Graphene oxide-based selection and identification of ofloxacin-specific single-stranded DNA aptamers†
Yuhong Zhang,
Yuanding You,
Ziwei Xia,
Xuyan Han,
Yaping Tian and
Nandi Zhou*
The Key Laboratory of Industrial Biotechnology, Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi 214122, China. E-mail: zhounandi@jiangnan.edu.cn; Fax: +86-510-85197831; Tel: +86-510-85197831
First published on 10th October 2016
Abstract
Graphene oxide (GO) was introduced in the process of systematic evolution of ligands by exponential enrichment (SELEX) to screen ofloxacin-specific single-stranded DNA aptamers. Four sequences with high homology, ap1, ap3, ap4 and ap5 were picked out. Their secondary structures were simulated, and the dissociation constants (Kd) were measured, which were 251.3, 130.1, 159.1 and 304.4 nM, respectively. The specificity of the aptamers was also evaluated.
Ofloxacin is a second-generation fluorinated quinolone antibiotic which belongs with orally-administered broad-spectrum antibacterial drugs against most Gram-negative bacteria and many Gram-positive bacteria.1,2 Ofloxacin has been widely used as a veterinary drug for therapeutic and prophylactic purposes.3,4 However, when it is overcommitted, it will be accumulated in animal tissues or excreted and emitted into the environment.5 The residual ofloxacin may be transmitted to human bodies by the food chain or other ways.6 Since serious side effects of ofloxacin have been reported,7 in order to protect human health, the European Union (EU) established the maximum residue limits (MRLs) of fluoroquinolones.8 So far, few methods have been reported for the detection of ofloxacin residue in food products. These established methods are mainly based on HPLC,9 solid-phase spectrofluorimetry,10 or CE-MS,11 which are time-consuming and expensive. Therefore, to establish rapid and high-sensitive methods for detection of ofloxacin is of great importance.
Aptamers are artificial single-stranded DNA (ssDNA) or RNA oligonucleotides. Similar to antibodies, aptamers can bind to their targets with high affinity and specificity.12,13 Meanwhile, aptamers have many advantages over antibodies, such as good renewability, chemical stability, easy to be synthesized and modified, etc.,14 therefore have great prospect in the fabrication of aptamer-based biosensors. Since the establishment of the systematic evolution of ligands by exponential enrichment (SELEX) in 1990 by Ellington15 and Tuerk,16 a large number of aptamers have been reported which are specific to different targets, including small organic molecules, peptides, proteins, even ions, cells or viruses.17 These aptamers have been applied in analytical, bioanalytical, diagnostic and therapeutic fields.18 In recent years, aptamers specific to antibiotics such as streptomycin,19 kanamycin,20 tetracycline21 and chloramphenicol22 have been reported. However, in consideration of diverse classes of antibiotics, aptamers specific for various types of antibiotics are highly desired for analysis and other purposes.
During the selection of the aptamers for small targets, these targets are generally difficult to be immobilized on magnetic beads or other carriers for aptamer screening. Therefore, some derivative SELEX techniques were developed. For example, for capture-SELEX, ssDNA library was alternatively immobilized on magnetic beads through the hybridization between a docking sequence in ssDNA library and a capture oligo-sequence immobilized on magnetic beads. It was successfully utilized to select aptamer for quinolone-type antibiotics.23 Graphene oxide (GO) has some unique characteristics including good water-solubility, versatile surface modification and superior fluorescence quenching ability.24 Recently, it is reported that the surface of GO can adsorb ssDNA via hydrophobic and π–π stacking interactions between the nucleobases of ssDNA and GO. However, the adsorption of dsDNA or well-folded ssDNA on GO is much lower.25 Based on this adsorption property, a variety of strategies have been explored for the detection of DNA,26 metal ions,27 small molecules,28 proteins,29 and even in situ cellular imaging.3 In order to improve the efficiency of SELEX, a novel immobilization-free SELEX method called GO-SELEX has been established based on the affinity of GO for ssDNA.30 It reduces a variety of steps involved in the conventional SELEX process and therefore has great prospect in the screening of the aptamers for different targets, such as pesticides,28 human C-reactive protein,29 gonyautoxin 1/4,31 T-2 toxin,25 patulin,32 H5Nx viruses,33 vaspin,34 etc. Compared to capture-SELEX, neither the target, nor ssDNA library needs to be immobilized during GO-SELEX. Herein we report the screening of ssDNA aptamers specific to ofloxacin from a 79-mer ssDNA library containing 35-nt random sequences by GO-SELEX. This is the first report on ofloxacin-specific ssDNA aptamer. The sequences and secondary structures of the screened aptamers were analyzed and the aptamer with high affinity and specificity was identified. It can be used as recognition element in aptasensors for ofloxacin in the future, and therefore has potential applications in various fields.
The individual steps of GO-SELEX round and GO-counter SELEX round are illustrated in Fig. 1. The random 79-mer ssDNA library (5′-TAGGGAATTCGTCGACGGATCC-N35-CTGCAGGTCGACGCATGCGCCG-3′) contained a central random sequence of 35 nucleotides, which was flanked by two primers binding sequences for PCR amplification and cloning, was employed as the initial ssDNA library to screen ofloxacin-specific aptamers. In the first step of selection, before ofloxacin was incubated with the initial library, the random ssDNA in binding buffer (20 mM Tris–HCl containing 100 mM NaCl, 2 mM MgCl2, 5 mM KCl, 1 mM CaCl2, and 0.02% Tween 20, pH 7.6) was denatured at 90 °C for 10 min, immediately cooled on ice for 10 min, then returned to room temperature (RT) for 10 min. ssDNA which has affinity for ofloxacin can bind to ofloxacin freely in solution to form ofloxacin/ssDNA complex. During the incubation with GO, the free ssDNA in the solution can be adsorbed on GO via π–π stacking, while ssDNA bound to ofloxacin remains in solution. GO and ssDNA without affinity for ofloxacin can be removed by centrifugation. Whereas ssDNA bound to ofloxacin can be recovered and amplified by PCR using a forward primer (5′-TAGGGAATTCGTCGACGGAT-3′) and a biotin-labeled reverse primer (5′-biotin-CGGCGCATGCGTCGACCTG-3′). Then streptavidin-modified magnetic beads were used to separate biotinylated oligonucleotide strands from their complementary strands in PCR products. The obtain ssDNA was used as the secondary library for next round of GO-SELEX (see Experimental in ESI†). In GO-counter SELEX, ssDNA from the previous SELEX round was incubated with a mixture of potential counter targets and then mixed with GO, ssDNA bound to the counter targets remained in the solution and was removed by centrifugation. ssDNA adsorbed on GO was potential aptamers of ofloxacin. Then ofloxacin was added into GO suspension. Free ofloxacin can bind to ssDNA adsorbed on GO, change their confirmation, and weaken the π–π stacking interactions between ssDNA and GO in turn, which finally desorb ssDNA from GO. The recovered ssDNA was purified and amplified.
 | ||
| Fig. 1 Schematic illustration of GO-based selection of the aptamers. Inset shows the recovery of ssDNA after each SELEX round. | ||
During the GO-SELEX process, the recovery of ssDNA was determined after each round. As expected, an increased recovery of ssDNA was obtained from round 1 to round 5, which means the increased amount of ssDNA in the ssDNA pool can bind to ofloxacin during selection and enrichment (Fig. 1, inset). Then the recovery leveled off, indicating the saturation of binding between ssDNA in the pool and the target. Therefore, GO counter-SELEX was performed after six rounds of selection. For the counter-SELEX, several fluoroquinolone antibiotics, such as ciprofloxacin, enrofloxacin and norfloxacin were chosen as counter targets to improve the specificity of the aptamers. After GO-counter SELEX, the final round of positive screening was performed, and the recovery of ssDNA in the final round was similar to round 5 and round 6. Compared to the conventional magnetic beads-based SELEX,19 GO-SELEX process can efficiently eliminate the unbound ssDNA and enrich the population of ssDNA aptamers specific to ofloxacin, therefore fewer rounds of selection were required before saturation was achieved. The recovered ssDNA from the last round of GO-SELEX was amplified with non-biotinylated primers, then cloned and sequenced.
Twenty-one positive clones were identified and sequenced, several among which were proven to possess the same sequences. Therefore, only 12 aptamers specific to ofloxacin were screened. The 35-mer variable regions of these aptamers are listed in Table 1. From sequence analysis, the variable regions of these aptamers possess conservative motifs ‘GGT’ or ‘GTT’. These aptamers can be classified into different families based on the homology analysis. Among them, aptamers from family 1 possess more identical sequences and higher homology, therefore, several aptamers from family 1 were chosen for further characterization.
| a The colored regions represent the conservative nucleotides in each family. |
|---|
 |
The secondary structures of the aptamers were predicted by using Mfold software (Fig. 2A). In mimic diagrams, yellow regions represent fixed sequences, while other regions represent random sequences. All the aptamers possess at least two stem-loop structures in their secondary structure patterns, which may be significant for their binding to ofloxacin. Then molecular docking was performed to predict the binding motif between aptamer ap3 and ofloxacin by using Autodock 4.0 software. Firstly, a model of the aptamer sequence was built by homology modeling. Then the simulating result was obtained through a series of calculation after hydrogenation, charge evaluation and combination of non-polar hydrogen. The simulated modeling was shown in Fig. 2B, which indicates that nucleotides T23, G24, G25, T55 and G56 in ap3 serve as recognition sites for ofloxacin.
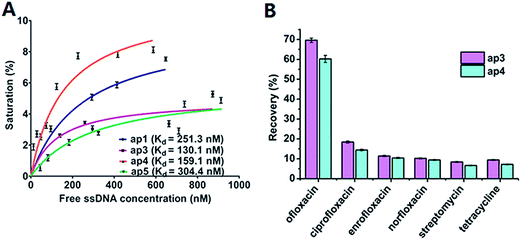
The affinity for the target undoubtedly represents the main characteristic of the aptamer. The dissociation constant (Kd) is commonly used to evaluate the affinity. To determine the Kd values of these aptamers, binding assays of the aptamers were performed by equilibrium filtration method.1 Fixed concentration of ofloxacin was mixed with a series of different concentration of aptamers. After ultrafiltration centrifugation, free ofloxacin in the filtrate was measured by UV-vis spectroscopy. By using non-linear regression analysis with the equation of y = Bmax[free ssDNA]/(Kd + [free ssDNA]) (y represents the degree of saturation, Bmax represents the maximum number of binding sites, [free ssDNA] is the concentration of unbound ssDNA),21 the Kd values of ap1, ap3, ap4 and ap5 were determined, which were 251.3 nM, 130.1 nM, 159.1 nM and 304.4 nM, respectively. The non-linear regression curves of the aptamers are shown in Fig. 3A. Among them, ap3 and ap4 possess comparatively low Kd values. With the lowest Kd value of 130.1 nM, ap3 has the highest affinity for the target.
The specificity of the aptamers decides the selectivity of aptamer-based bioanalysis, and therefore is the key characteristic of the aptamer. The specificity of ap3 and ap4 were investigated by determination of the rates of the bound ssDNA towards ofloxacin, ciprofloxacin, enrofloxacin, norfloxacin, streptomycin and tetracycline. As shown in Fig. 3B, for ofloxacin, the rate of bound aptamer (recovered ssDNA) was 69.6% for ap3 and 60.2% for ap4. The recoveries for other fluoroquinolone antibiotics such as ciprofloxacin, enrofloxacin and norfloxacin, are less than 20%. For other types of antibiotics, such as streptomycin and tetracycline, the recoveries are close to or less than 10%. Therefore, both ap3 and ap4 are highly specific to ofloxacin.
Conclusion
ssDNA aptamers that bind to ofloxacin with high affinity and high specificity were screened and identified from a random 79-mer oligonucleotides library by using GO-SELEX. After seven rounds of SELEX and one round of counter-SELEX, twelve aptamers with different sequences were identified. The sequences and the secondary structures of the aptamers were analyzed. Through the determination of Kd values and the estimation of the specificity, ap3 and ap4 were suggested to be the ideal aptamers for future analytical applications. To the best of our knowledge, it is the first report on ssDNA aptamers specific to ofloxacin. The screened aptamers may have potential applications in the fields like food safety control and environment monitoring.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 31271860), the Program for New Century Excellent Talents in University (NCET-12-0878) and the Fundamental Research Funds for the Central Universities (JUSRP51402A).Notes and references
- C. Andriole and V. Andriole, Mediguide Infect. Dis., 2001, 21, 1–5 Search PubMed.
- C. O. Agubata, C. Okereke, I. T. Nzekwe, R. I. Onoja and N. C. Obitte, Eur. J. Pharm. Sci., 2016, 89, 1–10 CrossRef CAS PubMed.
- J. F. Prescott, Anim. Health Res. Rev., 2008, 9, 127–133 CrossRef PubMed.
- C. Winckler and A. Grafe, J. Soils Sediments, 2001, 1, 66–70 CrossRef CAS.
- A. A. Boxall, in Comparative and Veterinary Pharmacology, ed. F. Cunningham, J. Elliott and P. Lees, Springer Berlin Heidelberg, Berlin, 2010, vol. 199, ch. 12, pp. 291–314 Search PubMed.
- K. He, X. Du, W. Sheng, X. Zhou, J. Wang and S. Wang, J. Agric. Food Chem., 2016, 64, 2627–2634 CrossRef CAS PubMed.
- J. P. Monk and D. M. Campoli-Richards, Drugs, 1987, 33, 346–391 CrossRef CAS PubMed.
- G. Cheng, X. Dong, Y. Wang, D. Peng, X. Wang, H. Hao, S. Xie, W. Qu, Z. Liu and Z. Yuan, Anal. Bioanal. Chem., 2014, 406, 7899–7910 CrossRef CAS PubMed.
- Y. K. Lv, L. Yang, X. H. Liu, Z. Y. Guo and H. W. Sun, Anal. Methods, 2013, 5, 1848–1855 RSC.
- O. Ballesteros, J. L. Vílchez and A. Navalón, J. Pharm. Biomed. Anal., 2002, 30, 1103–1110 CrossRef CAS PubMed.
- A. Juan-García, G. Font and Y. Picó, Electrophoresis, 2007, 28, 4180–4191 CrossRef PubMed.
- F. Yuan, H. Zhao, Z. Zhang, L. Gao, J. Xu and X. Quan, RSC Adv., 2015, 5, 58895–58901 RSC.
- M. Mckeague, A. Foster, Y. Miguel, A. Giamberardino, C. Verdin, J. Y. S. Chan and M. C. DeRosa, RSC Adv., 2013, 3, 24415–24422 RSC.
- K. Han, Z. Liang and N. Zhou, Sensors, 2010, 10, 4541–4557 CrossRef CAS PubMed.
- A. D. Ellington and J. W. Szostak, Nature, 1990, 346, 818–822 CrossRef CAS PubMed.
- C. Tuerk and L. Gold, Science, 1990, 249, 505–510 CAS.
- R. Stoltenburg, C. Reinemann and B. Strehlitz, Anal. Bioanal. Chem., 2005, 383, 83–91 CrossRef CAS PubMed.
- S. Wang, J. Liu, Y. Dong, H. Su and T. Tan, RSC Adv., 2015, 5, 53796–53801 RSC.
- N. Zhou, J. Wang, J. Zhang, C. Li, Y. Tian and J. Wang, Talanta, 2013, 108, 109–116 CrossRef CAS PubMed.
- K. M. Song, M. Cho, H. Jo, K. Min, S. H. Jeon, T. Kim and M. S. Han, Anal. Biochem., 2011, 415, 175–181 CrossRef CAS PubMed.
- J. H. Niazi, J. L. Su and B. G. Man, Bioorg. Med. Chem., 2008, 16, 7245–7253 CrossRef CAS PubMed.
- J. Mehta, B. V. Dorst, E. Rouah-Martin, W. Herrebout, M. L. Scippo, R. Blust and J. Robbens, J. Biotechnol., 2011, 155, 361–369 CrossRef CAS PubMed.
- C. Reinemann, U. F. von Fritsch, S. Rudolph and B. Strehlitz, Biosens. Bioelectron., 2016, 77, 1039–1047 CrossRef CAS PubMed.
- X. Xing, X. Liu, Y. Zhou, D. Xu, D. Pang and H. Tang, RSC Adv., 2016, 371, 355–396 Search PubMed.
- X. Chen, Y. Huang, N. Duan, S. Wu, Y. Xia, X. Ma, C. Zhu, Y. Jiang and Z. Wang, J. Agric. Food Chem., 2014, 62, 10368–10374 CrossRef CAS PubMed.
- C. H. Lu, J. Li, J. J. Liu, H. H. Yang, X. Chen and G. N. Chen, Chem.–Eur. J., 2010, 16, 4889–4894 CrossRef CAS PubMed.
- W. Y. Xie, W. T. Huang, N. B. Li and H. Q. Luo, Chem. Commun., 2012, 48, 82–84 RSC.
- V. T. Nguyen, Y. S. Kwon, J. H. Kim and M. B. Gu, Chem. Commun., 2014, 50, 10513–10516 RSC.
- X. Yang, Y. Wang, K. Wang, Q. Wang, P. Wang, M. Lin, N. Chen and Y. Tan, RSC Adv., 2014, 4, 30934–30937 RSC.
- J. W. Park, R. Tatavarty, D. W. Kim, H. T. Jung and M. B. Gu, Chem. Commun., 2011, 48, 2071–2073 RSC.
- S. Gao, B. Hu, X. Zheng, Y. Cao, D. Liu, M. Sun, B. Jiao and L. Wang, Biosens. Bioelectron., 2016, 79, 938–944 CrossRef CAS PubMed.
- S. Wu, N. Duan, W. Zhang, S. Zhao and Z. Wang, Anal. Biochem., 2016, 508, 58–64 CrossRef CAS PubMed.
- V. T. Nguyen, H. B. Seo, B. C. Kim, S. K. Kim, C. S. Song and M. B. Gu, Biosens. Bioelectron., 2016, 86, 293–300 CrossRef CAS PubMed.
- N. H. A. Raston and M. B. Gu, Biosens. Bioelectron., 2015, 70, 261–267 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18430h |
| This journal is © The Royal Society of Chemistry 2016 |