Evaluating the effectiveness of organic chemistry textbooks in promoting representational fluency and understanding of 2D–3D diagrammatic relationships†
Bryna C.
Kumi
a,
Jeffrey T.
Olimpo
b,
Felicia
Bartlett
a and
Bonnie L.
Dixon
*a
aDepartment of Chemistry and Biochemistry, University of Maryland, College Park, Maryland 20742, USA. E-mail: bdixon1@umd.edu; bclover@umd.edu; jeolimpo@umd.edu; fbartlet@terpmail.umd.edu
bDepartment of Teaching, Learning, Policy and Leadership, University of Maryland, College Park, Maryland 20742, USA
First published on 22nd February 2013
Abstract
The use of two-dimensional (2D) representations to communicate and reason about micromolecular phenomena is common practice in chemistry. While experts are adept at using such representations, research suggests that novices often exhibit great difficulty in understanding, manipulating, and translating between various representational forms. When used in conjunction with 2D illustrations, concrete and virtual models have been shown to increase novice understanding of 2D representations. However, such models are not always readily available. For this reason, it is vital that 2D textbook illustrations promote representational and diagrammatic competence. In this study, we use a self-designed rubric to evaluate the degree to which Newman (NPs) and Fischer Projections (FPs) are accurately introduced and described in seven frequently used Organic Chemistry textbooks. Additionally, we evaluate the success of these texts in including practice problems that encourage students to develop representational fluency by translating between different 2D diagrams. Results suggest that while most texts do a relatively thorough job of introducing the FP and NP, these introductions could be improved. Recommendations for how texts might increase novices' representational fluency through enhanced use of multi-representational examples, attention to molecule dynamics, and explicit characterization of the relationships between 3D molecules and their 2D projections are discussed.
Models and representations are used extensively in chemistry to communicate key knowledge of chemical phenomena and to make tangible the micromolecular world (Gilbert, 2005). To be successful in the field, one must therefore be able to understand, interpret, and readily translate between different forms and types of representation, as well as be able to choose the best representation for a given task. Research suggests that while experts are highly proficient at utilizing these skills, novices traditionally exhibit great difficulty in applying these same skills to solve visuospatial problems within the domain (Kozma and Russell, 1997). Specifically, studies have indicated that the abstraction of 3D information from 2D, discipline-specific diagrams has been found to be an especially difficult task for students to accomplish (Bodner and Domin, 2000; Ferk et al., 2003; Jones et al., 2005; Kuo et al., 2004).
For this reason, substantial research has been conducted focusing on identifying factors that affect novices' diagrammatic reasoning abilities (Carter et al., 1987; Pribyl and Bodner, 1987; Stieff, 2007; Stieff, 2011; Tuckey et al., 1991; Wu and Shah, 2004). These studies have concluded that the use of manipulatives, such as 3D physical models and virtual animations, increases novices' conceptual understanding of the 3D nature of molecules, thereby improving their level of representational competence (Appling and Peake, 2004; Copolo and Hounshell, 1995; Kozma et al., 2000; Wu et al., 2001; Yip et al., 2011). However, it is important to note that the use of these manipulatives does not predicate that students will transfer knowledge and understanding gained from these experiences to situations involving 2D representations (Copolo and Hounshell, 1995).
This caveat is compounded by the fact that few studies have focused on increasing novice understanding of molecular representations through the use of 2D representations alone. Although manipulatives appear to offer great support to the novice, there may be times when students do not have access to such tools and have only 2D visualizations at their disposal. Therefore, it is important that 2D illustrations, such as those in textbooks, also serve to increase students' domain-specific conceptual knowledge and representational fluency. Though the importance of illustrations in science textbooks has been discussed extensively (Cheng and Gilbert, 2009; Levie and Lentz, 1982; Mayer et al., 1995) and many have examined textbook illustrations in chemistry (Allendoerfer, 1990; Bonicamp and Clark, 2007; Stroud and Schwartz, 2010; Waner, 2010), we have found no studies which specifically address the role textbook illustrations and supporting text have in clarifying the conceptual and 3D spatial information implied in 2D projections in Organic Chemistry. In this study, we directly address this concern by using a self-designed rubric to evaluate the degree of success of several well-known Organic Chemistry texts in introducing and describing the Newman and Fischer Projections, as discussed below.
Theoretical framework and context of study
Several researchers, including Wu and Shah (2004) and Cook (2006), have offered guidelines for the design and use of instructional tools in chemistry whose purpose is to enhance students' representational fluency as they interact with visuospatial representations and diagrams. Collectively, these guidelines are grounded primarily in dual coding and cognitive load theories, and each is based on substantial empirical evidence. Given our own interest in analyzing the 2D depiction and supporting text description of Newman and Fischer Projections, we believe that the design principles offered by Wu and Shah, Cook, and others can be extended to assess the effectiveness of Organic Chemistry textbooks in promoting students' understanding of molecular representations.We have tailored the original five principles put forth by Wu and Shah (2004) to textual diagrams, as well as the corresponding chapter sections presented in the Organic Chemistry textbooks we surveyed (see Methods section below for a listing of these texts). Specifically, the five principles that we have used to instruct our assessment are: (1) using multiple representations (both two-dimensional and three-dimensional) when introducing new diagrams, (2) ensuring a referential framework between the diagrams, (3) conveying the dynamic nature of molecules and how this relates to diagram construction, (4) integrating diagrammatic and textual lessons, and (5) reducing cognitive load. This multifaceted analysis, we believe, best lends itself to establishing a holistic account of how texts discuss chemical representations.
In this study, we focus predominantly on two diagrams, the Newman projection and Fischer projection, and discuss the relationship between these forms of representation and other 2D and 3D diagrams (e.g., Dash–Wedge diagrams and pseudo-3D depictions of ball-and-stick models). As research suggests that 2D diagrams are the most frequently utilized form of representation in the field (Bodner and Domin, 2000), understanding the conventions and uses of such representations is critical. The Newman projection is used by chemists to identify spatial relationships between various substituents on adjacent carbons. These relationships directly impact and relate to the energy state of the molecule (Newman, 1995). Fischer projections, on the other hand, are traditionally used to illustrate the stereochemistry of molecules containing numerous chiral centers (McMurry, 2007). Each of these, with little or no manipulation, can be translated into a Dash–Wedge diagram, which, though also 2D in nature, perhaps best portrays the three-dimentionality of the molecule.
The relationships discussed above stress the important principle that numerous representations can be drawn of the same molecule, even if they highlight unique features of that molecule. We agree with Cook (2006) and Wu and Shah (2004) that the use of multiple representations in visualization tools is therefore essential in aiding the novice student in understanding the nuances of these domain-specific diagrams. Likewise, we acknowledge that it is vital to establish clear connections between these representations (Ainsworth, 2006; Brünken et al., 2003; Cook, 2006; Stieff et al., 2011; Wu and Shah, 2004). These elements have been captured in the first two criteria in our rubric.
The third criterion embodied by our rubric—that related to the dynamic nature of molecules—assesses how well textbooks establish connections between the intra-molecular dynamic nature of molecules and depiction of the Newman projection and Fischer projection. It is well known that all molecules are capable of undergoing internal sigma-bond rotations and that, for practical purposes, representations can be used to illustrate these different rotational states. The full 360° internal bond rotation, for instance, is most often viewed through use of a series of Newman projections. Each of these Newman projections can be translated to a Dash–Wedge structure, but only a certain set of them (those in an eclipsed conformation) can be directly converted to Fischer projections. This dynamic nature of molecules is a critical element, often overlooked, when discussing translation between various diagrammatic forms. To remedy this situation, Wu and Shah (2004), as well as Stieff and colleagues (2005), have called not only for the explicit depiction of the various, dynamic conformations a molecule may adopt, but also illustration of the 2D–3D relationship of that molecule as it transitions through these conformations and is viewed from multiple perspectives. Attentiveness to these elements, the authors contend, would serve to improve novices' representational fluency, including their ability to translate between different representational forms.
As we have alluded to previously, research suggests that the use of a multimodal learning approach—one involving 2D diagrams, virtual and concrete models, as well as text-based descriptions of chemical representations—can likewise serve to increase students' competency (Cheng and Gilbert, 2009; Kumi and Dixon, 2012; Stieff, 2007). Building from Paivio’s (1986) dual coding theory, which highlights the importance of effective integration of diagrams and linked text, the fourth and fifth criteria guiding the development of our rubric consider best practices for novices when learning about diagram construction and transformation. Several studies have suggested that, when designed correctly, presenting and discussing representations in a multimodal fashion can enhance students' understanding when neither mode of presentation is clear on its own (Chandler and Sweller, 1992; Cook, 2006; Kalyuga et al., 2003; Stieff et al., 2011; Wu and Shah, 2004). This, in turn, reduces students' task-specific cognitive load by making the implicit explicit (Brünken et al., 2003; Cook, 2006; Seufert, 2003). Connecting new information with prior knowledge in the long-term memory has also been suggested as a method of reducing cognitive load (Cook, 2006).
With specific regard to the depiction and discussion of NPs and FPs in textbook practice problems, each of the above five elements is, we believe, crucial to preventing student misconceptions. Though many factors can influence student success, previous studies have already demonstrated that textbooks may potentially promote the development of misconceptions in the field of stereochemistry and in students' understanding of basic concepts such as the structure and characteristics of molecules, for instance (Garnett et al., 1995; Smith and Jacobs, 2003). Attention to potential misconceptions regarding representations within the texts currently evaluated here is therefore of equal importance.
Methods
Selection of textbooks and identification of relevant textbook sections
The selection process was purposefully designed to provide a comparison of Organic Chemistry textbooks at the authors' institution and similar peer institutions. Evaluation of online university bookstore listings for these institutions during the winter and spring 2011 sessions resulted in the identification of five commonly used textbooks and one new textbook. These included texts by Wade (2010), McMurry (2007), Carey and Giuliano (2011), Bruice (2010), Loudon (2008) (used at our institution), and a new full textbook by Klein (2012). Two condensed guides by Klein (2006; 2008) were also evaluated given the frequency with which students at our institution and peer institutions utilize these resources. Given our primary interest in Newman and Fischer representations, book sections and chapters that focused on “Newman projections,” “Rotation and Conformation of Molecules,” “E2 reactions and mechanisms,” “Fischer Projections,” and “Carbohydrate Structure” were thoroughly examined. Analysis focused not only on the introduction of the FP and NP diagrams, but also on their use in practical contexts (e.g., practice problems).Rubric design
A 46-point rubric was designed to assess the degree to which written content and illustrations in those textbooks cited above promoted a thorough and comprehensive understanding of the Fischer Projection (FP) and Newman Projection (NP), as well as the translation between these and other representations, such as the Dash–Wedge diagram. Twenty-four of these points were associated with NP presentation, and the remaining twenty-two points accounted for the presentation of FPs. This rubric consisted of three categories: (1) Introduction to the Projection, in which points were awarded for an accurate explanation of either the NP or FP diagram and its relationship to other 2D and 3D representations, (2) Construction of the Projection, where points were awarded for the thorough demonstration of diagrammatic cues and conventions needed to understand the projection and the translation of that projection to a different representational form, and (3) Representations throughout the Text, in which points were given for the consistent presentation of the NP and FP diagrams and the effective connection of these diagrams to other relevant material within the book (see Table 1).| Area of analysis | Maximum number of points possible |
|---|---|
| 1. Introduction of the projection | 7 (NPs) or 6 (FPs) |
| 1.1 Purpose | 1 |
| 1.2 Definitions of conventions | 2 (NP) or 1 (FP) |
| 1.3 Relationship to DW | 2 |
| 1.4 Relationship to 3D representations | 1 |
| 1.5 Relationships to other 2D representations | 1 |
| 2. Construction of the projection | 11 (NPs) or 12 (FPs) |
| 2.1 Stepwise construction | 1 |
| 2.2 Diagrammatic and text-based examples | 1 |
| 2.3 Discussion of molecular conformation | 2 |
| 2.4 Illustrations and compound conformation | 1 |
| 2.5 Text discussion of viewing perspective | 1 |
| 2.6 Directional cues in illustrations | 1 |
| 2.7 Viewing perspective and relationship of substituents | 2 |
| 2.8 Carbon centers and diagram construction | 2 (NP) or 3 (FP) |
| 3. Representations throughout the text | 6 (NPs) or 4 (FPs) |
| 3.1 FPs in discussion of carbohydrates | 1 (FP only) |
| 3.2 FPs in discussion of R/S configuration | 1 (FP only) |
| 3.3 Viewing perspective throughout text | 1 |
| 3.4 Use of NPs in E2 reactions | 1 (NP only) |
| 3.5 Illustrations of molecule rotations | 2 (NP only) |
| 3.6 Textual discussion of rotations | 2 (NP) or 1 (FP) |
In addition, we developed a 20-point rubric designed to evaluate the effectiveness of practice problems within the texts at promoting representational fluency. Points were awarded for such items as providing stepwise instructions for translating between representations, encouraging students to construct representations from multiple viewing perspectives, and asking students to apply their understanding of chemical representations to assign stereochemistry and lowest/highest energy states. Items in both rubrics could receive a maximum of one, two, or three points depending on the degree to which it met the outlined criteria used for evaluation. A condensed summary of these criteria, including maximum total points per item, is provided in Tables 1 and 2.
| Area of analysis | Maximum number of points possible |
|---|---|
| 1. Presence of practice problems | 1 |
| 2. Viewing perspective(s) in practice | 3 (Total for section) |
| 2.1 Implied viewing perspective | 1 |
| 2.2 Variation of viewing perspective | 1 |
| 2.3 Viewing perspective and nomenclature | 1 |
| 3. Molecule conformations and diagram frameworks | 1 |
| 4. Reverse construction | 1 |
| 5. Carbon centers in translation and construction tasks | 2 (NPs) or 3 (FPs) |
| 6. Real-world applications of representations | 2 |
Both complete rubrics, including descriptions for each criteria, are available as ESI† (Tables S1 and S2).
Validity and reliability of scoring rubric
The validity of the rubric used in this study is grounded in and supported by our theoretical and empirical framework. Based specifically on the previously discussed principles of visualization design, the rubric awarded points for items that effectively demonstrated the relationship of the NP and FP diagrams to previously learned material, employed multiple modes of visual presentation (i.e., 2D vs. 3D) and viewing perspective, and reduced extraneous cognitive demands placed on students by forming clear connections between representations. One point was given, for instance, if a textbook identified the relationship between the NP or FP representation and the DW diagram. This technique allows the reader to connect to prior knowledge of the DW diagram and to use that knowledge to understand how the same molecule can be presented in multiple representations as either a NP or FP.Textbooks can also make distinct connections between representations by displaying and describing representations in multiple modalities. For instance, stepwise construction of either the NP or FP could be presented as a list of written steps, as well as a sequence of illustrations that align to each step. Similarly, in returning to the scenario described above, a second point would be awarded if the textbook employs a third representation, such as an illustration of a 3D ball-and-stick model, allowing for explicit connections to be made between the 3D perspective of a molecule and its 2D, diagrammatic representation.
The rubric was also designed to evaluate the accuracy of the material presented and the instructional techniques employed to present that material. Given the expertise of the authors, we believe a valid assessment of these items was achieved. Two authors hold doctoral degrees in chemistry, both have experience teaching these representations, and one has taught Introductory Organic Chemistry for 12 years. A third researcher is a doctoral student in the field of science education and also has relevant experience teaching these representations. Lastly, a specially-trained undergraduate research assistant offered the invaluable perspective of a recent student in the subject.
Textbooks were coded independently by two researchers to ensure reliability, and 92% agreement was achieved. Where appropriate, agreed upon descriptions and quotations were taken from the text to support scoring. Discrepancies in scoring were resolved by a third rater.
Results and discussion
For Organic Chemistry students in particular, knowledge gained through interaction with texts is a primary vehicle for acquiring information about chemical representations (Cheng and Gilbert, 2009). We found it imperative, therefore, to conduct a review of several commonly used textbooks in an effort to understand how they portray and discuss two commonly used representations in the field—the Newman projection (NP) and Fischer projection (FP). Specifically, we sought to evaluate the degree and success to which these textbooks (a) introduced NPs and FPs, (b) identified how these representations were constructed and how one translates between these different diagrams, including elements of stereochemistry and viewing perspective, and (c) used representations throughout the text to establish referential connections between previously presented representations and novel representations and processes. A summary of our findings is presented in Table 3 below (full point distributions can be found in Tables S3 and S5 within the ESI† accompanying the online article):| Textbook | |||||||
|---|---|---|---|---|---|---|---|
| Bruice | Carey | Kleina | Kleinb | Loudon | McMurry | Wade | |
| a Klein, Organic Chemistry, 2012. b Klein, Organic Chemistry as a Second Language, 2006 and 2008. | |||||||
| Analysis areas of Newman projections (total score possible) | |||||||
| Introduction (7) | 3 | 5 | 5 | 4 | 4 | 4 | 4 |
| Construction of diagrams (11) | 3 | 4 | 7 | 4 | 7 | 4 | 5 |
| Representation throughout text (6) | 3 | 1 | 4 | 4 | 3 | 3 | 4 |
| Total (24) | 9 | 10 | 16 | 12 | 14 | 11 | 13 |
| Analysis areas of Fischer projections (total score possible) | |||||||
| Introduction (6) | 3 | 4 | 4 | 4 | 3 | 4 | 5 |
| Construction of diagrams (12) | 2 | 8 | 4 | 9 | 11 | 6 | 5 |
| Representation throughout text (4) | 3 | 2 | 2 | 1 | 3 | 3 | 3 |
| Total (22) | 8 | 14 | 10 | 14 | 17 | 13 | 13 |
Generally, it was observed that, while most texts were roughly equivalent in making referential connections between representations, greater variation existed in how texts chose to introduce the NP and FP representations and how they portrayed the way in which one would construct these diagrams. In discussing the conventions of the Fischer Projection, for instance, Bruice (2010), Klein (2012), and Wade (2010) failed to make explicit the fact that FPs must be in an eclipsed conformation and provided few visualization cues (e.g., eyes, stick-figures) to indicate the viewing perspective readers should adopt to “see” the representation in this way. Conversely, in regard to the Newman Projection, the texts by Loudon (2008) and Klein (2012) were awarded the top two rankings largely because they were the only texts that clearly stated that one could observe NPs from multiple viewing perspectives—explicitly illustrating what the resultant NP would look like if the reader were to imagine the NP, in 3D space, being viewed from the perspective indicated by the visualization cue. This varying degree with which textbooks integrate diagrammatic and textual components is a salient and troublesome concern.
In speaking more specifically to textbooks' portrayal of NPs, two salient concerns were observed. The first of these was that only the texts by Klein (2006, 2008, 2012) and Loudon (2008) presented stepwise instructions for how to construct a Newman Projection. Given the cognitive demands imposed on novices attempting to appropriate novel representations in the field, approaching diagram construction in a stepwise manner may serve to make concrete each procedural action that must be performed to construct the complete representation (Seufert, 2003; Wu and Shah, 2004). This would, presumably, reduce cognitive load by making the implicit more explicit.
Furthermore, providing a stepwise method for constructing the NP (or any representation) demonstrates to students an explicit and successful strategy for completing that task. This is integral, particularly given that students are simultaneously devising strategies to work with representations as they are learning about them (Stieff, 2011; Stieff et al., 2010). Ongoing studies from our own group reinforce this claim, illustrating that students possess many incorrect strategies for constructing and translating between representations as a result both of difficulty understanding the conventions of each representation, as well as a weak understanding of the correct steps to perform the above tasks (Olimpo and Dixon, 2012).
This difficulty is often compounded by the types of molecules that students encounter within lecture materials and within the text. As our focus here is on textbooks, we find it critical to note secondly that the texts reviewed here, by and large, only provide students with illustrations of symmetric, non-chiral molecules. The exception among these texts is Klein (2012), who depicts NPs with two chiral centers and four different substituents on each carbon. We will discuss the importance of this feature at length below.
With regard to introduction and construction of the FP, similar trends emerge. Overall, only two texts, Klein (2006, 2008) and Loudon (2008), are successful at depicting the stepwise construction of Fischer Projections. The inclusion of these instructions is, perhaps, more critical here than in the case of the NP given students' expressed difficulty in understanding and manipulating FPs (Olimpo and Dixon, 2012). Furthermore, our evaluation suggests that texts are weak in their attention to the importance of viewer perspective and its relationship to molecule conformation when discussing FPs. While all texts except Klein (2012) and McMurry (2007) explicitly discussed the proper viewing perspective needed to translate a DW to a FP—one in which eclipsed substituents must point toward the viewer—only Loudon (2008) and McMurray (2007) illustrated this perspective from multiple directions. For example, one illustration within Loudon indicates a viewing perspective from above an eclipsed DW molecule while another shows the eclipsed substituents pointing towards the side (pp. 1168–1170). Additionally, only those texts by Carey and Giuliano (2011) and by Klein (2006, 2008) explicitly illustrate the rotation of staggered molecules into eclipsed conformations prior to constructing the FP. This latter point, though not necessary, would presumably serve to reduce students' cognitive load as they envision translating from the DW structure to the FP and reinforce the importance of the eclipsed conformation in the construction of FPs.
While the goal of Organic Chemistry textbooks may largely be to introduce key knowledge in the field (for instance, the types of representations discussed here), it is our belief that ideal texts should also encourage students to think critically about the meaning and implications of this knowledge. Therefore, an additional focal component of our rubric rested on the notion that textbooks should provide students with an opportunity to apply their understanding of chemical representations in the form of within-text assessments or practice problems.
The success of textbooks in accomplishing this task was evaluated using the same design and theoretical framework cited previously, with specific regard to the following dimensions: (a) inclusion of practice problems that required students to adopt multiple viewing perspectives of the problem; (b) inclusion of problems that illustrated non-symmetric, chiral molecules; (c) inclusion of problems that required students to perform translations between DW and NPs (or FPs) as well as from NPs (or FPs) to DW; and (d) the inclusion of problems linking chemical representations to concepts in stereochemistry and conformation (e.g., R/S stereochemistry). A summary of our findings is presented in Table 4 (see Tables S4 and S6 in the ESI† accompanying the online article for a full breakdown of how points were distributed for each text).
| Textbook | |||||||
|---|---|---|---|---|---|---|---|
| Bruice | Carey | Kleina | Kleinb | Loudon | McMurry | Wade | |
| a Klein, Organic Chemistry, 2012. b Klein, Organic Chemistry as a Second Language, 2006 and 2008. | |||||||
| Practice of projection construction (total score possible) | |||||||
| Newman projections (10) | 5 | 5 | 7 | 5 | 7 | 4 | 6 |
| Fischer projections (10) | 6 | 6 | 6 | 1 | 6 | 8 | 8 |
| Total (20) | 11 | 11 | 13 | 6 | 13 | 12 | 14 |
Generally, Klein's (2006, 2008) Second Language texts scored significantly lower than all other texts. This was largely a result of the fact that practice problems in the book already had skeletal structures of the NPs drawn, requiring only that the student simply fill in substituents. Furthermore, no practice problems were included on FPs. While the former may serve to scaffold students' comprehension of chemical representations, it does little to enhance representational fluency, which requires students to understand all dimensions of the conventions of representations and how they are constructed (Kozma and Russell, 1997). The latter is more troublesome given both the pervasive use of these supplemental resources by students and students lack of understanding of FPs (Olimpo and Dixon, 2012).
In addition to the above, several specific areas of concern were noted. For both FPs and NPs, few texts required students to translate the FP or NP to a DW—what we have termed reverse construction. Klein's (2012) Organic Chemistry text, as well as those texts by Loudon (2008) and Wade (2010) were the only three sources to do so in regard to Fischer Projections, and only the first two texts included reverse construction problems for NPs.
Similar to our findings regarding the introduction and construction of representations, we also found that most texts relied frequently on the use of symmetric molecules in presenting DWs for translation into NPs and FPs. While this practice does not incorrectly depict how one would translate between representations, research suggests that novices often have great difficulty in understanding the relationship between and placement of substituents in the initial and target representations when molecules are non-chiral (Bucat and Mocerino, 2009). Presumably, this difficulty would lead to increased misconceptions regarding how representations are constructed and manipulated—a key component of representational competence (Kozma and Russell, 1997).
Lastly, and with specific regard to Fischer Projections, the range distribution of scores among textbooks was significantly more variable than for NPs. This resulted for several reasons, including the absence of FP practice problems in the text (Klein, 2006, 2008) and practice problems that asked students to create FPs only from molecular names (Bruice (2010), Carey and Giuliano (2011), and Loudon (2008)), which eliminates the need to translate from another diagrammatic representation. Given empirical evidence that suggests students perceive FPs to be the most difficult of the three representations to understand (Olimpo and Dixon, 2012), lack of attention to the above items is problematic and should be considered in greater depth.
A critical question then becomes how textbooks should go about addressing these issues more broadly. We present two specific suggestions below, drawing on our own results as well as those in the literature to support our assertions.
Textbooks can increase referential connections between multiple types of representations
The presentation of molecules using multiple representations and descriptions allows novices to see connections between the representations, increases novices' translation skills, and may serve to decrease their cognitive load (Brünken et al., 2003; Cook, 2006; Stieff et al., 2011; Wu and Shah, 2004). We find that textbooks do use such multi-representational tactics but feel that the use of these tactics could be increased in the hope that this would lead to an augmentation in novices' understanding of chemical representations. Within both the NP and FP sections, 6 of the 7 textbooks analyzed related these new diagrams to the DW representation. Assuming the reader fully appreciates and understands the conventions of the DW, and that this representation has been stored as prior knowledge, depiction of the novel representation in this manner should serve to facilitate students' understanding of the new representation. As well, these same textbooks clearly related the information in the text with the illustrations present in a way that could presumably serve to reduce cognitive load for novices.Aside from the DW diagram, however, few other representations are used in the illustration of NPs and FPs. For example, only 3 out of 7 books employed a ball-and-stick model to illustrate FPs and NPs. Similarly, none of the textbooks examined here related the NP to the FP, though 4 out of 7 related other representations, such as the sawhorse, to the NP. To address this concern, we propose that a schematic, such as the one presented in Fig. 1 below, be utilized to illustrate the connections between all three of these diagrams (the DW, NP, and FP) and the various conformations they can adopt. The primary purpose of this schematic would be to increase students' representational fluency by making implicit elements of the representation explicit. As an extension, practice problems requiring students to create such schematics for various molecules would also have the potential to increase representational competence.
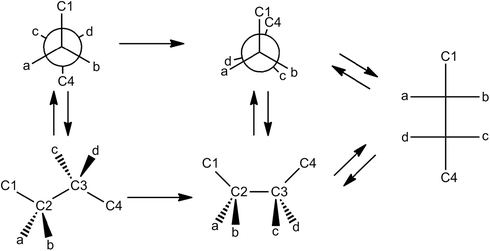 | ||
| Fig. 1 Illustration demonstrating the connections between Dash–Wedge diagram, Newman projection, and Fischer projection. This illustration also indicates the importance of rotating staggered molecules into an eclipsed conformation if they are to be projected as Fischer diagrams. Viewing perspective is such that the front of the NP becomes the left-hand side of the DW which becomes the top of the FP (and vice versa). | ||
It is likewise important that students are able to draw connections between 2D diagrams and the 3D molecules they represent if they are to develop metarepresentational skills in the domain (Bodner and Domin, 2000; Kozma and Russell, 1997). Though limited to a 2D medium, textbooks can demonstrate three-dimensionality in illustrations without relying exclusively on the use of conventions such as the dash and wedge. For instance, through use of illustrations of ball-and-stick models presented in conjunction with 2D diagrams, textbooks might encourage the reader to envision what a given molecule would look like in 3-dimensions and would presumably facilitate students' ability to connect these 3D representations with the more common 2D representations they encounter.
However, as we have stated above, few textbooks include such illustrations. With specific regard to the use of DW representations, for instance, textbooks often assume that the reader is able to abstract and understand the three-dimensionality depicted in the flat, 2D diagram. Bodner and Domin (2000) suggest, though, that this assumption is not always true. In other words, students do not create associations or referential links between 2D and 3D representations of the same molecule. In these cases, use of a ball-and-stick illustration would serve to ensure that readers connected these illustrations to the 3D molecules they represent, as opposed to seeing only lines and letters. Interestingly, two textbooks (Wade, 2010 and Loudon (2008)) did recommend that readers use modeling kits to compare different representations illustrated in the text. While research suggests that students would do well to heed this recommendation (Stieff, 2007; Stull et al., in press), it does little to directly enhance students' ability to visualize molecules in 3D simply by interacting with the text alone.
In addition to explicit illustration of 3D models (such as the ball-and-stick), visual cues, such as “eyes” and stick figures positioned in relation to molecules, can be effectively utilized to reinforce the three-dimensional nature of molecules and offer the viewer a perspective from which to view the molecule. As alluded to previously, the majority (6/7) of textbooks did include some form of visual cue to indicate the viewer perspective that one should adopt when constructing and interpreting both FPs and NPs. However, these textbooks typically utilized visual cues in a unidirectional manner. That is, in illustrations, translations to NPs were often portrayed with the left side of a DW becoming the front of the NP. McMurray (2007) and Loudon (2008) were the only texts to contradict this norm, and did so only within the introduction to FPs. We believe that such unidirectional translations may decrease students' awareness of the three-dimensionality of the molecules represented and encourage students to consistently adopt only one viewing perspective, regardless of its applicability to a given task. We have previously observed this phenomenon in students who are asked to perform representational translation tasks, for instance (Kumi and Dixon, 2012). In this regard, including illustrations of FP and NP translations from multiple perspectives may be beneficial in conveying relationships between representations and, with particular regard to the FP, showing the importance of proper alignment of the viewer with substituents in the molecule.
In exploring this latter recommendation through evaluation of the textbooks discussed here, we found that, on a whole, few textbooks (4/7 with FPs and 2/7 with NPs) showed an illustration of what the molecule would “look” like from the indicated viewing perspective, even if only one perspective was presented to the reader. To alleviate this concern in text discussing the translation of a DW to an FP, for instance, we offer that a textbook might address how to translate between these representations by showing a dash–wedge molecule with substituents pointing out of the page toward the reader when the reader is asked to view the molecule from below in constructing the FP. Cues such as these are important to illustrate the connection between perspective and conventions of diagrams, such as the characteristic that substituents in a FP must point towards the viewer, as is the case in our example above. Furthermore, by emphasizing these cues, the three-dimensionality and dynamic nature of the molecule is likewise stressed. Our group has previously observed that novices often misalign the viewer with respect to the substituents when translating FPs because they fail to understand that molecules can be rotated to form eclipsed conformations (if initially staggered) and/or fail to understand the relationship between substituents on the initial representation and how they map to substituent placement on the target representation (Kumi and Dixon, in preparation). Understanding the importance of viewing perspective is, therefore, something we believe texts should capitalize upon.
Textbooks should make more explicit the dynamic and complex nature of molecules, as well as the ability of molecules to have multiple chiral centers and to adopt multiple conformations
When using multiple representations, it is vital that textbooks explicitly illustrate the dynamic and complex nature of molecules in these representations if students are expected to utilize them effectively, for instance, to translate between DWs, NPs, and FPs (Ainsworth, 2006; Stieff et al., 2011; Wu and Shah, 2004). In regard to the discussion of NPs with multiple chiral centers specifically, we believe that textbooks can make such information clearer in their introduction to NPs. At present, our review suggests that though most textbooks illustrated the DW to NP translation, only one of the books (Klein, 2012) examined here used molecules with two chiral centers in introductory illustrations and practice problems. The remaining texts instead depict the translation using symmetric molecules, such as ethane or butane. As stated previously, we believe the use of such molecules potentially produces confusion regarding the translation of substituents between projections. For example, use of symmetric DW projections may leave students unable to determine where a substituent would reside within the NP projection—the front or back carbon, left or right of the diagram—and may lead students to believe there is no difference in the resulting NPs based on the end-on view that is used. Use of chiral centers in NP illustrations would therefore allow the reader to more easily follow the transposition of each substituent from the initial to the target representation and to identify the relationship between these two diagrams.Likewise, textbooks would do well to capitalize upon the dynamic nature of molecules (Stieff et al., 2005; Wu and Shah, 2004), a characteristic that is often vital to the translation between representations. Boukhechem and colleagues (2011) found that students often had difficulty comparing molecules in different representations because they did not consider rotations around carbon–carbon bonds, instead seeing the molecules as static. In this regard, the dynamic nature of molecules and the clear illustration of connections between representations could be increased, for instance, in many textbooks' introduction to FPs. Though it is vital that molecules be in an eclipsed conformation in the translation to an FP, only 3 out of 7 textbooks explicitly state this condition, and only two of the books (Carey and Giuliano (2011) and Klein (2006, 2008)) we evaluated included an illustration of the molecular rotations needed in order to translate a staggered molecule to an FP representation. Many textbooks only implied the importance of the eclipsed conformation within illustrations showing the translation of an eclipsed molecule to the FP, which may not be clear enough for students to realize the importance of conformation with regard to this projection. Similar to our NP findings, several textbooks also used only single-carbon chiral molecules in introductory FP illustrations, which presumably deemphasizes the importance of the eclipsed conformation.
Previously, we showed that students often simply flatten molecules to form an FP, failing to rotate staggered molecules into an eclipsed formation prior to the construction of the FP (Kumi and Dixon, in preparation). The textbooks evaluated here do not include any illustration or text to dissuade or address this misconception. Kabapinar (2009) has suggested the use of multi-frame illustrations to convey molecule movement, and we believe this technique could also be used to demonstrate the 3D rotations of molecules in alignment with 2D diagrams, such as that in Fig. 2. We acknowledge that this is not the only approach or series of viewing perspectives that one could adopt to translate between these representations. Regardless, stating the importance of rotations within the text and including a multi-frame illustration of the translation from a staggered molecule to an eclipsed molecule, and finally to an FP, could benefit students by clearly establishing connections between the representations, identifying the conventions of the FP, and highlighting the important role of the rotations of molecules (Wu and Shah, 2004).
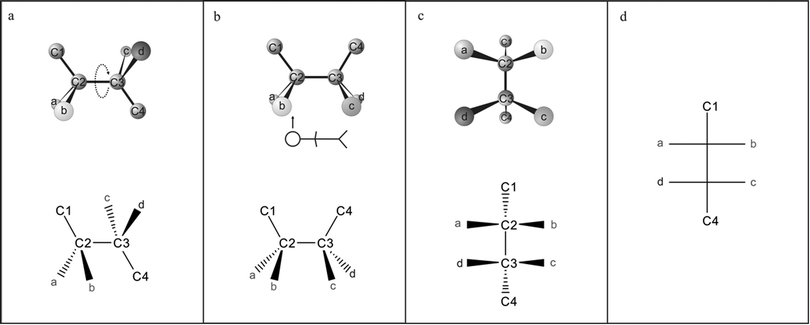 | ||
| Fig. 2 A suggested figure for the introduction of Fischer projections from a staggered conformation: Follow the 3D ball-and-stick (top) and dash–wedge (bottom) representations in the formation of a Fischer projection. A staggered molecule (a) must be rotated to an eclipsed formation prior to the formation of a Fischer projection; this is shown as an arrow depicting rotation of the C3 center to the eclipsed formation (b). Imagine the perspective of the stick figure to achieve the representations in (c). These transfer directly to the Fischer projection of this molecule (d). | ||
Given the aforementioned observations, it is perhaps not surprising that the dynamic nature of molecules could also be better illustrated in the introduction to NPs. We have previously demonstrated that students have difficulty identifying NPs of DW structures if the NP has undergone complex rotations of both the front and back carbons (Kumi and Dixon, in preparation). In most textbooks, discussion of the rotation around the carbon–carbon bond in a NP was used in combination with graphs to illustrate a molecule's energetic changes due to gauche and anti substituent relationships. Often, when such rotations were illustrated, one carbon of the NP was held still while the other was rotated, and the carbon held still was the same in all illustrations. While this may be an effective illustrative technique to clearly show the energetic effects of rotation, it does not introduce students to the concept that both carbons can (and do) rotate independently and gives students little experience visualizing complex rotations. In our sample, only 3 out of 7 texts explicitly stated that both carbons could rotate independently. We believe that the inclusion of such a statement, illustration of two-carbon rotation, or showing different single-carbon rotations throughout the text may serve to signify the possibility of complex rotations to students.
Conclusions and implications for teaching and learning
We have shown that textbooks, though ideal in many regards, could still benefit from placing further emphasis on the introduction, conventions, and dynamism of chemical visualizations, such as the Newman and Fischer projections. Additionally, we have illustrated the importance of textbooks in addressing, instead of potentially reinforcing, student misconceptions about these diagrams. This could be achieved both through better explanation of chemical representations, as well as the inclusion of within-text assessments and practice problems that would allow students to apply their knowledge of these representations in a practical context.Regardless, it is of practical concern also that instructors be aware of the benefits and shortcomings of the textbooks used within their classrooms. Ideally, instructors would seek to reinforce, or to rectify, material in the text, for instance by explicitly conveying the 2D–3D relationship of molecules in their teaching of chemical representations. Although this could certainly be accomplished through the use of 3D models and computer animations during lectures, it is our experience that students are often unable to appropriately depict these lecture models in their notes. As a result, they are therefore unable to fully apply the knowledge gained from these experiences when studying or doing homework in situations where such models and animations are unavailable. We believe that classroom intervention studies examining students model use and providing students with similar opportunities to build and work with models (not just observe lecture models) would be of value in addressing this need.
To address the broader depiction of science visualizations in textbooks across a wide array of scientific disciplines, we have also begun preliminary studies on the effects of illustrations used in Biology textbooks. Emerging results suggest that many similarities exist between Organic Chemistry and Biology books. For instance, our findings regarding the unidirectional illustration of Newman and Fischer projections aligns well to our observation that the transcription of RNA is most-often depicted in a left-to-right direction in Biology texts, though it could easily be rotated 180o and shown right-to-left. As we have stated previously, depicting representations in this manner may obfuscate important knowledge about the scientific process, instead causing students to focus only on superficial elements of the phenomenon. In this regard, future analyses of textbook illustrations and accompanying text across a host of scientific disciplines are necessary.
Acknowledgements
The authors acknowledge Mike Stieff and Mary Hegarty for their advice and input in discussions of this project and for their comments on early drafts of this manuscript. This project was supported by NSF grant DRL-0723313.References
- Ainsworth S., (2006), DeFT: A conceptual framework for considering learning with multiple representations. Learning & Instruction, 16(3), 183–198.
- Allendoerfer R. D., (1990), Teaching the shapes of the hydrogenlike and hybrid atomic orbitals. Journal of Chemical Education, 67(1), 37.
- Appling J. R. and Peake L. C., (2004), Instructional technology and molecular visualization. Journal of Science Education and Technology, 13(3), 361–365.
- Bodner G. and Domin D., (2000), Mental models: The role of representations in problem solving in chemistry. University Chemistry Education, 4(1), 24–30.
- Bonicamp J. M. and Clark R. W., (2007), Textbook error: Short circuiting an electrochemical cell. Journal of Chemical Education, 84(4), 731.
- Boukhechem, M.-S., Dumon, A. and Zouikri, M. (2011). The acquisition of stereochemical knowledge by Algerian students intending to teach physical sciences. Chemistry Education Research and Practice, 12(3), 331–343.
- Bruice P., (2010), Organic Chemistry (6th ed.): Prentice Hall.
- Brünken R., Plass J. and Leutner D., (2003). How instruction guides attention in multimedia learning. In H. Niegemann, R. Brünken and D. Leutner (ed.), Instructional design for multimedia learning (pp. 113–126). Münster: Waxmann.
- Bucat B. and Mocerino M., (2009), Learning at the sub-micro level: Structural representations. In J. K. Gilbert and D. Treagust (ed.), Multiple Representations in Chemical Education (Vol. 4, pp. 11–29). Netherlands: Springer.
- Carey F. and Giuliano R., (2011), Organic Chemistry (8th ed.). New York, NY: McGraw-Hill.
- Carter C. S., Larussa M. A. and Bodner G. M., (1987), A study of two measures of spatial ability as predictors of success in different levels of general chemistry. Journal of Research in Science Teaching, 24(7), 645–657.
- Chandler P. and Sweller J., (1992), The split-attention effect as a factor in the design of instruction. British Journal of Educational Psychology, 62(2), 233–246.
- Cheng M. and Gilbert J. K., (2009), Towards a better utilization of diagrams in research into the use of representative levels in chemical education. In J. K. Gilbert and D. Treagust (ed.), Multiple Representations in Chemical Education (Vol. 4, pp. 55–73). Netherlands: Springer.
- Cook M. P., (2006), Visual representations in science education: The influence of prior knowledge and cognitive load theory on instructional design principles. Science Education, 90(6), 1073–1091.
- Copolo C. F. and Hounshell P. B., (1995), Using three-dimensional models to teach molecular structures in high school chemistry. Journal of Science Education and Technology, 4(4), 295–305.
- Ferk V., Vrtacnik M., Blejec A. and Gril A., (2003), Students' understanding of molecular structure representations. International Journal of Science Education, 25(10), 1227–1245.
- Garnett P., Garnett P. and Hackling M., (1995), Students' alternative conceptions in chemistry: A review of research and implications for teaching and learning. Studies in Science Education, 25(1), 69–96.
- Gilbert J. K., (2005), Visualization: A metacognitive skill in science and science education. In J. K. Gilbert (Ed.), Visualization in Science Education (Vol. 1, pp. 9–27). Dordrecht, The Netherlands: Springer.
- Hawkes S. J., (1996), Salts are mostly NOT ionized. Journal of Chemical Education, 73(5), 421.
- Jones L. L., Jordan K. D. and Stillings N. A., (2005), Molecular visualization in chemistry education: The role of multidisciplinary collaboration. Chemistry Education Research and Practice, 6(3), 136–149.
- Kabapinar F., (2009), Multi-frame illustrations: A molecular visual strategy in learning and teaching chemistry concepts. Australian Journal of Education in Chemistry, 69, 11–16.
- Kalyuga S., Ayres P., Chandler P. and Sweller J., (2003), The expertise reversal effect. Educational Psychologist, 38(1), 23–31.
- King C. J. H., (2009), An analysis of misconceptions in science textbooks: Earth science in England and Wales. International Journal of Science Education, 32(5), 565–601.
- Klein D., (2006), Organic Chemistry II as a Second Language: Second Semester Topics (2nd ed.): John Wiley & Sons, Inc.
- Klein D., (2008), Organic Chemistry I as a Second Language: Translating the Basic Concepts (2nd ed.): John Wiley & Sons, Inc.
- Klein D., (2012), Organic Chemistry (1st ed.): John Wiley & Sons, Inc.
- Kozma R., Chin E., Russell J. and Marx N., (2000), The roles of representations and tools in the chemistry laboratory and their implications for chemistry learning. Journal of the Learning Sciences, 9(2), 105–143.
- Kozma R. and Russell J., (1997), Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena. Journal of Research in Science Teaching, 34(9), 949–968.
- Kumi B. and Dixon B., (2012), Models in the classroom – help or hindrance? A look at the variables that influence student success on representational translation tasks in the chemistry classroom. Paper presented at the American Education Research Association Annual Meeting, Vancouver, British Columbia, Canada.
- Kumi B. and Dixon B., (in preparation), Student misconceptions of Organic Chemistry diagrams.
- Kuo M.-T., Jones L., Pulos S. and Hyslop R., (2004), The relationship of molecular representations, complexity, and orientation to the difficulty of stereochemistry problems. The Chemical Educator, 9, 321–327.
- Levie W. and Lentz R., (1982), Effects of text illustrations: A review of research. Educational Technology Research and Development, 30(4), 195–232.
- Loudon M., (2008), Organic Chemistry (5th ed.). Greenwood Village, CO: Roberts and Company.
- Mayer R. E., Steinhoff K., Bower G. and Mars R., (1995), A generative theory of textbook design: Using annotated illustrations to foster meaningful learning of science text. Educational Technology Research and Development, 43(1), 31–41.
- McMurry J., (2007), Organic Chemistry (7th ed.). Belmont, CA: Brooks-Cole.
- Newman M., (1955), A notation for the study of certain stereochemical problems. Journal of Chemical Education, 32(7), 344.
- Olimpo J. T. and Dixon B. L., (2012), Exploring the role of pre-constructed concrete models versus authentic model building in promoting organic chemistry students' representational competence. Presented at the 2012 Biennial Conference on Chemistry Education.
- Paivio A., (1986), Mental representations: A dual coding approach. Oxford, UK: Oxford University Press.
- Pribyl J. R. and Bodner G., (1987), Spatial ability and its role in organic chemistry: A study of four organic courses. Journal of Research in Science Teaching, 24(3), 229–240.
- Sanger M. J. and Greenbowe T. J., (1997), Students' misconceptions in electrochemistry regarding current flow in electrolyte solutions and the salt bridge. Journal of Chemical Education, 74(7), 819.
- Seufert T., (2003), Supporting coherence formation in learning multiple representations. Learning and Instruction, 13(2), 227–237.
- Smith B. and Jacobs D., (2003), TextRev: A window into how general and organic chemistry students use textbook resources. Journal of Chemical Education, 80(1), 99–102.
- Stieff M., (2007), Mental rotation and diagrammatic reasoning in science. Learning & Instruction, 17(2), 219–234.
- Stieff M., (2011), When is a molecule three dimensional? A task-specific role for imagistic reasoning in advanced chemistry. Science Education, 95(2), 310–336.
- Stieff M., Bateman R. and Uttal D., (2005), Teaching and learning with three-dimensional representations. In J. Gilbert (Ed.), Visualization in Science Education (Vol. 1, pp. 93–120). Dordrecht, The Netherlands: Springer.
- Stieff M., Hegarty M. and Dixon B., (2010), Alternative strategies for spatial reasoning with diagrams. In A. Goel, M. Jamnik and N. Narayanan (ed.), Diagrammatic Representation and Inference (Vol. 6170, pp. 115–127): Springer Berlin/Heidelberg.
- Stieff M., Hegarty M. and Deslongchamps G., (2011), Identifying representational Competence with multi-representational displays. Cognition and Instruction, 29(1), 123–145.
- Stroud M., (2010), Summoning prior knowledge through metaphorical graphics: An example in chemistry instruction. Journal of Educational Research, 103(5), 351–366.
- Stull A. T., Hegarty M., Dixon B. and Stieff M., (in press), Representational translation with concrete models: It's not what you have, it's what you do.
- Sweller J., van Merrienboer J. and Paas F., (1998), Cognitive architecture and instructional design. Educational Psychology Review, 10(3), 251–296.
- Tuckey H., Selvaratnam J. and Bradley J., (1991), Identification and rectification of student difficulties concerning three-dimensional structures, rotation and reflection. Journal of Chemical Education, 68, 460–464.
- Wade L. G. Jr., (2010), Organic Chemistry (7th ed.). Upper Saddle River, NJ: Pearson Prentice Hall.
- Waner M. J., (2010), Particulate pictures and kinetic-molecular theory concepts: Seizing an opportunity. Journal of Chemical Education, 87(9), 924–927.
- Wu H.-K., Krajcik J. S. and Soloway E., (2001), Promoting understanding of chemical representations: Students' use of a visualization tool in the classroom. Journal of Research in Science Teaching, 38(7), 821–842.
- Wu H.-K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning. Science Education, 88(3), 465–492.
- Yip J. C., Jaber L. Z. and Stieff M., (2011), Examining changes in students' coordination of verbal and pictorial chemical representations in response to instruction. Paper presented at the American Education Research Association Annual Meeting, New Orleans, LA.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c3rp20166j |
| This journal is © The Royal Society of Chemistry 2013 |
