Self-aggregation mechanism of a naphthylamide cationic derivative of cholic acid. From fibers to tubules†
Juan V. Trilloa,
Francisco Meijidea,
Aida Jovera,
Victor H. Sotob,
Santiago de Frutosa,
Maria Chiara di Gregorioc,
Luciano Galantinic and
José Vázquez Tato*a
aDepartamento de Química Física, Facultad de Ciencias, Universidad de Santiago de Compostela, Avda. Alfonso X El Sabio s/n, 27002 Lugo, Spain
bEscuela de Química, Centro de Investigación en Electroquímica y Energía Química (CELEQ), Universidad de Costa Rica, San José, Costa Rica
cDipartimento di Chimica, Università di Roma “Sapienza”, P. le A. Moro 5, 00185 Rome, Italy
First published on 16th December 2013
Abstract
The aggregation behavior of a cationic derivative of cholic acid {[3β,5β,7α,12α]-3-(2-naphthoylamino)-7,12-dihydroxycholan-24-triethylamonium iodide} has been studied by surface tension measurements, fluorescence spectroscopy, transmission electronic microscopy (TEM), and circular dichroism. The critical aggregation concentration, the fraction of bound counterions and thermodynamic parameters for the formation of aggregates have been determined, as well as the morphology of the aggregates. TEM images support a consecutive transformation mechanism from fibers to tubules, these having a well-defined geometry and being the only structure observed at the end of the process. Intermediate observed structures are helical ribbons.
Introduction
Bile salts are biological surfactants that play important roles in several processes of the mammalian organisms. They are facially amphipathic molecules1 which self-associate in aqueous solution, forming aggregates which usually have low aggregation numbers and small sizes.2 However, the insertion of hydrophobic groups by chemical modification at the 3-position of natural bile salts (compounds 1–5, Fig. 1), gives anionic derivatives which lead to supramolecular structures as molecular tubes, lamellae (compound 6, Fig. 1), and other structures.3–9 Sodium lithocholate is the only unmodified bile salt which forms molecular tubes with diameters about 50 nm, in basic conditions.10–14 | ||
| Fig. 1 Structures of some anionic and cationic derivatives of bile salts, including compound 8 (napht-CCD) studied in this paper. | ||
In comparison to anionic derivatives, the aggregation behavior in aqueous solution of cationic derivatives of bile acids has received much less attention and, in general, the size and morphology of the aggregates, as well as the mechanisms of their formation, are almost unknown. However their high functionality allows the design of different positively charged derivatives.9,15,16 Overviews of bile salt and bile acid derivatives chemistry can be found elsewhere.1,17,18
There are several reasons to synthesize and characterize cationic derivatives of bile acids. For instance, the interaction between DNA and cationic surfactants has received a considerable attention during the last decade,19–24 and, consequently, the scientific interest on this kind of studies involving cationic bile salt derivatives will possibly raise in the near future. These derivatives are also important since several cationic steroid antibiotics have been obtained25,26 to mimic the properties of squalamine (an antibiotic first isolated from the dogfish shark)27 and peptide polymyxin B. Furthermore some cationic derivatives are excellent gelators as Maitra et al.28–31 have investigated.
On the other hand, catanionic mixtures are highly interesting because of their potential practical applications.32,33 Being negatively charged, natural bile salts have been mixed with long chain positively charged surfactants. This is the case of sodium deoxycholate with cetyltrimethylammonium bromide.34 Manna et al.35 have studied catanionic mixtures formed by long chain alkyltrimethylammonium bromides (CnTABr, n = 12, 14 and 16) in combination with sodium cholate (NaC) and sodium deoxycholate (NaDC). Liu et al.36 have observed that the free-salt catanionic mixture of lithocholate with tetradecyl- and cetyl-trimethylammonium hydroxides form unilamellar vesicles. In the second case, vesicles were transferred to helical ribbons after several days. The influence of cholic acid and deoxycholic acid on the properties of salt-free catanionic surfactant systems has been studied as well.37,38 Bile salts can also play important roles in the aggregation of catanionic surfactant systems as in the enlargement of the average surfactant headgroup area.39
As far as we know, only one catanionic mixture formed exclusively by bile salt derivatives has been carefully characterized by Manghisi et al.9 who used two opposite charged p-tert-butylphenyl derivatives of sodium cholate (compounds 1 and 7, Fig. 1). The mixture forms tubules with a charge which may be tuned controlling the proportion of the anionic and cationic derivatives. Another remarkable fact of this system is that at the low concentrations studied (<8.0 × 10−4 mol dm−3) each derivative alone does not form tubules.
Different mechanisms of the formation of molecular tubes of negatively charged hydrophobic derivatives of bile salts have been published,4–7 but neither the formation of these structures by positively charged derivatives nor the mechanism of their formation have been described. Those studies show that the mechanisms are dependent on the structure of the hydrophobic group and the number and location of the hydroxy groups, but predictions are not possible yet and each new derivative has to be fully characterized. The aim of this work is to study the aggregation and the morphology of the aggregates formed by a cationic derivative in aqueous solution. For this purpose the cationic derivative [3β,5β,7α,12α]-3-(2-naphthoylamino)-7,12-dihydroxycholan-24-triethylamonium iodide (8 or napht-CCD following other acronyms used in previous publications for this kind of compounds) was synthesized and the results of the study concerning its aggregation in aqueous solution are reported. This compound was chosen as the study of the anionic derivative has been recently published.8
Experimental
Equipment
Surface tension measurements were carried out in a Lauda TVT 2 volume drop tensiometer (Lauda GMBH, Lauda-Königshofen, Germany). Temperature was kept constant by recirculating water from a PolyScience 9100 thermostat (PolyScience, Niles, Illinois, USA).Steady-state fluorescence measurements, using pyrene as a probe, were recorded on a Cary Eclipse spectrometer (Agilent Technologies, Santa Clara, California). Experimental parameters: excitation wavelength 336 nm, excitation slit 2.5 nm, emission slit 2.5 nm. UV spectra were recorded on a Cary/1E spectrophotometer and reported in molar extinction coefficient, ε. Circular dichroism (CD) spectra were recorded on a JASCO (Jasco, Inc., Easton, MD, USA) model 715 and reported in molar ellipticity, [θ].
TEM images were obtained at room temperature in a JEOL JEM-1011 (Jeol Ltd., Tokyo, Japan), operated at 80 kV, equipped with a MegaView III camera. For sample preparation, a drop of the solution (kept at 25 or 50 °C) was deposited onto a carbon-coated copper grid and left for a few minutes to allow complete grid permeation. After that, the grid was deposited on filter paper to absorb the drop excess and measured immediately.
NMR experiments were performed in a Varian Mercury 300 instrument (Agilent Technologies).
Synthesis
The synthesis of napht-CCD 8 is similar to the one published for a p-tert-butylphenyl cationic derivative of cholic acid.9 The synthesis requires a 7-steps procedure, starting from the methyl ester of the 3β-aminocholic 9 (Scheme 1), whose synthesis is well known.40 The reaction of 9 with 2-naphthoyl chloride41 provides the amide 10 which leads the acid 11 after hydrolysis. After protecting the hydroxy groups at C7 and C12 (12), the acid function is reduced to the alcohol 13 which allows the synthesis of the iodide derivative 14.42 After deprotecting the hydroxy groups, the compound 15 is obtained which finally leads to the ammonium salt 8.431H NMR (300 MHz, DMSO-d6, δ/ppm) of 8: 8.38 (s, 1H, NHCO); 8.02–7.55 (m, 7H, H naphthalene); 4.13 (s, 1H, H12); 3.80 (s, 1H, H7); 3.67 (s, 1H, H3); 3.29–3.22 (q, 6H); 3.12–3.05 (m, 2H); 2.35–1.35 (m, H bile salt skeleton and lateral chain); 1.21–1.17 (t, J = 6.18 Hz, 9H); 1.00–0.98 (d, J = 6.24 Hz, 3H, H21); 0.91 (s, 3H, H19); 0.63 (s, 3H, H18).
13C NMR (75 MHz, DMSO-d6, δ/ppm) of 8: 167.13 (CONH); 134.72–125.31 (C naphthalene); 71.92 (C7); 67.19 (C12); 57.67; 53.12; 47.07; 46.67; 42.14; 37.23; 35.71; 35.46; 35.23; 34.05; 33.06; 31.73; 29.50; 27.96; 26.89; 25.12; 23.51; 23.39; 18.89; 18.06; 13.07; 8.00. Mass spectrum (MALDI-TOF) of 8: 631.53; theoretical [C41H63N2O3]: 631.95.
Results and discussion
In pure water at 25 °C, The solubility of napht-CCD is low and below of the critical aggregation concentration, cac. However, the solubility markedly increases when inert electrolytes are added, allowing to perform the determination of cac. For this reason, measurements were carried out in the presence of 0.100 mol dm−3 NaCl.Surface tension measurements were performed in a volume drop tensiometer at 25 and 50 °C. Some experimental results are plotted in Fig. 2, in which typical profiles for a surfactant are observed. Thus cac and γcac (surface tension value at cac) were determined from the breaking points of the dependence of surface tension, γ, with ln [napht-CCD]. Results are shown in Table 1. The cac values are close to those found for other negatively charged hydrophobic modified cholic acid derivatives, for which values around 4–5 × 10−4 mol dm−3 have been measured.3,4 Furthermore, the cac values for this derivative are lower than those for sodium cholate,2 evidencing a more hydrophobic nature.
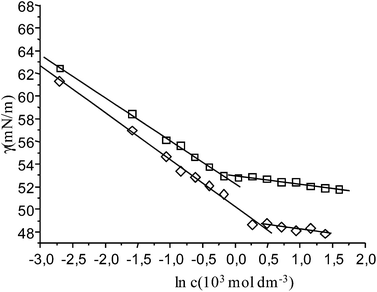 | ||
| Fig. 2 Plot of the surface tension data for napht-CCD in 0.100 mol dm−3 NaCl aqueous solutions at 25 °C (squares) and 50 °C (rhombs). | ||
| T (°C) | 103 cac (mol dm−3) | 106 Γ (mol m−2) | ao (Å2) |
|---|---|---|---|
| 25.0 | 0.80 ± 0.04 | 1.52 ± 0.03 | 109 ± 2 |
| 50.0 | 1.39 ± 0.09 | 1.55 ± 0.05 | 107 ± 3 |
The surface behavior may be analyzed in terms of the adsorption Gibbs equation, which relates the change in the equilibrium surface tension with changes in the chemical potentials of all of the solutes at constant temperature. From this isotherm, the surface excess concentration, Γ, is determined by eqn (1).
 | (1) |
 | (2) |
This equation shows that the prefactor n in the Gibbs equation depends on the concentration and stoichiometry of the surfactant (c, ν− and ν+) and also on both the stoichiometry (νs+) and the concentration, cs, of an added inert electrolyte. When the concentration of electrolyte is in a large excess with respect to the surfactant concentration, the prefactor becomes n = ν−. In this case, n = 1. Once Γ is known, the surface area per molecule can be determined by the eqn (3),
 | (3) |
The ao values for bile salts are highly dependent on experimental conditions. For instance, for sodium cholate, Swanson-Vethamuthu et al.45 have published values of 207 (in 0.050 mol dm−3 NaCl) and 288 Å2 (in 0.045 mol dm−3 NaCl and 5 × 10−3 mol dm−3 NaOH), while lower values (80–90 Å2) have been published for several bile acids.46,47 These last values have been accepted as an indication that the steroid salt anions lie flat at the interface with the polar groups projecting into the aqueous phase since the area of the steroid nucleus with a partially folded side chain may be estimated from the crystal structure as being 100 ± 3 Å2. For the p-tert-butylphenylamide derivatives of chenodeoxycholic and ursodeoxycholic acids, values of 77 and 54 Å2, respectively, have been published.6 These last low values would correspond to an upright orientation, attributed to the increase of the hydrophobic surface and to that the hydrophilic area (the cheno and urso derivatives only carry one α-hydroxy group) in the new derivative is not large enough for keeping flat the molecule in the air–water interface. These differences resemble the well-known orientations for monohydroxy lithocholate (upright) and trihydroxy and dihydroxy bile salts (flat).46
Recently, the crystal structures of the acid form of p-tert-butylphenylamide derivative of cholic acid, compound 1, in chlorobenzene and acetone have been published.48 The analysis of these structures indicates that the projected molecular areas for a molecule lying flat on a surface are 147 and 145 Å2, respectively. This is to say, the enlargement of the molecule by the hydrophobic group represents an increment of 46 Å2 respect to the area of the steroid nucleus, although the area of the p-tert-butylphenyl group lying flat on a surface is 55 Å2. The difference is due to that the phenyl group is forming an angle of 44–59° respect to the horizontal plane of the steroid nucleus. Since the area of the naphthoyl group is 62 Å2, lying flat, and 35 Å2, with a perpendicular orientation, it could be expected that the experimental value from surface tension experiments was around 135–162 Å2, clearly higher than the observed values of 108 Å2. The difference may be interpreted as the result of stacking of two-four naphthyl groups in the interface, thus reducing the contribution of this group to the total area per molecule by those factors. Therefore the surface area obtained from surface tension measurements suggests that molecules are lying flat on the interface with stacking of the aromatic groups, forming a closely packing layer, probably as in a liquid crystal. It is convenient to remember that an appropriate way to describe a bilayer lipid membrane is as a two-layer smectic-A liquid crystal.49 When the temperature is raised up to 50 °C, no effects are observed on the surface area but cac increases, now having a value of 1.39 × 10−3 mol dm−3.
Fluorescence spectra of pyrene solubilized in napht-CCD solutions in the presence of NaCl 0.100 mol dm−3 were also carried out at 50 °C. From them, the I1/I3 ratio of the intensities of the vibronic peaks of the probe were calculated and plotted against the surfactant concentration (Fig. 3). A typical sigmoid profile is obtained with an inflection point at (1.68 ± 0.06) × 10−3 mol dm−3. This value corresponds to the cac of the surfactant and compares well with the value obtained by surface tension measurements. At high surfactant concentrations I1/I3 reaches a plateau limiting value equal to 1.3 which is comparable to the one observed for compound 1 at 45–55 °C,4 and far from the observed values for NaDC (0.70) and NaC (0.75), which form small aggregates. This suggests that the probe is located in a more polar environment than in the natural bile salts, and that napht-CCD forms aggregates which are different from those of NaC and NaDC.2 The value mentioned above is compatible with less closed structures, as vesicles and molecular tubes, where the probe is located in less apolar environments.
From the results of cac at 25 and 50 °C, the thermodynamic magnitudes associated to the aggregation process in this interval of temperature can be determined using the following well-known equations for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 surfactants:
1 surfactants:
ΔG0agg = RT(1 + β)ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cac cac
| (4) |
 | (5) |
| ΔG0agg = H0agg − TΔS0agg | (6) |
 | (7) |
The morphology of aggregates of napht-CCD was studied by TEM.
Because of the experimental procedure, this technique can be only used to elucidate the mechanism of the aggregation process for some systems.4,7 On the other hand, for bile salt derivatives, this process crucially depends on time, concentration, pH, concentration of added inert salts, and temperature. Preliminary studies have shown that suitable experimental conditions are [napht-CCD] = 4.96 × 10−3 mol dm−3 in 0.100 mol dm−3 NaCl at 50 °C.
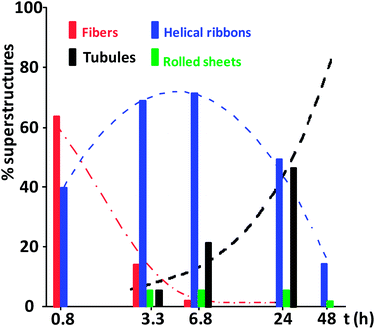
In these conditions, fibers (F) and helical ribbons (HR) coexist in solution (Fig. 4a) one hour (t = 0.8 h) after the preparation of the sample. The widths of fibers are in the range 20–60 nm, and the helical ribbons (with almost linear axis) have values of 60–100 nm and 500–750 nm for widths and pitches, respectively. The observed lateral fusion of narrow fibers may be the origin of the broader ones. It is known that neutral derivatives of bile acids also form fibers.55,56
After 3.3 h, helical ribbons are predominant (Fig. 4b). The linearity of the axis remains but widths are increasing (till 190 nm) while pitches are decreasing. This fact suggests the evolution of the helical ribbons towards molecular tubes (or tubules, T) and, in fact, some examples of this morphology can be observed in the TEM images. The formed tubules have diameters in the range 100–150 nm. In a less proportion, some rolled sheets (RS) are also observed. They have one or several turns, and their widths vary considerably since values from 190 to 1500 nm have been measured.
After t = 6.8 h (Fig. 4c), helical ribbons, tubules, and rolled sheets coexist. A high proportion of helical ribbons are in an advanced state in their transformation into tubules and helical marks are still evident. As a consequence, the proportion of tubules has increased. Furthermore, the widths of the rolled sheets have decreased.
Similar comments apply to TEM images collected after one day (Fig. 4d). The increase of the proportion of tubules and the contraction of rolled sheets are remarkable. The diameter of this last structure is now in the range 200–300 nm. After 48 h, the percentages of tubules, helical ribbons and rolled sheets are >85%, <14%, and <1%, respectively.
All these observations deduced from TEM experiments are summarized in Fig. 5.
The total number N of structures considered in the recounts were 24 (t = 0.8 h), 861 (t = 3.3 h), 762 (t = 6.8 h), 413 (t = 24 h) and 357 (t = 48 h). After four days neither rolled sheets, nor helical ribbons were observed, but residual helical marks are visible in some tubules (marked with arrows in Fig. 4e). The marks have fully disappeared after eight days. As observed for other systems,7,11,13,57 many tubules are parallelly aligned (Fig. 4f).
The evolution of the observed percentages of fibers, helical ribbons and tubules (Fig. 5) suggests that helical ribbons are intermediates in the conversion of fibers into tubules according to a sequential mechanism F → HR → T. Fig. 6 shows a model picture of the proposed mechanism.
A recount of the 593 tubules at this final stage leads to the size distribution of Fig. 7, and its statistics analysis gives a mean value of ![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif) = 125 ± 21 nm for the diameter. This average diameter is twice the one (= 59.0 nm) determined for 5, the anionic naphthyl derivative of cholic acid.8
= 125 ± 21 nm for the diameter. This average diameter is twice the one (= 59.0 nm) determined for 5, the anionic naphthyl derivative of cholic acid.8
Fig. 8 shows the UV and CD spectra of a solution of napht-CCD (in the same experimental conditions as TEM experiments) collected along the transformation process. UV spectra show three main absorptions at around 195, 225, 290 nm, and a weaker band at 330 nm. In analyzing the spectrum, it is necessary to recall that the iodide ion shows absorption peaks at 193 and 226 nm with molar absorptivities of 1.42 × 104 and 1.34 × 104 l mol−1 cm−1, respectively.58 Therefore the spectrum is a superimposition of the iodide absorption peaks and those corresponding to the naphthylamide residue at 230, 280 and 330 nm which are also present in the spectra of solutions of compound 5.8 Although the UV spectra do not show salient changes with time, changes are observed in the CD patterns.
 | ||
| Fig. 8 Evolution with time of the CD (top) and vis-UV (bottom) spectra of a 4.98 × 10−3 mol dm−3 solution of napht-CCD in 0.100 mol dm−3 NaCl at 50 °C. | ||
The just prepared sample presents a CD profile mainly characterized by an intense signal between 210 and 330 nm that can be interpreted as a superimposition of a conservative Cotton effect related to the absorption band at 230 nm and other negative signals from 250 to 330 nm, and those corresponding to the lower wavelengths distort the negative Cotton component. After 7 hours, the Cotton band has considerably decreased its intensity and the negative signals are now concentrated in a broad band centered around 300 nm. Thus, the main chiral structure responsible of the Cotton pattern has decreased its concentration and, accordingly with TEM experimental results this structure is the fiber one.
The absence of isosbestic points is in accordance with the presence of the several kinds of superstructures demonstrated by TEM experiments. Moreover, the negative band around 300 nm, should be related to the tubular emergent architecture. From 1 to 3 days molecular tubes is the predominant structure, and the CD spectra are better defined, with minima at around 240, 300 and 330 nm.
The transformation of aggregates is much slower at 25 °C. At this temperature, the proportion of fibers along time is much higher than at 50 °C, while the proportions of helical ribbons and rolled sheets are lower. On the other hand, tubules start to appear after five days but, after two months, tubule is again the major structure, still coexisting with helical ribbons and some fibers. Since the mean diameter of the tubules is ![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif) = 107 ± 22 nm (number of tubules = 60), it is very probable that the final state for napht-CCD aggregates is the same to the one at 50 °C, reinforcing the stability of the tubule architecture.
= 107 ± 22 nm (number of tubules = 60), it is very probable that the final state for napht-CCD aggregates is the same to the one at 50 °C, reinforcing the stability of the tubule architecture.
Conclusions
This study has shown that molecular tube is the final architecture for the aggregates of this cationic derivative of cholic acid. With the exception of fusion of vesicles, all other mechanisms reported in the literature for the formation of tubules (through fibers, helical ribbons or rolling sheets), operate in this particular case. The fact that at the end of the process only tubes are present with a well-defined geometry is an indication of a high thermodynamic stability of the arrangement of the surfactant molecules in this supramolecular architecture.Acknowledgements
The authors thank the Ministerio de Ciencia y Tecnología, Spain (Project MAT2010-19440) for financial support. J. V. Trillo also thanks for a scholarship.Notes and references
- S. Mukhopadhyay and U. Maitra, Curr. Sci., 2004, 87, 1666–1683 CAS.
- A. Coello, F. Meijide, E. Rodríguez Núñez and J. Vázquez Tato, J. Pharm. Sci., 1996, 85, 9–15 CrossRef CAS PubMed.
- V. H. Soto Tellini, A. Jover, L. Galantini, N. V. Pavel, F. Meijide and J. Vázquez Tato, J. Phys. Chem. B, 2006, 110, 13679–13681 CrossRef CAS PubMed.
- V. H. Soto Tellini, A. Jover, F. Meijide, J. Vázquez Tato, L. Galantini and N. V. Pavel, Adv. Mater., 2007, 19, 1752–1756 CrossRef.
- L. Galantini, C. Leggio, A. Jover, F. Meijide, N. V. Pavel, V. H. Soto, J. Vázquez Tato, R. Di Leonardo and G. Ruocco, Soft Matter, 2009, 5, 3018–3025 RSC.
- F. Meijide, J. V. Trillo, S. de Frutos, L. Galantini, N. V. Pavel, V. H. Soto, A. Jover and J. Vázquez Tato, Steroids, 2012, 77, 1205–1211 CrossRef CAS PubMed.
- F. Meijide, A. Antelo, M. Alvarez, A. Jover, L. Galantini, N. V. Pavel and J. Vázquez Tato, Langmuir, 2010, 26, 7768–7773 CrossRef CAS PubMed.
- M. C. di Gregorio, N. V. Pavel, A. Jover, F. Meijide, J. Vázquez Tato, V. H. Soto, A. Alfaro, O. Regev, Y. Kasavi, K. Schillen and L. Galantini, Phys. Chem. Chem. Phys., 2013, 15, 7560–7566 RSC.
- N. Manghisi, L. Galantini, C. Leggio, A. Jover, F. Meijide, N. V. Pavel, V. H. Soto, J. Vázquez Tato and R. Agostino, Angew. Chem., Int. Ed., 2010, 49, 6604–6607 CrossRef CAS PubMed.
- B. Jean, L. Oss-Ronen, P. Terech and Y. Talmon, Adv. Mater., 2005, 17, 728–731 CrossRef CAS.
- P. Terech, A. De Geyer, B. Struth and Y. Talmon, Adv. Mater., 2002, 14, 495–498 CrossRef CAS.
- P. Terech and S. Friol, Macromol. Symp., 2006, 241, 95–102 CrossRef CAS.
- P. Terech, S. Friol, N. Sangeetha, Y. Talmon and U. Maitra, Rheol. Acta, 2006, 45, 435–443 CrossRef CAS.
- P. Terech, B. Jean and F. Ne, Adv. Mater., 2006, 18, 1571–1574 CrossRef CAS.
- S. Broderick, A. P. Davis and R. P. Williams, Tetrahedron Lett., 1998, 39, 6083–6086 CrossRef CAS.
- Q. Guan, C. Li, E. J. Schmidt, J. S. Boswell, J. P. Walsh, G. W. Allman and P. B. Savage, Org. Lett., 2000, 2, 2837–2840 CrossRef CAS PubMed.
- Nonappa and U. Maitra, Org. Biomol. Chem., 2008, 6, 657–669 CAS.
- Y. Li and J. R. Dias, Chem. Rev., 1997, 97, 283–304 CrossRef CAS PubMed.
- S. Marchetti, G. Onori and C. Cametti, J. Phys. Chem. B, 2005, 109, 3676–3680 CrossRef CAS PubMed.
- R. S. Dias, L. M. Magno, A. J. M. Valente, D. Das, P. K. Das, S. Maiti, M. G. Miguel and B. Lindman, J. Phys. Chem. B, 2008, 112, 14446–14452 CrossRef CAS PubMed.
- E. Grueso, C. Cerrillos, J. Hidalgo and P. Lopez-Cornejo, Langmuir, 2012, 28, 10968–10979 CrossRef CAS PubMed.
- P. Di Profio, R. Germani, L. Goracci, R. Grilli, G. Savelli and M. Tiecco, Langmuir, 2010, 26, 7885–7892 CrossRef CAS PubMed.
- B. Sohrabi, V. Khani, A. A. Moosavi-Movahedi and P. Moradi, Colloids Surf., B, 2013, 110, 29–35 CrossRef CAS PubMed.
- P. Misiak, K. A. Wilk, T. Kral, E. Wozniak, H. Pruchnik, R. Frackowiak, M. Hof and B. Rozycka-Roszak, Biophys. Chem., 2013, 180–181, 44–54 CrossRef CAS PubMed.
- P. B. Savage, Current Medicinal Chemistry -Anti-Infective Agents, 2002, 1, 293–304 CrossRef CAS.
- P. B. Savage, Eur. J. Org. Chem., 2002, 759–768 CrossRef CAS.
- K. S. Moore, S. Wehrli, H. Roder, M. Rogers, J. N. Forrest, Jr, D. McCrimmon and M. Zasloff, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 1354–1358 CrossRef CAS.
- N. M. Sangeetha, R. Balasubramanian, U. Maitra, S. Ghosh and A. R. Raju, Langmuir, 2002, 18, 7154–7157 CrossRef CAS.
- S. Bhat, D. Leikin-Gobbi, F. M. Konikoff and U. Maitra, Biochim. Biophys. Acta, Gen. Subj., 2006, 1760, 1489–1496 CrossRef CAS PubMed.
- S. Bhattacharya, U. Maitra, S. Mukhopadhyay and A. Srivastava, Molecular Gels. Materials with Self-Assembled Fibrillar Networks, ed. G. Weiss and P. Terech, Springer, 2006, ch. 17, pp. 613–647 Search PubMed.
- U. Maitra and A. Chakrabarty, Beilstein J. Org. Chem., 2011, 7, 304–309 CrossRef CAS PubMed.
- J. Hao and H. Hoffmann, Curr. Opin. Colloid Interface Sci., 2004, 9, 279–293 CrossRef CAS PubMed.
- T. Bramer, N. Dew and K. Edsman, J. Pharm. Pharmacol., 2008, 59, 1319–1334 CrossRef PubMed.
- K. Manna and A. K. Panda, Spectrochim. Acta, Part A, 2009, 74, 1268–1274 CrossRef PubMed.
- K. Manna, C.-H. Chang and A. K. Panda, Colloids Surf., A, 2012, 415, 10–21 CrossRef CAS PubMed.
- C. Liu, J. Cui, A. Song and J. Hao, Soft Matter, 2011, 7, 8952–8960 RSC.
- C. Liu, J. Hao and Z. Wu, J. Phys. Chem. B, 2010, 114, 9795–9804 CrossRef CAS PubMed.
- C. Liu and J. Hao, J. Phys. Chem. B, 2010, 114, 4477–4484 CrossRef CAS PubMed.
- L. Jiang, K. Wang, M. Deng and Y. Wang, Langmuir, 2008, 24, 4600–4606 CrossRef CAS PubMed.
- P. L. Anelli, L. Lattuada and F. Uggeri, Synth. Commun., 1998, 28, 109–117 CrossRef CAS.
- J. Vázquez Tato, V. H. Soto Tellini, J. V. Trillo Novo, M. Alvarez, A. Antelo Queijo, J. Carrazana García, A. Jover Ramos and F. Meijide del Río, Spain, ES2296463A1, 2005.
- P. Babu and U. Maitra, Steroids, 2005, 70, 681–689 CrossRef CAS PubMed.
- N. M. Sangeetha, S. Bhat, U. Maitra and P. Terech, J. Phys. Chem. B, 2004, 108, 16056–16063 CrossRef CAS.
- A. J. Prosser and E. I. Franses, Colloids Surf., A, 2001, 178, 1–40 CrossRef CAS.
- M. Swanson-Vethamuthu, M. Almgren, P. Hansson and J. Zhao, Langmuir, 1996, 12, 2186–2189 CrossRef CAS.
- D. M. Small, in The Bile Acids, Chemistry, Physiology, and Metabolism, ed. P. P. Nair and D. Kritchevski, Plenum Press, New York, 1971, ch. 8, pp. 249–356 Search PubMed.
- M. C. Carey, J. C. Montet, M. C. Phillips, M. J. Armstrong and N. A. Mazer, Biochemistry, 1981, 20, 3637–3648 CrossRef CAS.
- F. Meijide, J. V. Trillo, V. H. Soto, A. Jover and J. Vázquez Tato, Chirality, 2011, 23, 940–947 CrossRef CAS PubMed.
- P. Helfrich and E. Jakobsson, Biophys. J., 1990, 57, 1075–1084 CrossRef CAS.
- M. Keisuke and M. Yoshikiyo, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2002, 1580, 189–199 CrossRef.
- K. Matsuoka, M. Suzuki, C. Honda, K. Endo and Y. Moroi, Chem. Phys. Lipids, 2006, 139, 1–10 CrossRef CAS PubMed.
- S. Paula, W. Sues, J. Tuchtenhagen and A. Blume, J. Phys. Chem., 1995, 99, 11742–11751 CrossRef CAS.
- P. Garidel, A. Hildebrand, R. Neubert and A. Blume, Langmuir, 2000, 16, 5267–5275 CrossRef.
- M. Álvarez, A. Jover, F. Meijide, L. Galantini, N. V. Pavel, Á. Antelo and J. Vázquez Tato, Langmuir, 2009, 25, 9037–9044 CrossRef PubMed.
- Nonappa and U. Maitra, Soft Matter, 2007, 3, 1428–1433 RSC.
- Nonappa, M. Lahtinen, B. Behera, E. Kolehmainen and U. Maitra, Soft Matter, 2010, 6, 1748–1757 RSC.
- P. Terech, N. M. Sangeetha, S. Bhat, J.-J. Allegraud and E. Buhler, Soft Matter, 2006, 2, 517–522 RSC.
- Y.-j. Wei, C.-g. Liu and L.-p. Mo, Guangpuxue Yu Guangpu Fenxi, 2005, 25, 86–88 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47160h |
| This journal is © The Royal Society of Chemistry 2014 |