Synthesis of a dextran-based bone tracer for in vivo magnetic resonance and optical imaging by two orthogonal coupling reactions†
Hiroshi Tanaka*a,
Sho Yamaguchia,
Jun-ichiro Job,
Ichio Aokib,
Yasuhiko Tabatac and
Takashi Takahashi*a
aDepartment of Applied Chemistry, Graduate School of Science and Engineering Tokyo Institute of Technology, 2-12-1-H-101 Ookayama, Meguro, Tokyo, 152-8552, Japan. E-mail: thiroshi@apc.titech.ac.jp; Fax: +81 357342884; Tel: +81 357342471
bMolecular Imaging Center, National Institute of Radiological Sciences, Anagawa 4-9-1, Inage, Chiba, 263–8555, Japan. E-mail: aoki@nirs.go.jp; Fax: +81432063276
cDepartment of Biomaterials, Institute for Frontier Medical Sciences, Kyoto University, 53 Shogoinkawaramachi, Sakyo-ku, Kyoto 606–8507, Japan. E-mail: yasuhiko@frontier.kyoto-u.ac.jp; Fax: +81-43-206-3276
First published on 14th November 2013
Abstract
Synthesis of dextran-based bone tracers for in vivo imaging from a dextran-template containing terminal acetylenes and amino groups though a diethylene glycol spacer is described. The tracer was used to visualize regenerated bone in a BMP-2 installed hydrogel by magnetic resonance and optical imaging.
Molecular imaging, such as magnetic resonance imaging (MRI) or in vivo optical imaging, is a promising technology, not only for the analysis of living cells, but also for diagnosis.1 In addition, this technology has the promise to strongly assist regenerative medicine by monitoring the regenerating process of target organs. Multimodal imaging tracers allow the same target to be evaluated with more than two different modalities and improve the efficiency of the monitoring process because no single modality is perfect and sufficient to obtain all the necessary information for a particular question.2 For example, in living subjects, it is very difficult to analyze data obtained using optical imaging using a near-infrared (NIR) fluorescent dye in deep tissues.3 On the other hand, MRI can be adapted to imaging tissues and organs in the human body with high resolution and contrast,4 although the sensitivity is lower than that for nuclear imaging such as PET5 and SPECT.6 Therefore, a combination of molecular imaging modalities can offer synergistic advantages over any single modality alone.7
Polysaccharides such as dextran, and pullulan can be used as biocompatible materials and are approved for clinical use and in food.8 The modification of the hydroxyl groups of dextran with various functionalities can be effective for the synthesis of biocompatible and functional materials such as DDS carriers and bioimaging agents.9 Tabata and co-workers recently reported on the synthesis of a dual-modal pullulan-based bone tracer by connecting pullulan with bisphosphonate (BP) as a ligand to bone and Cy5 and DTPA (diethylenetriamine-N,N,N′,N′′,N′′-pentaacetic acid) as a sensing device based on a CDI method that connects polysaccharides and amino derivatives through a carbamate linker.10 The tracer enabled the process of bone regeneration in a hydrogel to be visualized by magnetic resonance and optical imaging. In the case of the pullulan-based tracer, the BP density contributing to the binding affinity had a threshold (3.8%, based on hydroxyl groups). The synthesis of multifunctional imaging tracers requires the repeatable and suitable attachment of multiple functional molecules onto polysaccharides without any unexpected physical–chemical interference between the incorporated molecules. Herein we report on the synthesis of bone tracers using a dextran template possessing terminal acetylenes and amino groups for use in both fluorescence and MRI imaging.
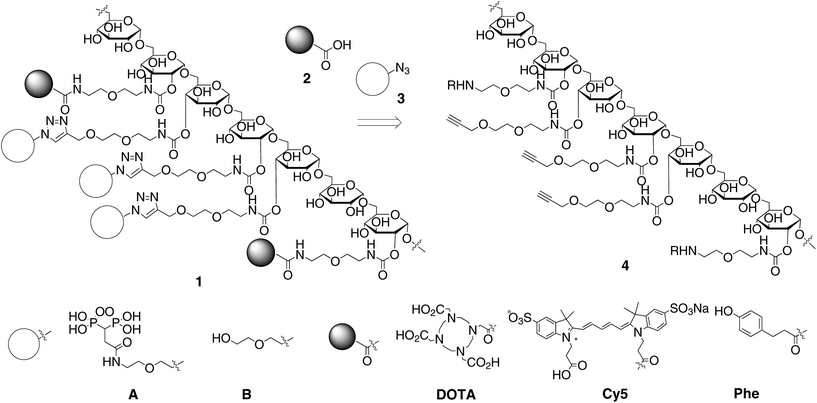
We designed the dextran derivatives 4 to possess amino groups and terminal acetylenes that are linked though a flexible and hydrophilic diethylene glycol spacer as a template for the synthesis of polysaccharide-based tracers (Scheme 1).11 The amino groups and terminal acetylenes can be independently coupled with the carboxylic acid 2 and azide derivative 3 via acylation and copper-catalyzed azide–acetylene coupling reactions, respectively.12 Installation of the carboxylic acids 2 and the azide derivatives 3 was tunable by appropriately adjusting the amount of the pre-installed amino groups and terminal acetylenes. Our objective was to synthesize the dextran-based bone tracer 1 so as to possess multiple BPs, Cy5, DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) and phenol from the dextran template 4. Multivalent interactions between the multiple BP units and hydroxyapatite at the bone surface would result in the tracer having a high affinity for bone. Cy5 is a near-infrared fluorescent probe for optical imaging.13 DOTA is used as a chelator of gadolinium ions and is used in MRI applications.14 Immobilizing the gadolinium complex on a macromolecule could effectively improve its sensitivity due to its enhanced relaxivity.15 The phenol was used as an acceptor for the highly sensitive radioactive iodonium. Radiolabeling of the tracer candidates permits the biological distribution to be determined during their structural optimization.
The synthesis of the dextran-based templates 4a–c from an acetal-modified dextran is shown in Scheme 2. We used dextran (5a–c) with varying molecular weights (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 15
000, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 6000) as a backbone since the biological distribution of a tracer largely depends on its molecular weight. According to a reported method,16 dextrans (5a–c) were treated with 2-methoxypropene in the presence of anhydrous pyridinium p-toluenesulfonate in DMSO at room temperature for 12 h, followed by precipitation of the resulting dextran from chloroform and hexane to provide the acetal-modified dextrans 6a–c. The percentages of the remaining hydroxyl groups of 6a–c, as determined by 1H NMR analysis of the acetylated products of 6a–c were 32%, 49% and 29% based on the hydroxyl groups of dextran. Treatment of the acetal-modified dextrans 6a–c with a mixture of the isocyanates 7 and 8 possessing an N-Boc protected amine and a terminal acetylene under basic conditions for 30 min, followed by removal of the acetal and N-Boc protecting groups under acidic conditions gave the dextran-based templates 4a–c. The percentages of the terminal acetylene and amino groups in 4a–c were estimated by 1H NMR analysis of the N-acetylated products 5a–c to be 18% and 9.3%, 26% and 7.8%, and 24% and 5% based on the hydroxyl groups in dextran, respectively. The dextran derivatives 4a–c were treated with a large excess of the azide derivatives 3A or 3B possessing a BP or a hydroxyl group with CuSO4 under basic conditions at room temperature for 48 h. An 1H NMR analysis of the coupling products indicated the disappearance of the terminal acetylene moieties and the generation of triazole units. Subsequent acylation of the amino groups with a mixture of the carboxylic acids 2 possessing Cy5, DOTA or phenol (Cy5
000 and 6000) as a backbone since the biological distribution of a tracer largely depends on its molecular weight. According to a reported method,16 dextrans (5a–c) were treated with 2-methoxypropene in the presence of anhydrous pyridinium p-toluenesulfonate in DMSO at room temperature for 12 h, followed by precipitation of the resulting dextran from chloroform and hexane to provide the acetal-modified dextrans 6a–c. The percentages of the remaining hydroxyl groups of 6a–c, as determined by 1H NMR analysis of the acetylated products of 6a–c were 32%, 49% and 29% based on the hydroxyl groups of dextran. Treatment of the acetal-modified dextrans 6a–c with a mixture of the isocyanates 7 and 8 possessing an N-Boc protected amine and a terminal acetylene under basic conditions for 30 min, followed by removal of the acetal and N-Boc protecting groups under acidic conditions gave the dextran-based templates 4a–c. The percentages of the terminal acetylene and amino groups in 4a–c were estimated by 1H NMR analysis of the N-acetylated products 5a–c to be 18% and 9.3%, 26% and 7.8%, and 24% and 5% based on the hydroxyl groups in dextran, respectively. The dextran derivatives 4a–c were treated with a large excess of the azide derivatives 3A or 3B possessing a BP or a hydroxyl group with CuSO4 under basic conditions at room temperature for 48 h. An 1H NMR analysis of the coupling products indicated the disappearance of the terminal acetylene moieties and the generation of triazole units. Subsequent acylation of the amino groups with a mixture of the carboxylic acids 2 possessing Cy5, DOTA or phenol (Cy5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOTA
DOTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phenol = 2
phenol = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by using DMT-MM17 resulted in the formation of the dextran-based tracers 1a–cA and 1a–cB. A Kaiser test of the resulting products indicated complete acylation of the amines. The ratio of the carboxylic acids was determined based on their sensitivity.
1) by using DMT-MM17 resulted in the formation of the dextran-based tracers 1a–cA and 1a–cB. A Kaiser test of the resulting products indicated complete acylation of the amines. The ratio of the carboxylic acids was determined based on their sensitivity.
We first examined the binding of the tracers to hydroxyapatite as a model for bone (Fig. 1).18 The hydroxyl derivatives 1a–cB were used as controls for the imaging. The tracers 1a–cA and 1aB (1.00 mg) were mixed with hydroxyapatite (5, 10 and 50 mg) in saline (2.0 mL) at room temperature. After 30 min, the amounts of 1a–cA and 1aB in the supernatants were estimated based on UV absorption. The binding ability of the tracers is dependent on the BP density. The molecular weight of the dextran used did not strongly affect the binding affinity of the tracers 1aA, 1bA and 1cA to hydroxyapatite. On the other hand, most of the non-BP tracer 1aB remained in solution after treatment with 50 mg of hydroxyapatite. These results clearly indicated that the installed BP units promoted selective adhesion of the tracer to hydroxyapatite.
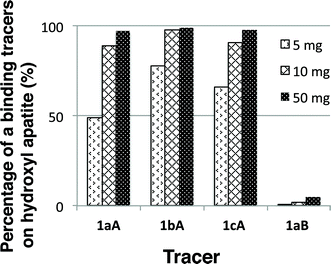 | ||
| Fig. 1 Percentage of the binding of tracers 1a–cA and 1aB to hydroxyapatite beads (5, 10 and 50 mg) (n = 3). | ||
We next examined the biological distribution of the 125I labeled bone tracers prepared using 1aA, 1bA and 1cA and 1a–cB in mice in which the BMP-2 containing hydrogel and the non-MBP-2 control hydrogel had been independently implanted. The dextran derivatives 1a–cA and 1a–cB were labeled with radioactive iodine by treatment with [125I]NaI and chloramine-T at room temperature for 2 min, followed by purification using a disposable gel filtration column (PD-10) to provide the 125I labeled tracers [125I]1a–cA and [125I]1a–cB.19 A solution of each tracer (20 μg) in potassium phosphate buffer (100 μL) was intravenously injected into the mice. No significant side effects were observed in the mice after the injection. The biological distribution of the 125I labeled tracers [125I]1a–cA and [125I]1a–cB on the hydrogels after 6 h was then estimated. Fig. 2 shows the accumulation ratios of tracers [125I]1a–cA and [125I]1a–cB on BMP-2 (+) and (−) hydrogels. The accumulation of the controlled tracers [125I]1a–cB on the implanted hydrogel were comparable. On the other hand, higher levels of the BP-installed tracers [125I]1a–cA accumulated on the BMP-2 containing hydrogel than on the non-BMP-2 hydrogel. In particular, a significant difference between the BP tracers (MW = 6000) [125I]1a–cA and the BP-tracers [125I]1aA, [125I]1bA with a higher molecular weight and its control tracers [125I]1cB was observed. Based on these results, the BP-tracer 1cA was used in further imaging studies.
 | ||
| Fig. 2 Accumulation of tracers [125I]1a–cA and [125I]1a–cB on the BMP-2 containing hydrogel and the control hydrogel. | ||
The dual sensing of the BMP-2 containing hydrogel in mice by magnetic resonance and optical imaging using the tracer 1cA was examined. The relative fluorescence intensity at 665 nm of tracer 1cA based on free Cy5 was measured by irradiation at 660 nm to be 65%. The BP-tracer 1cA (1 mg) was treated with GdCl3 (0.28 mg) in 0.1 M MES buffer at room temperate for 3 h, followed by purification on a disposable gel filtration column (PD-10). The solution was concentrated by freeze-drying to yield the BP-tracer [Gd3+]–1cA containing Gd(III) ions (Gd3+ 0.980 mmol g−1). The Gd-containing BP-tracer [Gd3+]–1cA (2.0 mg in 100 μL) was administered to the mice 3 weeks after the implantation of the hydrogel in the left leg. No significant side effects were observed in the mice. We first examined the in vivo optical imaging of the BMP-2 containing hydrogel in mice (Fig. 3). Accumulation of the tracer [Gd3+]–1cA was clearly observed in the left leg where the BMP-2 containing hydrogel was implanted from 1 h to 6 h after administration.
 | ||
| Fig. 3 Optical imaging of the BMP-2 containing hydrogel in mice with the dextran-based tracer [Gd3+]–1cA. | ||
We next examined the in vivo MR imaging of the hydrogel in which BMP-2 was incorporated with the Gd-containing BP-tracer [Gd3+]–1cA (Fig. 4). Gd-DTPA was used as the control tracer. The Gd-containing BP-tracer [Gd3+]–1cA and DTPA-Gd (2.0 mg) in potassium phosphate buffer (100 μL) were intravenously administered to the same mice. The Gd-containing BP-tracer [Gd3+]–1cA enhanced the outside of the BMP-containing hydrogel. These results indicate that bone regeneration in the BMP-2 hydrogel occurs on the outside of the gel. Fig. 4B shows the signal ratio of hydrogel based on the muscle tissue. It is noteworthy that the degree of enhancement by [Gd3+]–1cA was maintained for over 6 hours. On the other hand, no selective accumulation of DTPA–Gd in the hydrogel was observed. These results indicate that the bisphosphonates acted as an anchor for the regenerating bone.
We next performed microscopic imaging of subcutaneous tissue sections taken from around the implanted hydrogel incorporating BMP-2. The tissues were taken at 6 hours after the intravenous injection of the BP-tracer 1cA and the control tracer 1cB from the mice 4 weeks after implantation of the hydrogel. Fig. 5 shows the fluorescence imaging of tissue sections (A and D) and sections stained with Hematoxylin and Eosin (H&E) stain (B and E), and von Kossa stain (C and F).20 The fluorescent-labeled area for 1cA was stained by the H&E stain method. On the other hand, fluorescence of the control tracer 1cB was not observed in the H&E staining area. These results support the conclusion that the tracer 1cA can visualize bone regeneration by MRI and optical imaging modalities.
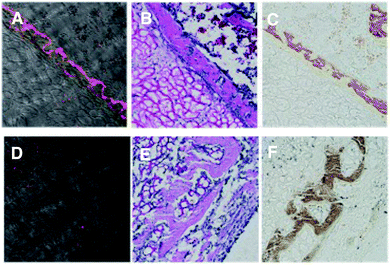 | ||
| Fig. 5 Microscopic images of subcutaneous tissue sections around the hydrogel incorporating implanted BMP-2. A, B, and C for 1cA. D, E and F for 1cB. | ||
Conclusions
In conclusion, we report on the synthesis of dextran-based bone tracers for in vivo magnetic resonance and optical imaging from a dextran-template containing terminal acetylenes and amino groups though a diethylene glycol spacer. The two functional groups chemoselectively reacted with the BP connected azide derivatives and the Cy5, DOTA or carboxylic acids connected via phenols, respectively. The affinity of the tracer for hydroxyapatite is largely dependent on the type of installed BP units. Limitations associated with the installed BP units are dependent on the solubility of the resulting tracers. Biological distribution of the tracers in mice implanted with the BMP-2 (+) and (−) installed hydrogel revealed that the tracer 1cA prepared from dextran (MW = 6000) effectively accumulated in the BMP-2 installed hydrogel. The corresponding Gd-containing BP-tracer [Gd3+]–1cA enabled the dual sensing of the BMP-2 containing hydrogel in mice by magnetic resonance and optical imaging.Notes and references
- V. Marx, Chem. Eng. News, 2005, 83(30), 25 Search PubMed.
- T. F. Massoud and S. S. Gambhir, Genes Dev., 2003, 17, 545 CrossRef CAS PubMed.
- J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626–634 CrossRef CAS PubMed.
- (a) R. B. Lauffer, Chem. Rev., 1987, 87, 901 CrossRef CAS; (b) P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293 CrossRef CAS PubMed; (c) V. Jacques and J. F. Desreux, Top. Curr. Chem., 2002, 221, 125 CrossRef; (d) H. Tanaka, Y. Ando, M. Wada and T. Takahashi, Org. Biomol. Chem., 2005, 3, 3311 RSC.
- M. M. Alauddin, Am. J. Nucl. Med. Mol. Imaging, 2012, 2, 55–76 CAS.
- (a) M. Jueptner and C. Weiller, Neuroimage, 1995, 2, 148 CrossRef CAS; (b) H. Tanaka, Y. Ando, T. Abe and T. Takahashi, Chem.–Asian J., 2008, 3, 2033 CrossRef CAS PubMed.
- (a) K. Chtourou, M. Maloul, F. Kallel, S. Charfedine, F. Hamza and F. Guermazi, Eur. J. Nucl. Med. Mol. Imaging, 2008, 35, 2343 CrossRef PubMed; (b) V. Ntziachristos, A. G. Yodh, M. Schnall and B. Chance, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 2767 CrossRef CAS PubMed; (c) T. Beyer, D. W. Townsend, T. Burn, P. E. Kinahan, M. Charron, R. Roddy, J. Jerin, J. Young, L. Byars and R. Nutt, J. Nucl. Med., 2000, 41, 1369 CAS; (d) O. Veiseh, C. Sun, J. Gunn, N. Kohler, P. Gabikian, D. Lee, N. Bhattarai, R. Ellenbogen, R. Sze, A. Hallahan, J. Olson and M. Zhang, Nano Lett., 2005, 5, 1003–1008 CrossRef CAS PubMed.
- (a) R. Duncan, Nat. Rev. Drug Discovery, 2003, 2, 347 CrossRef CAS PubMed; (b) P. A. Vasey, S. B. Kaye, R. Morrison, C. Twelves, P. Wilson, R. Duncan, A. H. Thomson, L. S. Murray, T. E. Hilditch, T. Murray, S. Burtles, D. Fraier, E. Frigerio and J. Cassidy, Cancer Research Campaign Phase I/II Committee, Clin. Cancer Res., 1999, 5, 83 CAS.
- (a) H. Hosseinkhani, T. Aoyama, O. Ogawa and Y. Tabata, J. Controlled Release, 2003, 88, 297 CrossRef CAS; (b) C. Schatz, S. Louguet, J. L. Meins and S. Lecommandoux, Angew. Chem., Int. Ed., 2009, 48, 2572 CrossRef CAS PubMed; (c) S. C. Abeylath and M. M. Amiji, Bioorg. Med. Chem., 2011, 19, 6167 CrossRef CAS PubMed.
- (a) J. Liu, J. Jo, Y. Kawai, I. Aoki, C. Tanaka, M. Yamamoto and Y. Tabata, J. Controlled Rel., 2012, 157, 398 CrossRef CAS PubMed; (b) G. T. Hermanson, Bioconjugate Techniques, Academic Press, 1996 Search PubMed.
- We first synthesized a dextran derivative possessing propargyl ethers by treatment of the partially protected dextran 8 with propargy bromide, followed by removal of the acetal protecting groups. However, the solubility of the products largely depended on the exhibited low solubility in water due to the hydrophobicity of the incorporated alkenyl units.
- (a) R. Huisgen, Angew. Chem., Int. Ed., 1963, 2, 565 CrossRef; (b) F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Shrpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210 CrossRef CAS PubMed.
- (a) P. L. Southwick, Cytochemistry, 1990, 11, 418 CAS; (b) M. W. Wessendorf, Histochemistry, 1992, 98, 81 CrossRef CAS; (c) R. B. Mujumdar, L. A. Ernst, S. R. Mujumdar, C. J. Lewis and A. S. Waggoner, Bioconjugate Chem., 1993, 4, 105–111 CrossRef CAS.
- N. Viola-Villegas and R. P. Doyle, Coord. Chem. Rev., 2009, 253, 1906 CrossRef CAS PubMed.
- (a) E. M. Bachelder, T. T. Beaudette, J. Dashe and J. Fre'chet, J. Am. Chem. Soc., 2008, 130, 10494 CrossRef CAS PubMed; (b) T. T. Beaudette, J. A. Cohen, E. M. Bachelder, K. E. Broaders, J. L. Cohen, E. G. Engleman and J. M. J. Fre'chet, J. Am. Chem. Soc., 2009, 131, 10360 CrossRef CAS PubMed; (c) J. A. Cohen, T. T. Beaudette, J. L. Cohen, K. E. Broaders, E. M. Bachelder and J. M. J. Fre'chet, Adv. Mater., 2010, 22, 3593 CrossRef CAS PubMed.
- G. Adam, J. Neuerburg, E. Spüntrup, A. Mühler, K. S. V. Surg and R. W. Günther, Chin. J. Magn. Reson. Imaging, 1994, 4, 462 CAS.
- M. Kunishima, C. Kawachi, K. Hioki, K. Terao and S. Tani, Tetrahedron, 2001, 57, 1551 CrossRef CAS.
- (a) H. Uludag, N. Kousinioris, T. Gao and D. Kantoci, Biotechnol. Prog., 2000, 16, 258 CrossRef CAS PubMed; (b) M. F. Jarvis, C. J. Burns, H. W. Pauls, A. Assal, J. S. Kim, D. L. Cheney and R. D. Youssefyeh, Calcif. Tissue Int., 1993, 52, 372 CrossRef CAS.
- D. S. Wilbur, Bioconjugate Chem., 1992, 3, 433–470 CrossRef CAS.
- R. Drury and E. Wallington, Carleton’s Histological Techniques, Oxford University Press, 1980 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46142d |
| This journal is © The Royal Society of Chemistry 2014 |