One-step synthesis of reactant-product-dual-template imprinted capsules as phosphotriesterase mimetic enzymes for pesticide elimination†
Yong Guo,
Ruiyu Wang,
Wenhao Chi,
Shuai Liu,
Heguang Shi and
Tianying Guo*
The Key Laboratory of Functional Polymer Materials (Ministry of Education), Institute of Polymer Chemistry, College of Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin, 300071, P. R. China. E-mail: tyguo@nankai.edu.cn
First published on 4th December 2013
Abstract
A capsule with catalysis activity for pesticides degradation as well as adsorption activity for corresponding product elimination was developed by a simple one-step polymerization method based on molecular imprinting technology. More importantly, the absorption behavior of the capsule was investigated in detail by an in situ method, which showed that the capsule could absorb product more quickly.
In nature, enzymes are biomacromolecules that catalyze chemical reactions with remarkable catalytic activity and specific selectivity under very mild conditions.1 The design and synthesis of catalytic systems that are able to simulate natural enzyme activity whilst overcoming some of its inherent drawbacks is a challenge that has interested scientists for many years.2 Among the different approaches for mimicking enzymes, molecular imprinted polymers (MIP) have shown considerable potential and led to significant results due to their better chemical stability, thermal stability and solvent resistance compared with natural counterparts. MIP are generally cross-linked polymers synthesized in the presence of the target compound (the template), and their application to catalytic fields as enzyme mimics was pioneered by Wulff.3 With a transition-state analogue (TSA) as the template, numerous imprinted MIP microgels for ester hydrolysis with impressive catalytic activity have been synthesized by Wulff and by Resmini et al.4 Despite the tremendous progress made in this area, many challenges still remain to be addressed. For example, some substrate-like pesticides, after undergoing hydrolysis, produce catalytic products that are also toxic. Thus, the development of MIP with high catalytic activity as well as absorption ability for the product generated in the medium is desirable. In our previous work,5 our group developed an efficient “reactant-product-dual-template imprinting capsule” strategy for MIP fabrication. The resultant MIP capsule mimicked natural phosphotriesterase for paraoxon hydrolysis. The results revealed the existence of a cavity, and the dual-template imprinting had a positive influence on the MIP's catalytic activity. More importantly, although the catalysis activity of the synthesized MIP capsule is still relatively low compared to the natural phosphotriesterase, it could absorb a certain amount of p-nitrophenol (the hydrolytic product of paraoxon), which showed the potential of the synthesized MIP for product absorption, which is difficult to achieve with natural phosphotriesterase.6 However, the kinetic uptake of p-nitrophenol in the paraoxon catalysis hydrolysis system is unclear at present. Therefore, the adsorption behavior of the dual-template imprinting capsule to p-nitrophenol was investigated in detail here by an in situ method, and the influence of the cavity's size on the catalytic activity for paraoxon hydrolysis and p-nitrophenol absorption ability was also discussed. In addition, the preparation of the dual-templates imprinting capsule was complicated in the previous work,5 which involved polymerization in two steps. This is not only a waste of time but also a waste of materials. So a one-step polymerization method for the synthesis of a dual-template imprinting capsule was developed here, and its catalytic activity was tested and compared with the one synthesized by a two-step polymerization method.
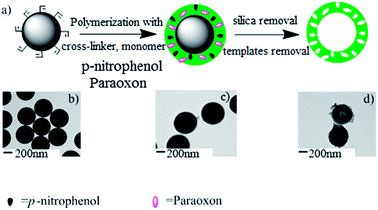
The simple procedure for synthesizing a dual-template imprinted capsule by a one-step polymerization method was described in Fig. 1. Using a vinyl-bearing silica microsphere (v-SiO2) as the seed, zinc dimethacrylate (MAA-Zn) as the functional monomer, divinylbenzene (DVB) as the cross-linking agent, and paraoxon and p-nitrophenol as the co-templates, the core–shell microsphere was prepared first, by precipitation polymerization. After removing the silica seed with hydrofluoric acid (HF), a hollow microsphere was obtained. Fig. 1(b–d) corresponds to the v-SiO2, the MIP core–shell microsphere and the MIP capsule respectively. The TEM result showed that the capsule was synthesized.
Firstly, the influence of different ratios of templates on the catalytic activity of the synthesized capsules was investigated. For this purpose, mole ratios of p-nitrophenol to paraoxon of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 8
1 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were chosen to synthesize the dual-template imprinted capsules, whilst we kept the other experiment conditions constant. The synthesized dual-template imprinted capsules were defined as H-V400P1, H-V400P2, H-V400P4 and H-V400P8 respectively. Then the dynamic curves of paraoxon hydrolysis catalyzed by these capsules were characterized by UV-Vis spectroscopy, as shown in Fig. 2. It can be seen that with the increase of the mole ratio of p-nitrophenol to paraoxon, the catalysis activity of the synthesized capsule was first increased, then decreased. When the mole ratio was 4
1 were chosen to synthesize the dual-template imprinted capsules, whilst we kept the other experiment conditions constant. The synthesized dual-template imprinted capsules were defined as H-V400P1, H-V400P2, H-V400P4 and H-V400P8 respectively. Then the dynamic curves of paraoxon hydrolysis catalyzed by these capsules were characterized by UV-Vis spectroscopy, as shown in Fig. 2. It can be seen that with the increase of the mole ratio of p-nitrophenol to paraoxon, the catalysis activity of the synthesized capsule was first increased, then decreased. When the mole ratio was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, it resulted in the highest catalysis activity. Our previous study5 showed that the dual-template imprinted MIP core–shell microspheres had better catalysis activity than the mono-template imprinted MIP core–shell microspheres, no matter whether the template was p-nitrophenol or paraoxon, which could be credited to the dual-template synergistic effect. In this situation, when the mole ratio was 4
1, it resulted in the highest catalysis activity. Our previous study5 showed that the dual-template imprinted MIP core–shell microspheres had better catalysis activity than the mono-template imprinted MIP core–shell microspheres, no matter whether the template was p-nitrophenol or paraoxon, which could be credited to the dual-template synergistic effect. In this situation, when the mole ratio was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the best dual-template synergistic effect occurred. The capsule synthesized at 4
1, the best dual-template synergistic effect occurred. The capsule synthesized at 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed the best catalysis activity towards paraoxon, and the value of the initial reaction rate (k) was 56.3 × 10−2 μM min−1. This is comparable with the catalysis activity of a dual-template imprinted capsule prepared using two-step polymerization, the k value of which is 59.6 × 10−2 μM min−1.5 The paraoxon degradation and p-nitrophenol elimination mechanism is also similar to the two steps of the polymerization strategy.5 The improved catalysis efficiency could be due to the synergistic effect of the catalysis sites of the reactant and adsorption sites of the product: paraoxon-imprinted sites could catalyze the hydrolysis of paraoxon, which generated p-nitrophenol in the medium; some of the p-nitrophenol could be adsorbed in the p-nitrophenol-imprinted sites and enriched in the void, which would result in the decrease of p-nitrophenol concentration; and the decrease of p-nitrophenol concentration in the medium would further prompt the hydrolysis of paraoxon. Therefore, paraoxon is degraded and p-nitrophenol is eliminated simultaneously.
1 showed the best catalysis activity towards paraoxon, and the value of the initial reaction rate (k) was 56.3 × 10−2 μM min−1. This is comparable with the catalysis activity of a dual-template imprinted capsule prepared using two-step polymerization, the k value of which is 59.6 × 10−2 μM min−1.5 The paraoxon degradation and p-nitrophenol elimination mechanism is also similar to the two steps of the polymerization strategy.5 The improved catalysis efficiency could be due to the synergistic effect of the catalysis sites of the reactant and adsorption sites of the product: paraoxon-imprinted sites could catalyze the hydrolysis of paraoxon, which generated p-nitrophenol in the medium; some of the p-nitrophenol could be adsorbed in the p-nitrophenol-imprinted sites and enriched in the void, which would result in the decrease of p-nitrophenol concentration; and the decrease of p-nitrophenol concentration in the medium would further prompt the hydrolysis of paraoxon. Therefore, paraoxon is degraded and p-nitrophenol is eliminated simultaneously.
 | ||
| Fig. 2 The hydrolytic kinetic curves of paraoxon catalyzed by H-V400P1, H-V400P2, H-V400P4 and H-V400P8. | ||
As mentioned before, not only the hydrolysis of paraoxon with remarkable catalytic activity, but also the effective absorption of p-nitrophenol by the synthesized capsule was expected. Here, the absorption ability of the capsule was tested and compared with the corresponding core–shell microsphere by an in situ method. Generally, the paraoxon concentration in the solution was first measured in different intervals, then the initial paraoxon concentration was deducted. Thus, the consumed paraoxon concentration at different intervals was calculated, which was equal in theory to the generated p-nitrophenol concentration in the solution at the same interval (Scheme S1 ESI†). Meanwhile, the p-nitrophenol concentration in the solution was also measured at the same time, which was the practical p-nitrophenol concentration generated in the solution. The discrepancy between the theoretical p-nitrophenol concentration and the practical p-nitrophenol concentration was the amount of p-nitrophenol that was adsorbed by the catalyst. Therefore, taking H-V400P4 and its corresponding core–shell microsphere V400P4 as an example, the dynamic curves of the theoretical p-nitrophenol concentration and the practical p-nitrophenol concentration of H-V400P4 and V400P4 respectively could be drawn, as shown in Fig. 3. The k value for paraoxon hydrolysis catalyzed by H-V400P4 and V400P4 could be calculated from the dynamic curves of the theoretical p-nitrophenol concentration that occurred when using them, and the values were 56.3 × 10−2 μM min−1 and 8.14 × 10−2 μM min−1 respectively, which is consistent with our previous results5. Indeed, the existence of a cavity had a positive influence on the MIP's catalytic activity. More importantly, it can be seen clearly from Fig. 3 that at the initial stage of catalysis reactivity, the discrepancy between the theoretical p-nitrophenol concentration and the practical p-nitrophenol concentration was low, no matter whether the catalyst used was H-V400P4 or V400P4. This can be explained by the fact that few p-nitrophenol molecules were produced at the initial stage, and few of them were adsorbed by the catalyst. As the catalysis reaction advanced, more p-nitrophenol was produced and more was adsorbed by the catalyst. As the H-V400P4 has higher catalytic activity for paraoxon hydrolysis than V400P4, it produced more p-nitrophenol in the solution in the same time. In our previous work,5 at the same p-nitrophenol concentration, the capsule had a higher adsorption ability than the corresponding core–shell catalyst. So in this catalysis system, H-V400P4 could adsorb more p-nitrophenol than V400P4 in the same time; therefore, the discrepancy between the theoretical p-nitrophenol concentration and the practical p-nitrophenol concentration for H-V400P4 was bigger than the one for V400P4, as shown in Fig. 3. This meant that the former had a higher absorption capacity and faster adsorption velocity for p-nitrophenol than the latter. In a word, the results of the absorption behavior of the capsule and corresponding core–shell microsphere for p-nitrophenol by an in situ method showed that not only the absorption capacity but also the absorption velocity of the capsule H-V400P4 was better than that of the corresponding core–shell microsphere V400P4. These results indicated that the introduction of a cavity could improve the absorption ability of MIP.
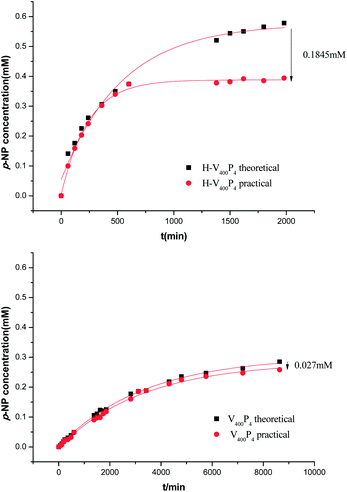 | ||
| Fig. 3 The kinetic adsorption of p-nitrophenol by H-V400P4 and V400P4. The quantities of H-V400P4 and V400P4 used were 0.01 g and 0.015 g respectively. | ||
Since the existence of the cavity could not only improve the MIP's catalysis activity for paraoxon hydrolysis, but also could promote the MIP's absorption ability for p-nitrophenol elimination, in this section the influence of different cavities’ size on the MIP's catalysis activity and absorption ability was investigated. Here two different silica microspheres with sizes of about 200 nm and 400 nm were used as core precursors, and the dual-template imprinted capsules were synthesized and defined as H-V200P4 and H-V400P4 respectively. Fig. 4 presents the time course of paraoxon hydrolysis catalyzed by H-V200P4 and H-V400P4. The values of k for paraoxon hydrolysis catalyzed by H-V200P4 and H-V400P4 are 120.13 × 10−2 μM min−1 and 56.3 × 10−2 μM min−1 respectively. As seen in Fig. 4, the catalysis activity for paraoxon hydrolysis of H-V200P4 was higher than that of H-V400P4.
 | ||
| Fig. 4 The hydrolytic kinetic curves of paraoxon catalyzed by H-V200P4 and H-V400P4. The quantity of H-V400P4 and H-V200P4 used was 0.01 g. | ||
In addition, Fig. 5 presents the time course of generated p-nitrophenol in theory and in practice, catalyzed by H-V200P4 and H-V400P4 respectively. As seen in Fig. 5, the discrepancy between the theoretical and practical p-nitrophenol concentrations of H-V200P4 was much higher than the one of H-V400P4 in the same time, especially during the late reaction stage. This meant that H-V200P4 could adsorb more p-nitrophenol, with a quicker absorption velocity, than H-V400P4. The results of Fig. 4 and 5 showed that the H-V200P4 had better catalysis activity for paraoxon hydrolysis and better absorption ability for p-nitrophenol elimination than that of H-V400P4. These results could be credited to the fact that H-V200P4 had a larger specific surface area for catalysis and absorption than H-V400P4.
 | ||
| Fig. 5 The kinetic adsorption of p-nitrophenol by H-V200P4 and H-V400P4. The quantity of H-V400P4 and H-V200P4 used was 0.01 g. | ||
It is of great interest to determine if the prepared capsule also shows other typical enzyme characteristics. Therefore, the kinetics of systems with strongly varying substrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratios were measured. Hydrolysis reactions with H-V400P4 and H-V200P4 all revealed typical Michaelis–Menten kinetics with saturation kinetics. In Michaelis–Menten kinetics, Km reflects the affinity of an enzyme for a particular substrate (the lower the value of Km the higher the affinity). The double-reciprocal Lineweaver–Burk plot, as shown in Fig. 6, indicated a strong substrate bonding, with a Michaelis constant of Km = 4.31 mM for H-V400P4 and Km = 2.91 mM for H-V200P4. This means that H-V200P4 has better affinity for paraoxon than H-V400P4.
catalyst ratios were measured. Hydrolysis reactions with H-V400P4 and H-V200P4 all revealed typical Michaelis–Menten kinetics with saturation kinetics. In Michaelis–Menten kinetics, Km reflects the affinity of an enzyme for a particular substrate (the lower the value of Km the higher the affinity). The double-reciprocal Lineweaver–Burk plot, as shown in Fig. 6, indicated a strong substrate bonding, with a Michaelis constant of Km = 4.31 mM for H-V400P4 and Km = 2.91 mM for H-V200P4. This means that H-V200P4 has better affinity for paraoxon than H-V400P4.
 | ||
| Fig. 6 A Lineweaver–Burk plot of kinetics data of hydrolysis for paraoxon catalyzed by H-V200P4 and H-V400P4. | ||
In summary, we developed here a simple one-step imprinting polymerization method for synthesizing multifunctional enzyme-mimetic nano-capsule MIP. The results showed that the catalysis activity for paraoxon degradation is comparable with the the activity of the one synthesized by a two-step polymerization method. More importantly, the dynamic absorption behavior towards p-nitrophenol in the catalysis system of the capsules was investigated, and the results showed that the capsule had better absorption capacity and absorption velocity for p-nitrophenol. In addition, the results also showed that the capsule with a smaller cavity (using 200 nm in diameter of silica as precursor) had a better catalysis activity for paraoxon hydrolysis and absorption ability for p-nitrophenol elimination than the one with a larger cavity (using 400 nm in diameter of silica as precursor). This novel strategy can be used to more easily synthesize more effective catalysts which have potential applications in environmental fields and, particularly, in herbicide and insecticide degradation.
Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (50978138), PCSIRT (IRT 1257) and NFFTBS (no. J1103306).Notes and references
- S. D. Aubert, Y. Li and F. M. Raushel, Biochemistry, 2004, 43, 5707–5715 CrossRef CAS PubMed.
- Y. Yin, Z. Dong, Q. Luo and J. Liu, Prog. Polym. Sci., 2012, 37, 1476–1509 CrossRef CAS PubMed; Y. Chen, H. Cao, W. Shi, H. Liu and Y. Huang, Chem. Commun., 2013, 49, 5013–5015 RSC; C. Hou, J. Li, L. Zhao, W. Zhang, Q. Luo, Z. Dong, J. Xu and J. Liu, Angew. Chem., Int. Ed., 2013, 52, 5590–5593 CrossRef PubMed; Y. Li, B. M. Wang, Y. Luo, D. W. Yang, P. Tong, J. F. Zhao, L. Luo, Y. H. Zhou, S. Chen, F. Cheng and J. P. Qu, Nat. Chem., 2013, 5, 320–326 CrossRef PubMed; Y. Tao, Y. H. Lin, Z. Z. Huang, J. S. Ren and X. G. Qu, Adv. Mater., 2013, 25, 2594–2599 CrossRef PubMed.
- G. Wulff, A. Sarhan and K. Zabrocki, Tetrahedron Lett., 1973, 14, 4329–4332 CrossRef.
- K. J. Shea, E. A. Thompson, S. D. Pandey and P. S. Beauchamp, J. Am. Chem. Soc., 1980, 102, 3149–3155 CrossRef CAS; G. Wulff, T. Gross and R. Schönfeld, Angew. Chem., Int. Ed., 1997, 36, 1962–1964 CrossRef; J. Q. Liu and G. Wulff, Angew. Chem., Int. Ed., 2004, 43, 1287–1290 CrossRef PubMed; J. Q. Liu and G. Wulff, J. Am. Chem. Soc., 2004, 126, 7452–7453 CrossRef PubMed; A. Servant, K. Haupt and M. Resmini, Chem.–Eur. J., 2011, 17, 11052–11059 CrossRef PubMed.
- Y. Guo and T. Y. Guo, Chem. Commun., 2013, 49, 1073–1075 RSC.
- D. P. Dumas, S. R. Caldwell, J. R. Wild and F. M. Raushel, J. Biol. Chem., 1989, 264, 19659–19665 Search PubMed; G. A. Omburo, J. M. Kuo, L. S. Mullins and F. M. Raushel, J. Biol. Chem., 1992, 267, 13278–13283 Search PubMed; S. D. Aubert, Y. Li and F. M. Raushel, Biochemistry, 2004, 43, 5707–5715 CrossRef CAS PubMed; A. Tamilselvi and G. Mugesh, Chem.–Eur. J., 2010, 16, 8878–8886 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of materials synthesis and characterisation. See DOI: 10.1039/c3ra45745a |
| This journal is © The Royal Society of Chemistry 2014 |