Assessing prospective chemistry teachers' understanding of gases through qualitative and quantitative analyses of their concept maps
Zeynep Bak Kibar*a,
Fatma Yaman*b and
Alipaşa Ayasc
aDepartment of Chemistry Education, Fatih Faculty of Education, Karadeniz Technical University, Trabzon, Turkey. E-mail: zeynepbak@gmail.com
bDepartment of Science Education, Bozok University Faculty of Education, Yozgat, Turkey. E-mail: fatma.yaman@bozok.edu.tr
cDepartment of Educational Sciences, Faculty of Education, Bilkent University, Ankara, Turkey. E-mail: alipasaayas@yahoo.com
First published on 28th August 2013
Abstract
The use of concept mapping as a tool to measure the meaningful learning of students is the focus of this study. The study was carried out with 24 last year students (22 years old) from the Department of Chemistry Teaching at Fatih Faculty of Education, Karadeniz Technical University (KTU). Prospective Chemistry Teachers (PCT) were asked to create concept maps using a list of given concepts related to gases. An examination of the PCTs' maps revealed that the students could not form hierarchical maps even after being shown examples of the basic elements and meaningful propositions between the concepts. After being provided with feedback about their concept maps and trained to form non-hierarchical concept maps, the students were asked to create new maps. This time they were allowed to use either hierarchical or non-hierarchical maps. When their new maps were examined, we found that most of the PCTs formed non-hierarchical maps. However, they still could not form meaningful relationships between the given concepts. We also found that the PCTs had some misconceptions about gases and kinetic molecular theory that explains gas behavior. The study ended up with some suggestions and implications for educators and researchers related to pre-service teachers' training.
Introduction
The concepts related to gases are the examples of chemistry concept that have attracted the attention of science education researchers (Çetin et al., 2009; Schuttlefield et al., 2012). Previous studies have mainly focused on students' understanding of the properties and structure of gases (Sawrey, 1990; Nakhleh, 1993). In addition, some studies have investigated the students' algorithmic or conceptual understanding of gases (Nurrenbern and Pickering, 1987; Pickering, 1990). The studies related to gases are quite problematic because of the abstract, mostly invisible nature of gases which raises the level of difficulty for student learning (Stavy, 1988). Likewise, students may have difficulty with observing gases at the macro level and explaining these observations at the molecular level (Nakiboğlu and Özkılıç-Arık, 2008).An example of research examining student misconceptions in the area of the ideal gas law is one conducted by Loverude et al. (2002). This study found that most students who took courses of first year university algebra-based introductory physics and second year thermal physics cited the ideal gas law and argued that changes in pressure and/or volume resulting from compression would lead to an increase in the gas temperature. Although they had studied the first law of thermodynamics and adiabatic compression of ideal gases, most students relied on a misinterpretation of the ideal gas law to explain this process. Some students focused on only two macroscopic gas variables at a time and assumed an invalid relationship between them. In addition, some students' arguments involved macroscopic quantities that were in fact tightly linked to microscopic ideas. They established an inappropriate connection between volume and temperature, apparently assuming a relationship between the density of gas particles and their average speed or energy, as measured by the temperature. Some students made convoluted explanatory statements to this effect, such as “the smaller volume forces the molecules of gas to increase in speed, therefore increasing the temperature,” or “the molecules of gas are closer together, making the energy higher”.
Kautz et al. (2008) also investigated student understanding of the ideal gas law. It was suggested that after instruction many students were not able to correctly interpret and apply the ideal gas law. In particular, difficulties with pressure, volume, and Avogadro's law were prevalent. In earlier studies, Kautz et al. (2005a, 2005b) investigated students' understanding of the ideal gas from a macroscopic and microscopic perspective. They studied more than 1000 students and interviewed 45 students individually to determine their macroscopic perspective about pressure, volume, and temperature and the relationship of these values through the ideal gas law. It was concluded that neither chemistry nor physics' students could explain the macroscopic variables of pressure, temperature, and volume in terms of mathematical expression of the ideal gas law. Kautz et al. (2005b) argued that the students' difficulties with the macroscopic level were linked to misinterpretations of microscopic processes.
One of the common findings of these studies is that students and even teachers have an inadequate understanding of gases. Since it is difficult for students to understand the abstract nature of gases, the importance of the particulate nature of matter, and the lack of students' perceptions of gases as connected to daily life; student conceptions of gases can be an interesting and important field of research. It is well known that one of the sources of students' inadequate understanding is the teachers' own lack understanding or alternative conceptions (Westbrook and Marek, 1992). Therefore, it is important to assess prospective chemistry teachers' understanding about gases because they will teach this topic in the future.
Assessment is one of the most important processes of educational settings, and the assessment of students' ideas and conceptual understanding is central to any curriculum and/or teaching–learning environment (Ruiz-Primo et al., 2001; Kaya, 2003b, 2008; Şen and Özgün-Koca, 2003). Students' ideas, concepts or misconceptions are difficult to determine because they are constructed as a schema in students' minds and are therefore not directly observable. The literature contains many methods used to determine and assess students' conceptions. These methods include interviews about instances and events, interviews about concepts (Renström et al., 1990; Abdullah and Scaife, 1997; Eshach and Garik, 2001), drawings (Longden et al., 1991; Smith and Metz, 1996), predict–observe–explain (POE) activities (Kearney, 2004; Liew, 2004; Coştu et al., 2012), and concept mapping (Novak and Gowin, 1984; Mason, 1992; White and Gunstone, 1992; Pendly et al., 1994; Nicoll et al., 2001; Francisco et al., 2002; Smith, 2003; Cassata et al., 2004; Striebel, 2005). Although many methods have been used in science education, traditional tests are usually performed as the assessment tool in university science courses for informing the students related to their grade (Kaya, 2008). Therefore, it is important to use authentic assessment methods in university courses. For this study, we decided to use concept maps because they are one of the key strategies being used to encourage meaningful learning and to assess students' understanding (Novak and Gowin, 1984; White and Gunstone, 1992; Kaya, 2003a, 2008; Bak and Ayas, 2008). Thoughtful analysis of concept maps can reveal meaningful knowledge (Şen and Özgün-Koca, 2003; Kaya, 2003a), but there are few published studies illustrating how concept maps can be used as measurement and assessment strategies.
Research has shown that concept maps are useful assessment methods for eliciting and enhancing individuals' conceptual understanding (Novak and Gowin, 1984; White and Gunstone, 1992; Markham et al., 1994; Kinchin et al., 2000; Kaya, 2008; Özmen et al., 2009). Kaya (2008) argued that concept maps of prospective science teachers done before and after a series of chemistry laboratory courses are valid and reliable for explaining their conceptual understanding. Özmen et al., (2009) investigated high school students' conceptual understanding using concept mapping in conjunction with laboratory activities. The results of this study indicated that using concept mapping helped students to link the concepts and reduced their alternative concepts related to acid–base chemistry. Besides, research has shown that evaluating concept maps can be done quantitatively and qualitatively (White and Gunstone, 1992; Ruiz-Primo and Shavelson, 1996; Kinchin et al., 2000; Van Zele et al., 2004). Quantitative analysis of concept maps is based on the scoring protocol devised by Novak and Gowin (1984), and subsequent quantitative analysis is based on minor modifications of the scoring component such as number of concepts, cross-links, hierarchy and so on (Kinchin et al., 2000; Kaya, 2008). On the other hand, qualitative analysis of concept maps generally relies on the structural framework referred to as “chain”, “spoke” and “net” concept maps (Kinchin et al., 2000). In this study, we focused on concept mapping as an assessment tool to assess Prospective Chemistry Teachers' (PCTs) conceptual understanding of gases. When the related literature is investigated it can be seen that quantitative and qualitative analyses of concept mapping have been done separately. Therefore, there is a need for combining quantitative and qualitative assessment of concept maps for testing conceptual understanding. Furthermore, connection level analysis has been added to concept map analysis in this study, which has not been reported in the literature before.
Purpose
This study was designed to assess PCTs’ understanding of gas concepts through the use of concept maps. In this study, PCTs were asked to use the term gas at the top or center of the page and 19 terms were provided that they could relate to the concept of gases. PCTs could use any of these words, all of these words, or add other related terms to their maps. These given terms were gas, pressure, volume, temperature, mole number, gas pressure, effusion, diffusion, kinetic theory, real gas, ideal gas, manometer, open-ended manometer, closed-ended manometer, barometer, P–V law (Boyle’s law), T–V law (Charles’ law), T–P law (Gay Lussac’s law), Avogadro’s law. The researchers chose these terms because they were headings in textbooks and because chemistry courses and university level curriculums typically focused on these concepts in relation to gases.Methodology
A case study research methodology was chosen for this study to conduct a detailed investigation of the participants using a mixture of qualitative and quantitative techniques (Patton, 2002; Merriam, 2009). One of the key characteristics of the case study is that it provides a better understanding of the case studied in detail. It is often used once we want to probe the things happening in some complex situation or to have explicit information about something (Taber, 2007). We chose this method since it enabled the detailed examination of a single subject, and in depth study with a small number of participants. In this context, the case of this study is the conceptual understanding of gases by one cohort of PCTs. We selected this case because these PCTs were enrolled in our institution and the information that will emerge from this study will provide an insight and feedback related to our teaching to these PCTs. Besides, this case looks at a typical example which will provide us with an insight that will be considered as worth testing out in other similar cases (Taber, 2007).Description of participants
The participants were 24 (11 females and 13 males) PCTs who were in their final year in the Department of Chemistry Teaching at Karadeniz Technical University, in Turkey. These PCTs had undertaken five years of study. Whilst the PCTs took chemistry courses for the first three and half years, later they studied pedagogical knowledge for one and half years. Each participant was familiar with concept maps through their Chemistry Teaching Methods I–II courses which were taught in the PCTs' last one and half year program. Both courses content includes learning theories and approaches to learning, teaching and learning processes, practices of teaching methods in the subject area, an examination of textbooks and their relation to subject teaching methods and strategies, micro teaching practices, and assessment of teaching (Higher Education Council [HEC], 1998). In the context of the chemistry education program, these pre-service teachers have taken certain general and advanced chemistry courses, such as General Chemistry I, General Chemistry II, Analytical Chemistry, Physical Chemistry, Organic Chemistry, and Inorganic Chemistry. Some of these courses, especially, General Chemistry studied in their first year and Physical Chemistry studied in their third year, include the subject of gases. Consequently, these PCTs should be familiar with gases from their previous chemistry courses and also from their high school chemistry courses.Data collection
Data sources included concept maps drawn by 24 PCTs. The PCTs were asked to construct their concept maps using a list of given terms (19 terms provided) related to the concept of gases. In the literature, this type of concept map is referred to as a guided concept map (Şen and Özgün-Koca, 2003; Özgün-Koca and Şen, 2004). Concept maps can be formed in different ways such as using a list of terms provided by the teacher, completing a skeleton map, completing a partially formed map, using terms from a textbook or document, after a discussion in a group of two or three, or using self conceptions not based on any resources (Ruiz-Primo and Shavelson, 1996; Kaya, 2003b; Özgün-Koca and Şen, 2004). We used the provided list of terms for this study but also informed PCTs that they could add additional terms or elect to not use some of the ones provided. This enabled us to better identify how PCTs constructed their maps and the connections they made between concepts.Although the participants were familiar with concept map construction from the “Chemistry Teaching Methods Courses”, one session in a class period which is a two hour lesson was used to summarize the key components of concept mapping such as concepts, propositions, hierarchy, cross-linking and examples and how to construct a hierarchical concept map prior to the application. The PCTs were then asked to create their own concept maps using the provided list of terms. The PCTs were told that they were free to use additional terms related to gases that were not on the list and that they were not required to use all of the given terms. The PCTs had difficulty in forming hierarchical maps and meaningful propositions between concepts. Therefore, in another session of a class period (which was also a two hour lesson), the PCTs were trained to create non-hierarchical concept maps and then they were asked to form new maps. This time they were allowed to use either hierarchical or non-hierarchical maps. At the end of these two sessions (which were four hours of lessons), the PCTs constructed their final concept maps, and the PCTs' final concept maps were taken into consideration for analysis in the study. In other words, the data were taken from the PCTs' final concept maps.
Analysis of data
Each PCT's concept map was analyzed qualitatively and quantitatively (White and Gunstone, 1992; Ruiz-Primo and Shavelson, 1996; Kinchin et al., 2000; Van Zele et al., 2004). Concept maps can be assessed in as many different ways as they can be formed. The fundamental criteria used to evaluate concept maps are dependent on the aim of the assessment. Quantitative analysis is suitable if you want to reveal the development of the students' learning processes and to see individual differences in these processes. However, qualitative analysis of concept maps is more appropriate if your aim is to reveal students' preconceptions, to identify difficulties in students' understanding, or to investigate the students' ideas related to a specific field (Şen and Özgün-Koca, 2003).Science educators typically use three different methods to evaluate concept maps (Kaya, 2003b). The first method utilizes four criteria based on evaluation of the content; propositions, hierarchy, cross-linking, and examples (Novak and Gowin, 1984; White and Gunstone, 1992). According to this method propositions refer to consistent and meaningful relationships between two concepts that are signified by connecting words and lines and every relationship is assigned 1 point. Hierarchy is determined by whether the concepts are placed from the general to specific and concepts of the same scope are placed at the same level. Every hierarchical level is assigned 5 points. Cross linking focuses on whether the connections between concepts in different parts of the map are both valid and important with appropriate cross linking assigned 10 points. Finally, every valid example of a concept is awarded 1 point (Novak and Gowin, 1984). The second evaluation method commonly used includes a comparison of the students' maps to teacher's or an expert's concept map (Kaya, 2003b). The points awarded for every component (proposition, hierarchy and so on) of the criterion map can be compared to those of the students. Percent values can be calculated to provide an evaluation of the students' conceptual understanding in terms of the components compared (Kaya, 2003a). The last method of evaluation is the combination of the two previous two approaches. Initially, the criterion concept map and students' maps are assessed according to four components as described in the first approach. Then, the total points for the students' concept map are divided by the total points for the criterion concept map and reported as a percent value (Kaya, 2003b). We used a third assessment approach combining the other two in this study.
In the qualitative analysis, the PCTs' concept maps were analyzed by considering the connection levels of the concepts with the key term gas and other concepts (see, Table 1). For this, the maps were examined one by one and the concepts were categorized by looking at the connecting statements written on the arrows. The connection levels detailed in Table 1 were used to classify the connection levels of the concept maps. If a PCT connected one concept (e.g. volume, temperature, mole number…) directly to the key term gas, this was considered to be a first level connection. If a more specific concept was connected to a more general concept then it was labelled as a second level, this concept was considered to be part of the third hierarchical level. These levels show the connection proceeding from general concepts to specific concepts. This allowed us to use a connection level to study how the PCTs linked the concepts at the same importance level to the key concept of gas. Besides, this allowed us to determine which concepts were most important in a PCT's mind in relation to the gas key concept. The following figure shows an example of a concept map of the PCT numbered 2 in Table 2.
| Concepts | Connection level | Number of PCTs on related concepts | Related concept | |||||
|---|---|---|---|---|---|---|---|---|
| Factors affecting the gas pressure | Volume | 1 level | 8(42%) | Gas | ||||
| 2 level | 8(42%) | Gas pressure (4 = 21%) | Gas laws(1) | Ideal gas(2) | Temperature (1) | |||
| 3 level | 2(11%) | P–V law, T–V laws(1) | Measure results(1) | |||||
| 4 level | 1(5%) | Gas pressure(1) | ||||||
| Temperature | 1 level | 8(38%) | Gas | |||||
| 2 level | 10(47%) | Gas laws(1) | Gas pressure (7 = 33%) | Ideal gas(2) | ||||
| 3 level | 2(10%) | T–V law, T–P law(1) | Measure results(1) | |||||
| 4 level | 1(5) | Gas pressure(1) | ||||||
| Mole number | 1 level | 7(35%) | Gas | |||||
| 2 level | 9(45%) | Ideal gas(1) | Gas pressure(7) | Gas laws(1) | ||||
| 3 level | 3(15%) | P–V law, T–V law(1) | Avogadro law(1) | Measure results(1) | ||||
| 4 level | 1(5) | Avogadro law(1) | ||||||
| Type of gases | Real gas | 1 level | 24(100%) | Gas | ||||
| Ideal gas | 1 level | 23(96%) | Gas | |||||
| 2 level | 1(4) | Real gas | ||||||
| Gas laws | Avogadro’s law | 1 level | 3(13%) | Gas | ||||
| 2 level | 16(70%) | Ideal gas(10) | Gas laws(2) | Mole number(1) | Real gas(2) | Gas pressure(1) | ||
| 3 level | 4(17%) | Kinetic theory(1) | Volume, mole number(1) | Gas laws(1) | Diffusion(1) | |||
| P–V law | 1 level | 3(13%) | Gas | |||||
| 2 level | 17(71%) | Ideal gas(12) | Real gas(1) | Pressure, volume(2) | Gas laws(2) | |||
| 3 level | 4(16%) | Diffusion(1) | Gas laws(1) | Gas pressure, temperature(1) | Kinetic theory(1) | |||
| T–V law | 1 level | 3(13%) | Gas | |||||
| 2 level | 17(71%) | Ideal gas(12) | Real gas(1) | Gas laws(2) | Temperature, volume(2) | |||
| 3 level | 4(16%) | Volume, temperature(1) | Kinetic theory(1) | Gas laws(1) | Diffusion(1) | |||
| T–P law | 1 level | 1(5%) | Gas | |||||
| 2 level | 14(74%) | Ideal gas(10) | Real gas(1) | Pressure, temperature(2) | Gas laws(1) | |||
| 3 level | 4(21%) | Diffusion(1) | Gas laws(1) | Kinetic theory(1) | Gas pressure(1) | |||
| Partial pressure law | 1 level | 2(25%) | Gas | |||||
| 2 level | 5(62%) | Mole number, pressure(1) | Ideal gas(3) | Real gas(1) | ||||
| 3 level | 1(13%) | Diffusion | ||||||
| Diffusion of gases | Diffusion | 1 level | 14(74%) | Gas | ||||
| 2 level | 4(21%) | Kinetic theory(3) | Temperature(1) | |||||
| 3 level | 1(5%) | Kinetic theory | ||||||
| Effusion | 1 level | 13(72%) | Gas | |||||
| 2 level | 4(22%) | Kinetic theory(3) | Temperature(1) | |||||
| 3 level | 1(6%) | Kinetic theory | ||||||
| Kinetic theory | 1 level | 7(33%) | Gas | |||||
| 2 level | 14(67%) | Real gas(6) | Effusion, real gas, diffusion, ideal gas(1) | Temperature(1) | Ideal gas(6) | |||
| Measurement of gas pressure | Barometer | 1 level | 0 | |||||
| 2 level | 17(77%) | Gas pressure | ||||||
| 3 level | 4(18%) | Atmospheric pressure(2) | Gas pressure(2) | |||||
| 4 level | 1(5) | Gas pressure(1) | ||||||
| Manometer | 1 level | 0 | ||||||
| 2 level | 18(78%) | Gas pressure | ||||||
| 3 level | 4(18%) | Gas pressure(2) | Closed gas pressure(1) | Gas pressure in closed container(1) | ||||
| 4 level | 1(4%) | Gas pressure(1) | ||||||
| Open-ended manometer | 1 level | 0 | ||||||
| 2 level | 1(4%) | Gas pressure | ||||||
| 3 level | 18(75%) | Manometer | ||||||
| 4 level | 4(17%) | Manometer | ||||||
| 5 level | 1(4%) | Manometer | ||||||
| Closed-ended manometer | 1 level | 0 | ||||||
| 2 level | 2(10%) | Gas pressure | ||||||
| 3 level | 14(66%) | Manometer (13) | Barometer(1) | |||||
| 4 level | 4(19%) | Manometer | ||||||
| 5 level | 1(5%) | Manometer | ||||||
| R | VCN | P | TPN | P | Con.LN | P | Cross. Con. N | P | EN | P | IRV | PCTTP | (PCTTP/RTP) × 100b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCT | 19 | 19 | 23 | 23 | 6 | 30 | 7 | 70 | 3 | 3 | 368.42 | 145 | |
| a R, researcher; PCT, prospective chemistry teacher; VCN, valid concept number; P, point; TPN, true proposition number; Con.LN, connection level number; CCN, cross connection number; EN, example number; IRV, internal relation value; PCTTP , PCT total point.b (PCTTP/RTP) × 100 (PCTTP: PCT total point, RTP: researcher total point). | |||||||||||||
| 1 | 18 | 18 | 18 | 18 | 3 | 15 | 51 | 35.2 | |||||
| 2 | 20′′ | 20 | 3 | 3 | 3 | 15 | 1* | 2 | 10 | 40 | 27.6 | ||
| 3 | 19′′ | 19 | 8 | 8 | 3 | 15 | 2* | 4 | 21 | 46 | 31.7 | ||
| 4 | 19′′ | 19 | 8 | 8 | 3 | 15 | 42 | 28.9 | |||||
| 5 | 17′′ | 17 | 11 | 11 | 3 | 15 | 43 | 29.6 | |||||
| 6 | 20′′ | 20 | 10 | 10 | 3 | 15 | 45 | 31.03 | |||||
| 7 | 19′′ | 19 | 11 | 11 | 3 | 15 | 45 | 31.03 | |||||
| 8 | 16′′ | 16 | 10 | 10 | 2 | 10 | 36 | 24.8 | |||||
| 9 | 16′′ | 16 | 8 | 8 | 3 | 15 | 39 | 26.9 | |||||
| 10 | 17′′ | 17 | 13 | 13 | 5 | 25 | 1 + 4* | 18 | 105.88 | 73 | 50.34 | ||
| 11 | 19′′ | 19 | 7 | 7 | 3 | 15 | 41 | 28.3 | |||||
| 12 | 20′′ | 20 | 15 | 15 | 4 | 20 | 55 | 37.9 | |||||
| 13 | 18 | 18 | 7 | 7 | 3 | 15 | 40 | 27.6 | |||||
| 14 | 21′′ | 21 | 18 | 18 | 3 | 15 | 4* | 8 | 38.1 | 62 | 42.7 | ||
| 15 | 19′′ | 19 | 13 | 13 | 4 | 20 | 5* | 10 | 52.6 | 62 | 42.7 | ||
| 16 | 17′′ | 17 | 16 | 16 | 3 | 15 | 4* | 8 | 47.06 | 56 | 38.6 | ||
| 17 | 15′′ | 15 | 8 | 8 | 3 | 15 | 1* | 2 | 13 | 40 | 27.6 | ||
| 18 | 10′′ | 10 | 9 | 9 | 5 | 25 | 3* | 6 | 11 | 11 | 60 | 61 | 42.06 |
| 19 | 16′′ | 16 | 10 | 10 | 3 | 15 | 3* | 6 | 37.5 | 47 | 32.4 | ||
| 20 | 15′′ | 15 | 6 | 6 | 3 | 15 | 2* | 4 | 26.7 | 40 | 27.6 | ||
| 21 | 19′′ | 19 | 11 | 11 | 3 | 15 | 45 | 31.03 | |||||
| 22 | 16′′ | 16 | 14 | 14 | 3 | 15 | 45 | 31.03 | |||||
| 23 | 21′′ | 21 | 12 | 12 | 4 | 20 | 53 | 36.5 | |||||
| 24 | 17′′ | 17 | 15 | 15 | 3 | 15 | 47 | 32.4 | |||||
On the concept map, 1.L shows the first level connection, 2.L shows the second level connection, 3.L shows the third level connection, and CCN shows the cross-connection number. As seen on the concept map, the PCT correlated the concepts of real gas, diffusion, effusion, gas laws, ideal gas and gas pressure to the key concept of gas directly. Therefore, these concepts are considered first level connections since they are directly connected to the gas key term. This means that these concepts were more specific than the general concept of gas for this PCT. The concepts of manometer, barometer, kinetic theory, mol number, pressure–volume, temperature–volume, Avogadro’s law, temperature, volume, and pressure are considered to be second level connections since they are connected to the concepts designated as first level connections. Moreover, the terms open-ended manometer and closed-ended manometer are considered to be third level connections since they are connected to the concepts designated as second level connections.
The other qualitative analysis of PCTs' concept maps is shown in Table 3. We analyzed PCTs' concept maps whether or not they established correct relationships and wrote meaningful statements on the arrow. For instance, when we look at the example concept map in Fig. 1, it can be seen that the PCT “thought that kinetic theory explained behavior of real gas”, “Real gas and ideal gas concept were correlated with gas concept and gas and gas pressure was correlated but connection expressions were not written”, “gas pressure connected with barometer and manometer but direction of arrow was drawn inversely. Also, pressure and liquid pressure were added by this PCT to the concept map”, and “gas laws concept was connected with pressure, temperature and mole number but meaningful relationship could not established”.
| Analysis of their concept maps |
|---|
| – The concept of effusion and diffusion correlated with kinetic theory but connection propositions are not meaningful (4). Likewise, one PCT has misconceptions about effusion that the effusion is collecting of gases (1)/kinetic theory is measured with diffusion, kinetic theory is measured with a velocity of effusion (1). |
| – Ideal gas and kinetic theory connected with each other but connection was not meaningful. (PCT has misconception like ideal gas forms kinetic theory) (2). |
| – The real gas concept was related to kinetic theory but in this relationship connection expression includes misconception that real gas is explained with kinetic theory (6). |
| – Ideal and real gases were related to gas concept directly (5), but connection expression was not written (4). And also, ideal and real gas were connected but the relationship was not meaningful (2). |
| – Gas key concept related to ideal and real gas separately and were considered as a kind of gas (9). |
| – Kinetic theory connected with gas but the direction of the arrow was drawn inversely (2). |
| – Gas and kinetic theory connected with each other but connection expression was not written (1). |
| – Effusion and diffusion concepts correlated with gas concept but connection expression is not meaningful (3). |
| – Effusion was associated with gas (3) but connection expression has the misconception that gases collect with effusion (4). |
| – Propositions were not meaningful among given gas laws and P–V, T–V, T–P and Avogadro’s law (2). |
| – Given gas laws (P–V, T–V, Avogadro’s law, partial pressure law) connected with the gas key concept separately but connection expression was not meaningful (2). |
| – Manometer was related to open-ended and closed ended manometer concepts, but connection expressions are not meaningful (1). |
| – Added a concept on the map such as measurement results and correlated barometer, open-ended and closed-ended manometer concepts with this concept (1). |
| – Established a relationship between barometer and closed-ended manometer, but connection expression includes misconception such as “barometer is closed-ended nanometer” (1). |
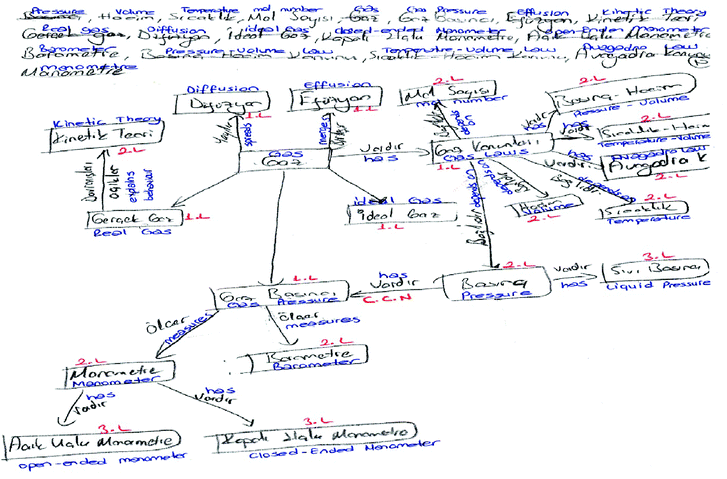 | ||
| Fig. 1 2 numbered PCT's concept map. | ||
In the quantitative analysis as summarized in Table 2, the researchers' concept maps were compared with the PCTs' concept maps. The researchers' concept maps were considered to be the criterion maps which were constructed by the authors of this article after the negotiations were done. The criterion maps and PCTs' concept maps were given points according to Novak and Gowin's (1984) scoring system. However, there are certain changes we made when we analyzed the PCTs’ concept maps and criterion concept map. Whilst the PCTs' concept maps and criterion maps were analyzed according to four components of concepts, cross-links, hierarchies, and examples as described in Novak and Gowin (1984), we analyzed three components except hierarchies as detailed in the literature. We used connection levels instead of hierarchy since all the PCTs did not construct hierarchical concept maps. On the other hand, every connection level was awarded 5 points. Also, the PCTs' concept maps were examined by considering the number of valid concepts (1 point each), the number of true propositions (1 point each), the number of valid cross-linking (10 points each), and the number of examples (1 point each). Also, the “internal relation value (IRV)” was calculated and shown in Table 2. This IRV express the ratio of the “cross-links” to “concept scores” or “cross-links/concepts × 100” in percentage. The numerical values obtained from the IRV show differences between rote and meaningful learning. The IRV is calculated using the following equation for this study. IRV = [(significant cross-links × 10 points)/(valid concepts × 1 point) × 100]. If the cross-links established by PCTs is significant but the proposition is not valid, then the IRV is calculated as [(cross-links × 2 points)/(valid concepts × 1 point) × 100].
The other part of the quantitative analysis of PCTs' concept maps is also shown in Table 2. Ruiz-Primo and Shavelson (1996) mentioned that the teacher's criterion concept map could be compared separately with the PCTs' maps in terms of proposition, hierarchy, cross-linking and so on. However, we used connection level number instead of hierarchy. We scored the PCTs' and the researcher' concept maps, and calculated the PCT's total points (PCTTP) and divided by the researcher total point (RTP) to determine meaningful learning and the analysis of relationship between concepts. The PCTs' rates of (PCTTP/RTP) × 100 are given in Table 2 and indicate the PCTs' level of understanding. Kaya (2003b) indicated that these percent values could indicate the students' conceptual understanding from the point of being compared to the elements. For example; scores of 90–100% indicate a sound understanding, 65–89% a considerable understanding, 33–64% partial understanding, 1–32% low understanding, and a score of 0% indicates no understanding (Kaya, 2003b). We chose this scale because we wanted to determine which understanding levels the PCTs had.
With the light of the quantitative analysis, the scores of the totals, IRV and the rate of (PCTTP/RTP) × 100, which are represented in Table 2 related to the PCT shown in Fig. 1, were calculated as follows:
The PCT that was numbered as 2 in Table 2, used 20 valid concepts, stated 3 true propositions, made 3 connection levels, connected 1 cross-link but the proposition on the arrow of this connection was not valid. Therefore, this PCT's cross-link was awarded 2 points. The total points for PCT were calculated as follows:
| PCT total point = (valid concept × 1) + (true proposition × 1) + (connection level × 5) + (significant cross-connection × 10) or (cross-connection × 2) + (example number × 1) |
| PCT total point = (20 × 1) + (3 × 1) + (3 × 5) + (1 × 2) + (0 × 1) = 40 |
| IRV = [(cross-links × 2 points)/(valid concepts × 1 point) × 100]. |
| IRV = [(1 × 2 points)/(20 × 1 point) × 100] = 10 |
| Researchers' total point (RTP) = (valid concept × 1) + (true proposition × 1) + (connection level × 5) + (significant cross-connection × 10) + (example number × 1) |
| RTP = (19 × 1) + (23 × 1) + (6 × 5) + (7 × 10) + (3 × 1) = 145 |
| The rate of (PCTTP/RTP) × 100 = (40/145) × 100 = 27.6 |
The followings represent some examples of PCTs. Fig. 2 shows the PCT's concept map numbered 23 in Table 2.
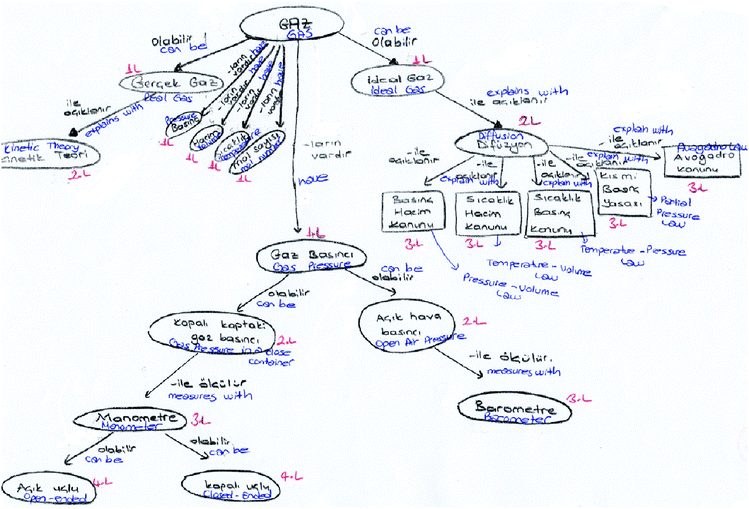 | ||
| Fig. 2 23 numbered PCT's concept map. | ||
Findings
Findings from analysis of PCTs' concept maps
Table 1 shows how the PCTs correlated given concepts with the key term gas or the other provided terms. The PCTs' concepts were separated into levels as indicated in the data analysis. These levels show the connection levels proceeding from general concepts to specific concepts. Concepts connected to a general concept coming off of the key concept are labeled as in Table 1. The term volume was correlated to gas in the first level by 8 PCTs; meaning that volume was more specific than the general concept of gas for 8 PCTs. The other 8 PCTs correlated the volume term in the second level. Four of 8 PCTs related the volume term to gas pressure, 1 of 8 PCTs to gas laws term, 2 of 8 to ideal gas term and 1of 8 to temperature term in the second level. And here, gas pressure was a secondary general concept for 4 PCTs, gas laws concept was a secondary general concept for 1 PCT, the ideal gas concept was a secondary general concept for 2 PCTs, and temperature concept was a secondary general concept for 1 PCT and so on. This showed us how PCTs established the connections between gas concepts from general to specific. Besides, it provided an initial understanding of the PCTs' thought regarding gases .As seen in Table 1 the PCTs' concept maps were analyzed using five categories factors affecting gas pressure, type of gases, gas laws, diffusion of gases, and measurement of gas pressure were formed by the researchers according to relationships between concepts in maps. The PCTs' concepts were mostly in two hierarchical levels. In the first category-factors affecting gas pressure, 8 of the 19 PCTs (42%) established the relationship with volume to gas key concept in the first hierarchical level. The PCTs considered the volume as a property of gases in the analysis of their maps. Eight PCTs connected the volume concept from second hierarchical level, and 4 (21%) of them correlated it with gas pressure from the second level. In this level the PCTs perceived the volume as one of the factors effecting gas pressure. Two PCTs (11%) connected volume concept with gas key concept from the third hierarchical level. One PCT (5%) connected the volume concept to pressure–volume law concept and temperature–volume law concept at the same time, more general concepts, according to him. And he related these two concepts to ideal gas concept, more general, and at the end he correlated the ideal gas concept to most general gas key concept. Besides, one PCT correlated volume concept to the gas pressure from fourth level.
Eight of 21 PCTs (38%) connected temperature concept with gas concept form the first level. In this connection most of the PCTs perceived the temperature as one of the properties of gases. Ten of 21 PCTs (48%) connected temperature from the second level, and 7 (33%) of them connected it with gas pressure. In this level, the temperature concept was perceived as one of the factors affecting gas pressure from their maps. Twenty of 24 PCTs (83%) used mole number concept in their maps. Seven of 20 PCTs (35%) connected the mole number to gas concept from the first level. Nine of 20 PCTs (45%) connected it from the second level, and seven of 9 PCTs (78%) correlated it to gas pressure from second level.
Looking at the category of type of gases in Table 1, while all PCTs correlated real gas concept with the gas key concept, that is, gas was the most general concept at the center of the paper and 23 of the 24 PCTs (96%) correlated ideal gas to gas key concept from the first hierarchical level. Only one PCT correlated ideal gas to real gas from second level. But they did not establish a relation word according to what they thought by correlating between ideal and real gases. They correlated both ideal and real gas to gas key concept without indicating propositions on the arrows.
Four concepts related to the gas laws (Avogadro’s law, pressure–volume law (Boyle’s law) concept, pressure–temperature law concept, volume–temperature law (Charles’ law)) were given to the PCTs. But 8 of the 24 PCTs (33%) added the law of partial pressure to their maps. Twenty-three of 24 PCTs (96%) used Avogadro’s law in their maps. Three of 23 PCTs (13%) correlated it to gas the key concept from the first hierarchical level. Sixteen of 23 PCTs (70%) correlated this concept from the second level and 10 of the 16 PCTs (63%) related it with ideal gas. In P–V law, while 3 of 24 PCTs (13%) connected this law to gas concept from the first level, 17 PCTs (71%) correlated it from the second level. Twelve of 17 PCTs (71%) correlated it to ideal gas. In T–V law, while 3 of the 24 PCTs (13%) correlated this law to gas concept from the first level, 17 PCTs (71%) connected it from the second level. Twelve of 17 PCTs (71%) correlated it with ideal gas. In the T–P category, 19 of the 24 PCTs (79%) used this concept in their maps. While 1 of 19 PCTs (5%) connected it with the gas key concept from the first level, 14 PCTs (74%) connected it from the second level. Ten of 14 PCTs (71%) connected it to ideal gas. In the category of partial pressure, 8 of 24 PCTs (33%) added this concept to their maps. Two of 8 PCTs (25%) connected it to gas concept from the first level, and 5 PCTs (62%) correlated it from the second level and 1 of 8 (13%) connected it to the diffusion from the third level.
Looking at the diffusion, effusion and kinetic theory concepts in Table 1, 19 of 24 PCTs (79%) used diffusion in their maps and 14 of 19 PCTs (74%) correlated it with the gas concept from the first hierarchical level. 18 of 24 PCTs (75%) used effusion in their maps and 13 of 18 PCTs (72%) connected it to gas concept from the first level. 21 of 24 PCTs (88%) used kinetic theory in their maps and 7 of 21 PCTs (33%) correlated it to gas concept from the first level. 14 of 21 PCTs (67%) connected it from the second level. While 6 of 14 PCTs (43%) connected it to real gas, 6 of them (43%) connected it to ideal gases.
In the measurement of gas pressure category in Table 1, 22 of 24 PCTs (92%) used barometer concept in their maps. 17 of 22 PCTs (77%) connected it with gas pressure from the second level. 4 of 22 PCTs (18%) correlated it from the third level. Whilst 2 of them (9%) related it to atmospheric pressure, 2 of them (9%) related it to gas pressure from the third level. Also, one of 22 PCTs (5%) correlated it to gas pressure from the fourth level. 23 of 24 PCTs (96%) used the manometer concept in their maps. 18 of 23 PCTs (78%) related it to gas pressure from the second level. Whilst four of 23 PCTs (18%) correlated it from the third level, one of 23 PCTs (4%) correlated it from the fourth level. All PCTs (100%) used the open-ended manometer in their maps, and one of 24 PCTs related it to gas pressure from the second level. 18 of 24 PCTs (75%) related it to manometer from the third level. Besides, whilst four of them correlated it to manometer from the fourth level, one of them correlated it from the fifth level. 21 of 24 PCTs (88%) used the closed-ended manometer in their maps, and 14 of 19 PCTs (66%) related it to manometer from the third level. Whilst four of them related it to manometer from the fourth level, one of them correlated it to manometer from the fifth level. Table 2 shows the quantitative analysis of the PCTs' concept maps.
The PCTs were given 19 terms before preparing their map to correlate each other on their maps. 13 of the 24 PCTs (54%) used under 19 concepts. While the researcher formed 23 valid propositions between concepts, the PCTs' valid propositions changed from 3 to 18. As seen from Table 2, while the researchers established six connection levels between concepts, PCTs mostly formed three connection levels. The PCTs' valid concepts in Table 2 with (′′) shows their number of deficient or extra concepts related to gas from 19 concepts. While the researcher established 7 crossing correlation, only 10 PCTs established crossing correlation. The number of these correlations ranged from 1 to 5. On the other hand, the number of PCTs' cross-connection levels (*) from Table 2 shows that the PCTs mostly could not write either correlation expressed between concepts on arrows or draw the direction of arrows correctly but did not write the relation statement correctly. In that case, the PCTs' cross-links were graded with 2 points. Also, when we look at IRV which determines the rote or meaningful learning, the researcher's point of IRV is 368.42, but only one PCT could take 105.88 point of IRV. The point of IRV for 14 PCTs could not be calculated because the PCTs' could not establish cross-correlation. Also, Table 2 shows the PCTs' rate of points and researcher's point from their maps and, analysis of the relationship between concepts. These rates range from 50.34 to 24.8. 8 of the PCTs show partial understanding and 16 PCTs show low understanding.
Table 3 describes how the PCTs established relationships between the gas concepts. From this table, it was found that relationships between concepts were mostly not meaningful, relationships were established but connection statements were not written on the arrows in some cases, the PCTs thought the ideal and real gases as two different kinds of gases, and effusion and diffusion concepts were correlated with gas key concept but this relationship was not meaningful such as in explaining diffusion with P–V law (Boyle’s law).
Discussion, conclusions and suggestions
The results of our analysis of the PCTs' concept maps are discussed in this section. Besides, based on our study we suggest the following for each assertion;Assertion 1: PCTs' concept maps show that PCTs explained gas properties using the P × V = nRT equation and they could not relate gas concepts to each other.
Volume, temperature and mole number concepts were correctly correlated with gas concept or gas pressure. These concepts were covered in lectures as gas properties, factors affecting gas pressure in the traditional teaching process. Therefore, this result is not surprising. The PCTs explained the gas properties with the P × V = nRT equation, and they constructed relationships using this equation; however, they could not relate these concepts to each other. Loverude et al. (2002) mentioned that the students established invalid relationships between the variables in this equation. This does not indicate meaningful learning; their learning could have been rote or superficial. From Table 1, most of the PCTs established some connections between concepts at different hierarchical levels, but these levels mostly changed from first to third levels. This showed us that their connections were superficial, and also not detailed from their maps. Analysis of their maps showed that the PCTs used mostly their rote knowledge while they were constructing their maps due to their simple propositions such as “gas pressure is effected by mole number”, “ideal and real gases have pressure”. For more specific example; most of the students correlated effusion and diffusion concepts to gas key concept from the first hierarchical level as forming the proposition that gases effused and diffused. This showed us that they established superficial connections and their learning was rote.
From Table 2, the internal relationship value of the PCTs from their maps supports the idea that their learning was not meaningful but superficial because for most of the students this IRV could not be calculated. Although the PCTs were given concepts related to gases, they could not correlate these concepts with each other meaningfully. From Tables 2 and 3, qualitative analysis of PCTs' concept maps, some PCTs (3, 6, 7, 8, 13, 17, 18, 19, 20, 21, 23) correlated these concepts with either gas key concept or gas laws inappropriately. The reason for this could be that propositions established between concepts are not meaningful. While our study revealed this conclusion, Kautz et al. (2005a, 2005b) revealed in their studies that the students had inappropriate understanding of the macroscopic variables of pressure, volume and temperature, and relation with each other, and they concluded that this situation stemmed from misinterpretation of microscopic processes.
It could be concluded that the PCTs perceived the volume, temperature and mole number concepts as either factors affecting gas pressure or one of the properties of gases. While most of the PCTs considered the temperature and mole concepts as factors affecting gas pressure from second hierarchical level, volume was seen as one of the properties of gases from the first level. It could be concluded that the PCTs' learning was rote and superficial. Therefore, teaching should be supported with examples from daily life for learning to be meaningful. The students should be given homework related to daily life.
Assertion 2: Table 3 showed that they have misconceptions related to an ideal gas, real gas and kinetic theory. Moreover, their knowledge regarding the gas laws and ideal gases are superficial and that they also have difficulties in understanding ideal and real gases.
PCTs (except 1, 9, 16, 17, 22 and 23) related ideal and real gas to gas key concept separately as a kind of gas and at this point they have misconception as seen at Table 3. These misconceptions could stem from the fact that differences between ideal and real gases are not explained in detail during the formal teaching process. As a result, the PCTs perceived them as if there were two kinds of gases. PCTs could not correlate ideal gases with real gases, possibly because they forgot the ideal gas conditions. These PCTs took chemistry courses for three and half years, and then they studied pedagogical knowledge for one and half years. Therefore, they kept away from chemistry courses directly when they came to the Faculty of Education. Table 3 also shows some PCTs (14, 20, and 23) have the misconception that the kinetic molecular theory explains real gas behavior.
From analysis of their maps it seems that the PCTs used these laws to explain ideal gas behavior. In these maps, the PCTs established a superficial relationship between the laws and ideal gases. That is, they gave some explanations about the relationships between effects of these factors by using the ideal gas equation in an inappropriate way as determined in the literature (Loverude et al., 2002; Kautz et al., 2008). Therefore, it was shown that they forgot their knowledge on gases and their learning was not meaningful.
Some of the PCTs thought that kinetic theory explained the behavior of ideal gas and some of them believed that this theory explained the behavior of real gas from the PCTs' (3, 4, 11, 12, 14, 19, 20, 21, 24) concept map analysis as shown in Table 2. In addition, as shown in Table 1, almost all the PCTs correlated kinetic theory to ideal and real gases as types of gas of first or second hierarchical level. They could not establish meaningful connections relating kinetic theory on their maps. This showed us that they could not perceive the kinetic theory of gases, and how it is used to explain the behavior of gases. Possibly, they did not know the assumptions of kinetic theory or perhaps their learning was superficial. They were not aware of what they said about this relationship. This showed that they did not assimilate the knowledge they had learned.
It was concluded that the PCTs thought ideal and real gases related to the gas concept as types of gas. Therefore, during the teaching process, PCTs should learn first about real gas concepts, and the PCTs should learn that all gases have real, attractive forces between gas particles. And then, the condition under which real gases behave as ideal gases should be explained. This explanation should not be only verbal. This concept should be thought first on the macroscopic level and then be supported by microscopic level animations. Besides, visual materials should be developed to help students understand how these laws explain ideal gas behavior at both the macro and molecular levels (Oh et al., 2012).
Assertion 3: PCTs' concept maps showed that they do not know the meaning of effusion and diffusion. On the other hand, they know what a manometer and a barometer are.
Diffusion and effusion concepts were thought to be the movement of gases in the maps. The PCTs did not know the definition of effusion, but they compared effusion to diffusion. On the other hand, the PCTs mostly related manometer and barometer to gas pressure correctly. Their relationship established was as “gas pressure was measured with a manometer or barometer”. However, they did not emphasize the discrimination of these two concepts in their maps. The PCTs thought open- and closed-ended manometers were types of manometers in their maps.
The PCTs confused effusion and diffusion because the words seemed to be similar. Consequently, these concepts should be taught comparatively, and an analogy or examples related to daily life should be given while teaching. Visual activities in animations would help in this area as well. While the PCTs related the barometer and manometer to gas pressure, they related the open- and closed-ended manometers to manometer concept.
In this study, we found that concept maps used as assessment methods are effective in terms of identifying PCTs' conceptual understanding. We also found that PCTs have a superficial understanding and misconceptions about this topic. These results might show that the PCTs could not remember this subject. They took chemistry courses related to the gas subject deeper at grade 10 (at the age of 16 or 17), first year university introductory chemistry course, and later thermo chemistry courses. These PCTs took chemistry courses for three and half years, and then they studied pedagogical knowledge for one and half years. Therefore, they might not have studied chemistry directly when they came to the Faculty of Education. In this context, our case study can suggest similar results for those who have the same educational experience.
In the literature, the research has addressed that assessing students' concept maps allows teachers to identify students' misconceptions or conceptual understanding effectively (Kaya, 2008; Özmen et al., 2009). Moreover, the qualitative and quantitative analysis of concept maps provided rich data in this study. Therefore, teachers and the researchers can use this kind of analysis in other topics of chemistry as well as in other science fields. Whilst we used qualitative and quantitative analysis, we understood that most of the PCTs preferred constructing the non-hierarchical concept map, and this showed us that although these PCTs are familiar with constructing hierarchical concept maps in their Chemistry Teaching Methods I–II courses, they are not familiar with constructing them in relation to gas concepts. This result might be because of the difficulty of drawing these kinds of maps using these concepts. In other words, since hierarchical concept maps require constructing concepts from more general to most general, the PCTs might find it difficult to construct a map from more general to most general hierarchically. There has been some research which has argued that students prefer to use non-hierarchical concept maps rather than hierarchical concept maps (Novak and Gowin, 1984; Ruiz-Primo and Shavelson, 1996; Ebenezer and Haggerty, 1999; Kaya and Ebenezer, 2003; Kaya, 2003a, 2008). The results of these studies are compatible with our study. The connection level of which PCTs correlated the concepts with each other showed which concepts are most important in a PCT's mind and PCTs' understanding related to gas. In the light of the findings of this study, we may suggest that PCTs should be taught how to construct concept maps in a different way such as hierarchical, non-hierarchical, chain or spoke (Novak and Gowin, 1984; Ruiz-Primo and Shavelson, 1996; Kinchin et al., 2000; Kaya, 2003a), and teachers should be taught how to assess concept maps as an assessment tool qualitatively and quantitatively.
Finally we recommend that PCTs should be provided with facilities in a learning environment, materials supporting both macro and micro dimensions where they construct their learning, and reflect what they have learned. Lecturing to the PCTs does not encourage meaningful learning. These students are prospective teachers, and they should be gaining skills in using different methods to construct their knowledge, so that they can be more effective teachers in the future. Meaningful learning requires applying and using what has been learned, in different ways. In education faculties, the learning environment should focus more on chemistry content knowledge and pedagogical content knowledge.
Acknowledgements
The authors are grateful to Dr Mary B. Nakhleh retired from Purdue University Department of Curriculum and Instruction Division of Chemical Education for her valuable input and insight in preparing this manuscript.References
- Abdullah A. and Scaife J., (1997), Using interviews to assess children's understanding of science concepts, School Sci. Rev., 78(285), 79–84.
- Bak Z. and Ayas A., (2008), Identifying understanding levels related to atom concept of chemistry students through concept mapping, 8. International Educational Technology Conference, Anadolu University, 6–9 May, Eskişehir-Turkey.
- Cassata A. E., Himangshu S. and Juli R. J., (2004), What do you know? Assessing change in student conceptual understanding in science, concept maps: theory, methodology, Technology Proc. of the First Int. Conference on Concept Mapping, Spain.
- Çetin P. S., Kaya E. and Geban O., (2009), Facilitating conceptual change in gases concepts, J. Sci. Educ. Technol., 18(2), 130–137.
- Coştu B., Ayas, A. and Niaz, M., (2012), Investigating the effectiveness of a POE-based teaching activity on students' understanding of condensation, Instr. Sci., 40, 47–67.
- Ebenezer J. V. and Haggerty M. S., (1999), Becoming a Secondary School Science Teacher, New Jersey: Merill Press.
- Eshach H. and Garik P., (2001), Students' conceptions about atoms and atom-bonding, available online at: www.bu.edu/smec/qsad/ed/QM-NARST-finalpg.pdf (accessed 20 December, 2005).
- Francisco J. S., Nakhleh M. B., Nurrenbern S. C. and Miller M. L., (2002), Assessing student understanding of general chemistry with concept mapping, J. Chem. Educ., 79(2), 248–257.
- Higher Education Council, (1998), Education Faculty Teacher Training Programs, March, Ankara.
- Kautz C. H., Heron P. R. L., Loverude M. E. and McDermott L. C., (2005a), Student understanding of the ideal gas law. Part I: a macroscopic perspective, Am. J. Phys., 73, 1056–1063.
- Kautz C. H., Heron P. R. L., Schaffer P. S. and McDermott L. C., (2005b), Students' understanding of the ideal gas law, Part II: A microscopic perspective, Am. J. Phys., 73(11), 1064–1071.
- Kautz C. H., Loverude M. E., Heron P. R. L. and McDermott L. C., (2008), Research on student understanding of ideal gas law, http://www.ipn.uni-kiel.de/projekte/esera/book/b027-kau.pdf, access date: 18/04/2008.
- Kaya O. N., (2003a), Concept maps in science education, Pamukkale University Faculty of Education Journal, 1(13), 70–79.
- Kaya O. N., (2003b), An alternative assessment method in education; concept maps, Hacettepe University J. Educ., 25, 265–271.
- Kaya O. N., (2008), A student-centered approach: assessing the changes in prospective science teachers' conceptual understanding by concept mapping in a general chemistry laboratory, Res. Sci. Educ., 38, 91–110.
- Kaya O. N. and Ebenezer J., (2003), A longitudinal study of the effects of concept mapping and Vee diagramming on senior university students' aAchievement, attitudes and perceptions in the science laboratory. Paper presented at the annual meeting of the National Association for Research in Science Teaching (NARST), Philadelphia, PA.
- Kearney M., (2004), Classroom use of multimedia-supported predict–observe–explain tasks in a social constructivist learning environment, Res. Sci. Educ., 34, 427–453.
- Kinchin I. M., Hay D. B. and Adams A., (2000), How a qualitative approach to concept map analysis can be used to aid learning by illustrating patterns of conceptual development, Educ. Res., 42(1), 43–57.
- Liew C. W., (2004), The effectiveness of predict–observe–explain technique in diagnosing students' understanding of science and identifying their level of achievement, Unpublished doctorate thesis, Australia: Curtin University of Technology.
- Longden K., Black P. and Solomon J., (1991), Children's interpretation of dissolving, Int. J. Sci. Educ., 13(1), 59–68.
- Loverude M. E., Kautz C. H. and Heron P. R. L., (2002), Student understanding of the first law of thermodynamics: Relating work to the adiabatic compression of an ideal gas, Am. J. Phys., 70(2), 137–148.
- Markham K., Mintzes J. and Jones G., (1994), The concept map as a research and evaluation tool: Further evidence of validity, J. Res. Sci. Teach., 31(1), 91–101.
- Mason C. L., (1992), Concept mapping: A tool to develop reflective science instruction, Sci. Educ., 76(1), 51–63.
- Merriam S. B., (2009), Qualitative research: A Guide to Design and Implementation, San Francisco: Jossey-Bass.
- Nakhleh M. B., (1993), Are our students conceptual thinkers or algorithmic problem solvers? Identifying conceptual students in general chemistry, Chem. Educ., 70(1), 52–55.
- Nakiboğlu C. and Özkılıç-Arık R., (2008), Determining 4th grade students' misconcepts about gases via V-diagram, http://www.yeditepe.edu.tr/yeditepe/Yeditepe%20UniverSiteSi/EGitim/LiSanS/EGitim%20FakulteSi/EDU7/Makaleler/Cilt%201%20Sayi%202.aspx?cacheid=/Yeditepe%20UniverSiteSi/EGitim/LiSanS/EGitim%20FakulteSi/EDU7/Makaleler/Cilt%201%20Sayi%202, access date 23/06/2008.
- Nicoll G., Francisco J. S. and Nakhleh M. B., (2001), An investigation of the value of using concept maps in general chemistry, J. Chem. Educ., 78(8), 1111–1117.
- Novak J. D. and Gowin, D. B., (1984), Learning how to learn, New York and Cambridge, UK: Cambridge University Press.
- Nurrenbern S. C. and Pickering, M., (1987), Concepts learning versus problem solving: is there a difference? J. Chem. Educ., 64(6), 508–510.
- Oh E. Y. Y., Treagust D. F., Koh T. S., Phang W. L., Lit Ng. S., Sim G. and Chandrasegaran, A. L., (2012), Using visualisations in secondary school physics teaching and learning: evaluating the efficacy of an instructional program to facilitate understanding of gas and liquid pressure concepts, Teach. Sci., 58(4), 34–42.
- Özgün-Koca S. A. and Şen A. İ, (2004), The development of a qualitative analyzing method for concept maps, Hacettepe University J. Educ., 27, 165–173.
- Özmen H., Demircioğlu G. and Coll R. K., (2009), A comparative study of the effects of a concept mapping enhanced laboratory experience on Turkish high school students' understanding of acid-base chemistry, Int. J. Sci. Math. Educ., 7, 1–24.
- Patton M. Q., (2002), Qualitative evaluation and research methods, 3rd edn, Thousand Oaks, CA: Sage Publications, Inc.
- Pendly B. D., Novak J. D. and Bretz R. L., (1994), Concept maps as a tool to assess learning in chemistry, J. Chem. Educ., 71(1), 9–15.
- Pickering M., (1990), Further studies on concept learning versus problem solving, J. Chem. Educ., 67, 254–255.
- Renström L., Björn A. and Marton F., (1990), Students' conceptions of matter, J. Educ. Pschol., 2(3), 555–569.
- Ruiz-Primo M. A. and Shavelson R. J., (1996), Problems and issues in the concept maps in science assessment, J. Res. Sci. Teach., 33(6), 569–600.
- Ruiz-Primo M. A., Schultz S. E., Li M. and Shavelson R. J., (2001), Comparison of the reliability and validity of scores from two concept-mapping techniques, J. Res. Sci. Teach., 38, 260–278.
- Sawrey B. A., (1990), Concept learning versus problem solving: Revisited, J. Chem. Educ., 67, 253–254.
- Schuttlefield J. D., Kirk J., Pienta N. J. and Tang H., (2012), Problem difficulty in ideal gas law problems, J. Chem. Educ., 89(5), 586–591.
- Şen A. I. and Özgün-Koca A., (2003), Using and comparing qualitative and quantitative methods on concept map analysis, Çukurova University Faculty of Education Journal, 2, 1–9.
- Smith R., (2003), Creating meaningful learning through the implementation of concept mapping, www.montana.edu/msse/2003%20Capstone%20Projects.html, access date 20/03/2005.
- Smith K. J. and Metz, P. A., (1996), Evaluating student understanding of solution chemistry through microscopic representations, J. Chem. Educ., 73(3), 233–235.
- Stavy R., (1988), Children's conception of gas, Int. J. Sci. Educ., 10(5), 553–563.
- Striebel J. F., (2005), Improving student metacognition and the assessment of conceptual understanding through pre and post unit concept mapping. www.montana.edu/msse/2003%20Capstone%20Projects.html, access date 20/03/2005.
- Taber K. S., (2007), Classroom-Based Research and Evidence-Based Practice: A Guide for Teachers, London: SAGE Publications.
- Van Zele E., Lenaerts J. and Wieme W., (2004), Improving the usefulness of concept maps as a research tool for science education, Int. J. Sci. Educ., 26(9), 1043–1064.
- Westbrook S. L. and Marek, E. A., (1992), A cross-age study of student understanding of the concept of homeostasis, J. Res. Sci. Teach., 29, 51–61.
- White R. and Gunstone R., (1992), Probing understanding, London: The Falmer Press.
| This journal is © The Royal Society of Chemistry 2013 |
