and Mg2+ to PPi, ATP and ADP (log K′s ∼ 3–4);42–44 and the higher affinity for PPi by receptors 2·Zn22222, 3·Zn222, 5·Zn22, 6·Zn222 and 7·Zn22 compared to ATP or ADP that enables this ion to displace the strongly bound PV. Similarly, addition of 6 equiv. of PPi to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 5·Zn22 and PR led to ∼70% restoration of PR's absorption while addition of 10 equiv. of either ATP or ADP gave only minor changes (<8%, Fig. 5b).
1 mixture of 5·Zn22 and PR led to ∼70% restoration of PR's absorption while addition of 10 equiv. of either ATP or ADP gave only minor changes (<8%, Fig. 5b).
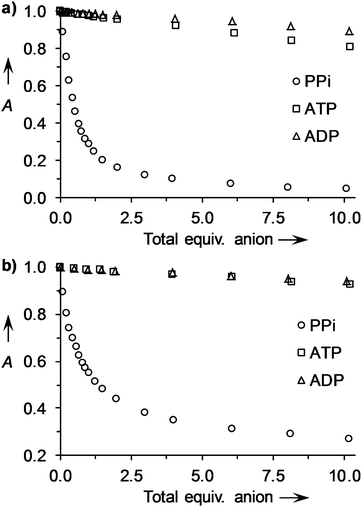 | ||
Fig. 5 (a) Changes in absorbance at 640 nm for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 5·Zn22 1 mixture of 5·Zn22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PV (20 μM each) upon addition of up to 10 equiv. of anions (sodium salts); (b) changes in absorbance at 600 nm for 1 PV (20 μM each) upon addition of up to 10 equiv. of anions (sodium salts); (b) changes in absorbance at 600 nm for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 5·Zn22 1 mixture of 5·Zn22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PR (20 μM each) upon addition of up to 10 equiv. of anions (sodium salts). Measurements were carried out in Krebs saline solutions (pH 7.4) at 25 °C. A = relative absorbance (arbitrary units). PR (20 μM each) upon addition of up to 10 equiv. of anions (sodium salts). Measurements were carried out in Krebs saline solutions (pH 7.4) at 25 °C. A = relative absorbance (arbitrary units). | ||
Given the excellent levels of selectivity for PPi observed for the cyclic peptide receptors 2·Zn22222, 3·Zn222, 5·Zn22, 6·Zn222 and 7·Zn22 in Krebs saline, it was of interest to examine whether these receptors would be useful for PPi sensing applications. Using the 3·Zn222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PV chemosensing ensemble (20 μM each), we carried out calibration experiments for PPi detection in Krebs buffer. The first calibration was obtained over the range of [PPi] between 1 and 36 μM without any ATP present, and the second calibration was carried out the same way but in the presence of 0.25 mM ATP. In both cases, a strong absorption response to PPi was observed as PV was displaced from the sensing ensemble and the presence of a large excess of ATP and other competing anions and cations did not appear to have a significant impact on the displacement capability of PPi (see ESI†). Ratiometric absorbances at 444 nm and 640 nm afforded linear calibration curves over the measured range of PPi concentrations in both experiments with similar slopes and R2 values being obtained (R2 = 0.999 for the calibration plot without ATP – see ESI;†R2 = 0.998 for the calibration plot with ATP – Fig. 6a). The results indicate that less than 2 μM of PPi can be detected even in the presence of >125 times excess of ATP in the same solution. Similar results were obtained with 2·Zn22222
PV chemosensing ensemble (20 μM each), we carried out calibration experiments for PPi detection in Krebs buffer. The first calibration was obtained over the range of [PPi] between 1 and 36 μM without any ATP present, and the second calibration was carried out the same way but in the presence of 0.25 mM ATP. In both cases, a strong absorption response to PPi was observed as PV was displaced from the sensing ensemble and the presence of a large excess of ATP and other competing anions and cations did not appear to have a significant impact on the displacement capability of PPi (see ESI†). Ratiometric absorbances at 444 nm and 640 nm afforded linear calibration curves over the measured range of PPi concentrations in both experiments with similar slopes and R2 values being obtained (R2 = 0.999 for the calibration plot without ATP – see ESI;†R2 = 0.998 for the calibration plot with ATP – Fig. 6a). The results indicate that less than 2 μM of PPi can be detected even in the presence of >125 times excess of ATP in the same solution. Similar results were obtained with 2·Zn22222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PR chemosensing ensemble in HEPES buffer (Fig. 6b), indicating that these IDAs are useful for sensing applications where the detection of low amounts of PPi is required in the presence of a large excess of ATP, such as the monitoring of DNA polymerase reactions.
PR chemosensing ensemble in HEPES buffer (Fig. 6b), indicating that these IDAs are useful for sensing applications where the detection of low amounts of PPi is required in the presence of a large excess of ATP, such as the monitoring of DNA polymerase reactions.
 | ||
Fig. 6 (a) Calibration plot for PPi obtained from ratiometric absorbances at 444 nm and 640 nm using the 3·Zn222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PV chemosensing ensemble (20 μM each) in the presence of ATP (250 μM). UV–vis measurements were carried out in Krebs saline solutions (pH 7.4) at 25 °C. R2 = 0.998. (b) Calibration plot for PPi obtained from absorbance at 565 nm using the 2·Zn22222 PV chemosensing ensemble (20 μM each) in the presence of ATP (250 μM). UV–vis measurements were carried out in Krebs saline solutions (pH 7.4) at 25 °C. R2 = 0.998. (b) Calibration plot for PPi obtained from absorbance at 565 nm using the 2·Zn22222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PR chemosensing ensemble (20 μM each) in the presence of ATP (250 μM). UV–vis measurements were carried out in HEPES buffer (5 mM, pH 7.4, 145 mM NaCl) at 25 °C. R2 = 0.991. PR chemosensing ensemble (20 μM each) in the presence of ATP (250 μM). UV–vis measurements were carried out in HEPES buffer (5 mM, pH 7.4, 145 mM NaCl) at 25 °C. R2 = 0.991. | ||
To investigate the reason for the remarkable selectivity observed for PPi with these receptors, we investigated the mode of binding between the cyclic peptide-based bis(ZnII-DPA) complexes and polyphosphate anions. 31P NMR experiments were carried out in D2O for receptor 1·Zn2222 with PPi and ATP. A change in chemical shift from −6.5 to −3.9 ppm for the 31P (202 MHz) signal for PPi was observed upon addition of 1 equiv. 1·Zn2222 (see ESI†), indicating strong binding and that all four negatively charged O–P oxygen atoms bind to the two Zn2+ centers in the symmetrical fashion. On the other hand, the three 31P signals of ATP behave differently upon binding to 1·Zn2222 (Fig. 7). While the signals attributable to Pβ and Pγ gradually moved downfield (from −22.7 and −10.5 ppm to −21.1 and −8.5 ppm for Pβ and Pγ, respectively at 2 equiv. 1·Zn2222), that of Pα shifted slightly upfield from −11.1 to −12.3 ppm. This indicated that only the two outer phosphate groups play a significant role in binding to the Zn2+ ions. These results suggest the binding modes illustrated for PPi and ATP by 1·Zn2222 in Scheme 1. The selectivity of the cyclic peptide derived receptors for PPi over ATP can be attributed in part to the difference in the total anionic charge density involved in the binding, as proposed by Lee et al.10 for other bis(ZnII-DPA) receptors. The relatively smaller total negative charge of the four O–P oxygen atoms of ATP complexed to the Zn2+ ions compared to those of PPi results in reduced binding affinity.
![31P NMR spectra (202 MHz) of ATP (10 mM) in the absence and presence of receptor 1·Zn2222 in D2O at 300 K. (a) [1·Zn2222] = 0. (b) [1·Zn2222] = 0.5 equiv. (c) [1·Zn2222] = 1 equiv. (d) [1·Zn2222] = 2 equiv.](/image/article/2013/SC/c3sc22233k/c3sc22233k-f7.gif) | ||
| Fig. 7 31P NMR spectra (202 MHz) of ATP (10 mM) in the absence and presence of receptor 1·Zn2222 in D2O at 300 K. (a) [1·Zn2222] = 0. (b) [1·Zn2222] = 0.5 equiv. (c) [1·Zn2222] = 1 equiv. (d) [1·Zn2222] = 2 equiv. | ||
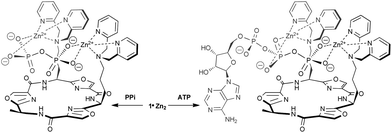 | ||
| Scheme 1 Possible binding modes for 1·Zn2222 with PPi and ATP suggested by 31P NMR data. | ||
However, there is an additional factor that contributes to the enhanced selectivity for PPi by the cyclic peptide receptors compared to the m-xylene based receptor 9·Zn2, which is related to the size and geometry of the receptors themselves. The high affinity of 9·Zn2 for all three anions (PPi, ATP and ADP) can be attributed to a tighter fit due to the shorter and more rigid side-arms of the ZnII-DPA binding sides compared to the cyclic peptide derived receptors. The increased selectivity for PPi observed for 1–8·Zn2 may be attributed to the presence of the additional side chains on the cyclic peptide-based receptors, which can interfere with ATP binding through steric blocking. Amongst the cyclic peptide receptors, those based on the larger tetraoxazole scaffolds generally exhibit higher binding affinities and selectivities than the smaller trioxazole based receptors. This difference in selectivity can be explained by additional steric interference with the larger ATP and ADP ions due to the second Ala residue on the tetraoxazole scaffolds. For the tetraoxazole scaffolds, where the two ZnII-DPA binding sites are positioned either adjacent to each other or on opposite sides of the macrocycle, there is no significant difference on PPi binding affinity or selectivity. This suggests that the relatively flexible bis(ZnII-DPA) side-arms can readily accommodate PPi in an induced-fit binding manner.
Conclusions
The synthesis of a library of cyclic peptide receptors has enabled the identification of chemosensing ensembles with excellent selectivity for PPi over other polyphosphate anions. Chemosensing ensembles comprising the cyclic peptide derived receptors 1–8·Zn2 and pyrocatechol violet show enhanced discrimination between PPi and other polyphosphate ions (ATP, ADP) in comparison to those containing the simple bis(ZnII-DPA) complex 9·Zn2. The increased selectivity can be attributed to steric blocking of the larger anions by additional side chains on the cyclic peptide scaffold. The best discrimination between anions was observed for receptors 5·Zn22 and 6·Zn222 in which the (ZnII-DPA) binding sites are appended to a tetraoxazole scaffold, with spacers of two methylene units between the scaffold and the anion binding sites. These structures provide the best compromise between the flexibility required for induced-fit binding of the PPi guest and the steric blocking of other anions by the scaffold. Notably, the selectivity of the chemosensing ensembles can be further tuned by changing the indicator, with pyrogallol red and bromopyrogallol red both providing enhanced discrimination between polyphosphate ions when compared to pyrocatechol violet, despite binding with different stoichiometries to the cyclic peptide receptors. Ensembles of all three indicators with the cyclic peptides 1–8·Zn2 provide colour changes visible to the naked eye. Importantly, we have found that such chemosensing ensembles are effective in Krebs saline solution and enable the ratiometric detection of PPi in the presence of a large excess of ATP. This will be applied in the monitoring of enzymatic reactions and detection of PPi levels in biological samples in due course.Acknowledgements
We thank the Australian Research Council for financial support.Notes and references
- A. E. Hargrove, S. Nieto, T. Zhang, J. L. Sessler and E. V. Anslyn, Chem. Rev., 2011, 111, 6603–6782 CrossRef CAS.
- H. T. Ngo, X. Liu and K. A. Jolliffe, Chem. Soc. Rev., 2012, 41, 4928–4965 RSC.
- P. A. Gale, Chem. Soc. Rev., 2010, 39, 3746–3771 RSC.
- C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520–563 RSC.
- J. L. Sessler, P. A. Gale and W.-S. Cho, Anion Receptor Chemistry, Royal Society of Chemistry, Cambridge, 2006 Search PubMed.
- Supramolecular Chemistry of Anions, ed. A. Bianchi, K. Bowman-James and E. García-España, Wiley-VCH, New York, 1997 Search PubMed.
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef.
- D.-H. Lee and J.-I. Hong, Bull. Korean Chem. Soc., 2008, 29, 497–498 CrossRef CAS.
- S. K. Kim, D. H. Lee, J.-I. Hong and J. Yoon, Acc. Chem. Res., 2009, 42, 23–31 CrossRef CAS.
- D. H. Lee, S. Y. Kim and J.-I. Hong, Angew. Chem., Int. Ed., 2004, 43, 4777–4780 CrossRef CAS.
- H. N. Lee, K. M. K. Swamy, S. K. Kim, J.-Y. Kwon, Y. Kim, S.-J. Kim, Y. J. Yoon and J. Yoon, Org. Lett., 2007, 9, 243–246 CrossRef CAS.
- H. N. Lee, Z. Xu, S. K. Kim, K. M. K. Swamy, Y. Kim, S.-J. Kim and J. Yoon, J. Am. Chem. Soc., 2007, 129, 3828–3829 CrossRef CAS.
- J. H. Lee, A. R. Jeong, J.-H. Jung, C.-M. Park and J.-I. Hong, J. Org. Chem., 2011, 76, 417–423 CrossRef CAS.
- J. F. Zhang, S. Kim, J. H. Han, S.-J. Lee, T. Pradhan, Q. Y. Cao, S. J. Lee, C. Kang and J. S. Kim, Org. Lett., 2011, 13, 5294–5297 CrossRef CAS.
- W.-H. Chen, Y. Xing and Y. Pang, Org. Lett., 2011, 13, 1362–1365 CrossRef CAS.
- R. G. Hanshaw, S. M. Hilkert, H. Jiang and B. D. Smith, Tetrahedron Lett., 2004, 45, 8721–8724 CrossRef CAS.
- B. P. Morgan, S. He and R. C. Smith, Inorg. Chem., 2007, 46, 9262–9266 CrossRef CAS.
- M. K. Coggins, A. M. Parker, A. Mangalum, G. A. Galdamez and R. C. Smith, Eur. J. Org. Chem., 2009, 343–348 CrossRef CAS.
- M. J. McDonough, A. J. Reynolds, G. W. Y. Lee and K. A. Jolliffe, Chem. Commun., 2006, 2971–2973 RSC.
- J. H. Lee, J. Park, M. S. Lah, J. Chin and J.-I. Hong, Org. Lett., 2007, 9, 3729–3731 CrossRef CAS.
- Z. Chen, Y. Lu, Y. He and X. Huang, Sens. Actuators, B, 2010, 149, 407–412 CrossRef.
- S. J. Butler and K. A. Jolliffe, Chem.–Asian J., 2012, 7, 2621–2628 Search PubMed.
- L. You and E. V. Anslyn, in Supramolecular Chemistry: From Molecules to Nanomaterials, ed. J. W. Steed and P. A. Gale, Wiley, Hoboken, NJ, 2012, pp. 135–160 Search PubMed.
- L. Fabbrizzi, N. Marcotte, F. Stomeo and A. Taglietti, Angew. Chem., Int. Ed., 2002, 41, 3811–3814 CrossRef CAS.
- S. L. Wiskur, P. N. Floriano, E. V. Anslyn and J. T. McDevitt, Angew. Chem., Int. Ed., 2003, 42, 2070–2072 CrossRef CAS.
- S. J. Butler and K. A. Jolliffe, Org. Biomol. Chem., 2011, 9, 3471–3483 RSC.
- R. M. C. Dawson, D. C. Elliot, W. H. Elliot and K. M. Jones, Data for Biochemical Research, Clarendon Press, Oxford, 1986 Search PubMed.
- P. G. Young and K. A. Jolliffe, Org. Biomol. Chem., 2012, 10, 2664–2672 RSC.
- P. Young, J. Clegg and K. Jolliffe, Supramol. Chem., 2012, 24, 77–87 Search PubMed.
- V. J. Dungan, H. T. Ngo, P. G. Young and K. A. Jolliffe, Chem. Commun., 2013, 49, 264–266 RSC.
- Y. Mito-oka, S. Tsukiji, T. Hiraoka, N. Kasagi, S. Shinkai and I. Hamachi, Tetrahedron Lett., 2001, 42, 7059–7062 CrossRef CAS.
- M. S. Han and D. H. Kim, Bull. Korean Chem. Soc., 2004, 25, 1151–1155 Search PubMed.
- K. D. Karlin, Y. Gultneh, T. Nicholson and J. Zubieta, Inorg. Chem., 1985, 24, 3725–3727 CrossRef CAS.
- P. Gans, A. Sabatini and A. Vacca, Talanta, 1996, 43, 1739–1753 CrossRef CAS.
- R. G. Hanshaw, E. J. O'Neil, M. Foley, R. T. Carpenter and B. D. Smith, J. Mater. Chem., 2005, 15, 2707–2713 RSC.
- S. L. Wiskur, H. Ait-Haddou, J. J. Lavigne and E. V. Anslyn, Acc. Chem. Res., 2001, 34, 963–972 CrossRef CAS.
- K. A. Connors, Binding Constants: The Measurement of Molecular Complex Stability, John Wiley and Sons, New York, 1987 Search PubMed.
- Apparent association constants (log Ka) of 9·Zn2 with ATP and ADP were previously determined to be 8.8 and 7.1, respectively (ref. 32). These are comparable to the values obtained in this study after taking into consideration the different conditions (higher ionic strength, higher pH) used in the current study.
- M. A. Hortalá, L. Fabbrizzi, N. Marcotte, F. Stomeo and A. Taglietti, J. Am. Chem. Soc., 2003, 125, 20–21 CrossRef CAS.
- J. Cacho, A. Lopez-Molinero and J. E. Castells, Analyst, 1987, 112, 1723–1729 RSC.
- J. V. Carolan, S. J. Butler and K. A. Jolliffe, J. Org. Chem., 2009, 74, 2992–2996 CrossRef CAS.
- R. K. Airas, Biophys. Chem., 2009, 131, 29–35 Search PubMed.
- C. T. Burt, H.-M. Cheng, S. Gabel and R. E. London, J. Biochem., 1990, 108, 441–448 Search PubMed.
- J. E. Wilson and A. Chin, Anal. Biochem., 1991, 193, 16–19 CrossRef CAS.
Footnotes |
† Electronic supplementary information (ESI) available: Experimental details; 1H and 13C spectra of new compounds; selected 1H and 31P spectra of receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anion complexes; anion titration data. See DOI: 10.1039/c3sc22233k anion complexes; anion titration data. See DOI: 10.1039/c3sc22233k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2013 |
