Design and synthesis of ERα/ERβ selective coumarin and chromene derivatives as potential anti-breast cancer and anti-osteoporotic agents†
Abstract
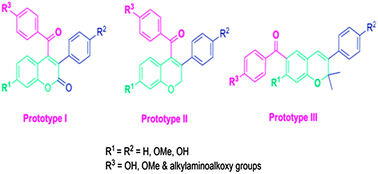
Several new coumarin and chromene prototype derivatives have been synthesised and evaluated for their ERα and ERβ selective activity. Coumarin prototype compounds 18 & 19 were found to be ERα selective and the most active, exhibiting potential antiproliferative activity against both ER +ve & ER −ve breast cancer cell lines. The surprise finding of the series, however, are the novel prototype III chromenes 45 & 46, with aroyl substitution at the 6th position. Both the compounds have shown potent antiproliferative activity against both the breast cancer cell lines, promote alkaline phosphatase activity, enhance osteoblast mineralization in vitro, significantly decrease ERE–ERα dependent transactivation and induce ERβ activity. This specific upregulation of ERβ isoform activity of compound 45 may be responsible for the antiosteoporotic activity at picomolar concentration. In addition, both the compounds were also devoid of any estrogenic activity, which correlates to their antiestrogenic behaviour in the two breast cancer cell lines. Assessment of selectivity using specific SiRNAs for ERα and ERβ revealed that most of the compounds showed ERα and ERβ-mediated action, except compound 28, which showed selectivity to ERα only. Computational docking analysis of active compounds 18 and 45 was conducted to correlate the interaction with the two receptors and it was found that the docked conformations of the coumarin prototype, compound 18 at ERα and ERβ active sites were more or less superimposable on each other. However, the unique orientation of the aminoalkoxy side chain of novel chromene (prototype III) compound 45 in the ERβ binding cavity may be responsible for its potential biological response.

 Please wait while we load your content...
Please wait while we load your content...