Toxicity and biodegradability of dicationic ionic liquids†
Stephanie Steudteab,
Steve Bemowskya,
Maria Mahrovac,
Ulrike Bottin-Webera,
Emilia Tojo-Suarezc,
Piotr Stepnowskib and
Stefan Stolte*ab
aDepartment Sustainable Chemistry, Centre for Environmental Research and Sustainable Technology, University of Bremen, Leobener Str. UFT, 28359 Bremen, Germany. E-mail: sstolte@uni-bremen.de; Fax: +49 421 218-98-63370; Tel: +49 421 218-63370
bDepartment of Environmental Analytics, Faculty of Chemistry, University of Gdańsk, Wita Stwosza 56, 80-308 Gdańsk, Poland
cDepartment of Organic Chemistry, University of Vigo, 36310 Vigo, Spain
First published on 10th December 2013
Abstract
Ionic liquids (ILs) formed by multivalent cations are generally of higher thermal and electrochemical stability, which makes them attractive for use in high-temperature applications. Whereas the influence of structural elements on the physicochemical properties of dicationic ILs (DILs) is well established, such systematic investigations on their ecotoxicity and biodegradablility are still lacking. The present study investigates the influence of the dicationic structural elements on these characteristics and addresses the question whether already established structure–activity relationships of common ILs can be applied to DILs. Therefore, a set of 10 DILs with different linkage chain length, terminal alkyl side chain length, linkage chain polarity and head groups were synthesized and studied in several biodegradation and toxicity tests. The results showed that the acute toxicity was in many cases below the levels observed for monocationic ILs. However, none of the DILs could be degraded within the performed biodegradation experiments. Hence, DILs are a potential less toxic alternative to monocationic ILs, but further work on their design is necessary.
1 Introduction
Ionic liquids (ILs), salts with low melting points, have been increasingly studied in recent years. They are mostly composed of a bulky organic cation and an inorganic or organic anion. Based on the appropriate combination of these structural elements, ILs can be designed with desired properties and possess unique properties like high thermal stability, a wide liquid range or a broad electrochemical window.ILs are applicable in various fields, e.g. as solvent,1 catalyst,2 in lithium ion batteries3 or dye-sensitized solar cells.4 Some ILs are already used in industrial processes.5 In terms of the “Green Chemistry concept”, ILs have the advantage of enhanced operational safety, based on their low vapor pressure and therefore lower evaporation rate. However, their toxicity and the environmental hazards that they could pose are also of the highest importance. Many reports on the influence of the cationic head group, length and functionalization of the side chain and type of anion have been published in the last 10 years. The results are summarized in various review articles.6–8 In brief, ILs exhibit a varying hazard potential, depending on the structural elements they consist of. Regardless of the investigated test system, lipophilicity appears to have the highest impact on such a compound's toxicity. In biodegradation studies, ILs with long and/or functionalized (e.g. alcohol or carboxylic acid) alkyl chains often show better biodegradation rates.9
Recently, multivalent cations have also been used to form ILs.10,11 Here two or more cationic head groups are linked by alkyl chain(s). Because it is possible to form either symmetrical or unsymmetrical ILs, containing different head groups and substituents, the number of compounds and the design potential is further enlarged. Compared to monocationic ILs, multicationic ones can have a higher melting point, viscosity, surface tension, thermal stability and a wider liquid range.10,12–14 The higher thermal stability in particular makes them attractive for use as high-temperature lubricants14–16 or as the stationary phase in gas chromatography.17,18 They have also been used as electrolytes in secondary batteries19 and in dye-sensitized solar cells.20,21 The influence of structural elements like the type of head group and anion, length of side or linkage chain and symmetry on the physicochemical properties of ILs has already been studied.10,14,22–24 Such systematic studies of their environmental impact are still lacking. To the best of our knowledge, examinations have just been done for selected representatives. 1,3-Bis[4-methylpyridinium] dibromide, for instance, was found to own a lower microbial activity among all tested species (Gram-positive and Gram-negative bacteria and yeast) compared to the tested monocations.25 However, substitution of the anions to tetrafluoroborate led to compounds with similar growth inhibition as traditional ILs.25 Ford et al. showed that an acetal-linked bis-pyridinium IL was not biodegraded in a CO2 headspace test (OECD 310).26
In this study, we focused on whether the dicationic structural element changes the toxicity and/or the biodegradability in comparison with traditional IL structures. Moreover, we wanted to address the question whether already established structure–activity relationships of monocationic ILs could be applied to dicationic ILs (DILs). To do so, we synthesized a set of 10 DILs (Table 1) in order to perform a systematic investigation into the influence of:
| No. | Structure | IC50 [μM]a | EC50 [μM]b | |||
|---|---|---|---|---|---|---|
| AChE | IPC-81 | S. vacuolatus | D. magna | |||
| a Half maximal inhibition concentration (IC50), values in parentheses: 97.5% confidence intervals.b Half maximal effective concentration (EC50), values in parentheses: 97.5% confidence intervals.c Data taken from ref. 49.d Data taken from ref. 32.e Data taken from ref. 47.f Data taken from ref. 50.g Data taken from ref. 51.h Data taken from ref. 34.i Not available.j Data taken from ref. 52. | ||||||
| 1 |  |
5420 (4750–6230) | 6230 (4980–8160) | 1000 (777–1410) | 9.99 (9.40–10.7) | |
| 2 |  |
3240 (2980–3530) | 7470 (6860–8210) | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
276 (265–287) | |
| 3 | 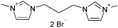 |
865 (782–957) | 9670 (8700–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
242 (231–253) | |
| 4 |  |
13.3 (11.9–14.9) | 9090 (8460–9800) | 1960 (1370–2700) | 59.7 (51.5–67.2) | |
| 5 | 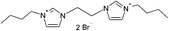 |
533 (467–610) | 614 (490–782) | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
191 (172–210) | |
| 6 |  |
192 (169–217) | 93.4 (82.3–105) | 327 (226–484) | 52.4 (47.4–57.2) | |
| 7 |  |
27.9 (24.0–32.5) | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>5000 | 171 (150–192) | |
| 8 |  |
28.2 (24.7–32.2) | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>5000 | 302 (274–324) | |
| 9 | 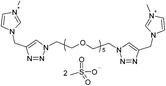 |
52.6 (49.5–55.8) | 1680 (1550–1830) | >1000 | 323 (301–347) | |
| 10 |  |
37.0 (33.8–40.5) | 533 (465–613) | >1000 | 123 (110–138) | |
| 11 | 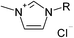 |
R = ethyl | 120 (110–130)c | 9900 (6000–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)c 000)c |
600 (530–690)c | 770 (720–810)c |
| 12 | R = butyl | 83.2 (74.1–93.3)d | 3580 (3030–4300)e | 177 (148–220)e | 84.7 (73.0–97.5)f | |
| 13 | R = hexyl | 82.6 (75.4–90.5)e | 661 (535–843)e | 1.2e | 6.02 (2.00–16.2)g | |
| 14 | R = octyl | 39.4 (36.7–42.3)e | 102 (91.2–115)h | 0.00175e | 0.047 (0.022–0.083)g | |
| 15 | R = decyl | 12.3 (11.0–13.6)e | 21.9 (20–24)h | 0.000272 | n.a.i | |
| (0.000242–0.000308)e | ||||||
| 16 |  |
R = butyl | 82.9 (74.8–91.9)e | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000j 000j |
2430 (1860–3320)e | n.a.i |
| 17 | R = hexyl | 303 (278–330)e | 844 (770–933)e | n.a.i | n.a.i | |
| 18 | R = octyl | 230 (211–251)e | 387 (339–446)e | n.a.i | n.a.i | |
• the linkage chain length (ILs 1–4)
• the terminal alkyl side chain length (ILs 2, 5, 6)
• the linkage chain polarity (IL 4, 7, 9)
• the head group (IL 7, 8 and 9, 10) on the biodegradability and toxicity of the DILs.
The selection of halides and methylsulfonates enables the observed effects to be related solely to the cation moiety, owing to the low intrinsic toxicity of these anions.27–29
For our preliminary hazard assessment of DILs we carried out different biodegradation tests, investigated the influence of DILs on a waste water treatment plant microbial community and performed toxicity tests at different levels of biological complexity, including enzyme inhibition tests (acetylcholinesterase), promyelocytic rat leukemia cells (IPC-81), limnic green algae (Scenedesmus vacuolatus) and water fleas (Daphnia magna). These test systems are well established and data for monocationic ILs as well as other reference compounds are available, which allows for a comparative hazard assessment.
This study is addressed to the users and developers of ILs, especially DILs, in all application and research fields in order to extend knowledge of their environmental properties and to create inherently safer products.
2 Experimental part
2.1 Chemicals
1H-Imidazole (>99.5%), 1-methylimidazole (>99.0%), 1-butylimidazole (>98.0%), 1-methylpyrrolidinium (>99.0%), tetraethylene glycol (TEG) (99%), hexaethylene glycol (HEG) (>97.0%), thionyl bromide, thionyl chloride (>99.0%), sodium azide (>99.0%), N,N-dimethylformamide (DMF) (>99.0%), N,N-diisopropylethylamine (DIPEA) (>95.0%), copper(I) bromide, diiodomethane (>99.0%), 1-bromohexane (>98.0%), 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), bovine serum albumin (BSA), K2HPO4, FeSO4·7H2O, (NH4)2Mo7O24·4H2O, CaCl2·2H2O and urea were purchased from Sigma Aldrich (Steinheim, Germany); dibromoethane (>99.0%), dibromopropane (>98.0%) and dibromohexane (>98.0%) from Acros Organics (Geel, Belgium); phosphorus tribromide (>98.0%), propargyl alcohol (>99.0%), Na2HPO4, NaH2PO4, Na2MoO4·2H2O, Titriplex III and 3,5-dichlorophenol from Merck (Darmstadt, Germany); methanesulfonyl chloride (>99.0%), acetylcholinesterase (from the electric eel), acetylthiocholine, NaCl, MgSO4·7H2O, H3BO3, ZnSO4·7H2O, FeCl3·6H2O, KNO3, peptone from soybean and meat extracts from Fluka (Buchs, Switzerland); NaHCO3 from GIBCO BRL (Eggenstein, Germany); KH2PO4, Ca(NO3)2·4H2O and MnCl2·4H2O from Riedel-de Haën (Seelze, Germany); cell culture media, fetal bovine serum and phosphate buffer from Invitrogen Life Technologies (Frankfurt, Germany); WST-1 reagent from Roche Diagnostics GmbH (Mannheim, Germany).2.2 Synthesis
The synthesis of 1 was performed as described in,24 2–6 were synthesized according to23 and the preparation of 7–10 was previously described in.30 Detailed information as well as NMR and MS- data are given in the ESI.† All DILs could be easily dissolved in water (>3 g L−1). The miscibility with water was also described in other publications.10,23,242.3 Biodegradation tests
2.4 Toxicity tests
The cytotoxicity assay was carried out according to Ranke et al.34 Cell viability was measured using a colorimetric assay for 96 well plates with 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt (WST-1) reagent. Each plate contained blanks (no cells), controls (no toxicants), and substance in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dilution series. Stocks of ILs were prepared in culture medium with 0.5% dimethylsulfoxide (DMSO) to improve the solubility of the substances. This DMSO concentration has been proven to be non-cytotoxic.35 For the test, IPC-81 cells in a concentration of 15 × 105 cells per mL−1 (in RPMI with 8% fetal calf serum) were incubated for 44 h in 96-well plates in the presence of substance and for an additional 4 h in the presence of WST-1 reagent. Cell viability, measured as the ability to reduce WST-1, was observed photometrically at 450 nm in a microplate reader (MRX, Dynatech Laboratories, Chantilly, USA). The cytotoxicity of the compounds was expressed as a percentage of the cell viability measured as WST-1 reduction compared to controls. Each dose–response curve was recorded for at least 9 parallel dilution series on three different 96 well plates. Positive controls with carbendazim were checked at regular intervals.
1 dilution series. Stocks of ILs were prepared in culture medium with 0.5% dimethylsulfoxide (DMSO) to improve the solubility of the substances. This DMSO concentration has been proven to be non-cytotoxic.35 For the test, IPC-81 cells in a concentration of 15 × 105 cells per mL−1 (in RPMI with 8% fetal calf serum) were incubated for 44 h in 96-well plates in the presence of substance and for an additional 4 h in the presence of WST-1 reagent. Cell viability, measured as the ability to reduce WST-1, was observed photometrically at 450 nm in a microplate reader (MRX, Dynatech Laboratories, Chantilly, USA). The cytotoxicity of the compounds was expressed as a percentage of the cell viability measured as WST-1 reduction compared to controls. Each dose–response curve was recorded for at least 9 parallel dilution series on three different 96 well plates. Positive controls with carbendazim were checked at regular intervals.
For toxicity tests, autospores (young algal cells at the beginning of the growth cycle) were exposed to the test substances in different concentrations for one growth cycle (24 h). The tests were performed in sterilized glass tubes (20 mL Pyrex tubes sealed with caps containing a gas-tight Teflon membrane), the algae were stirred over the whole test period, and the test conditions were the same as for the stock culture, except the CO2 source. Therefore, 150 μL of 0.15 M NaHCO3 solution were added to each test tube. The initial and final cell number was measured using a Z2 Coulter Counter (Beckmann, Nürnberg, Germany). The relative toxicity of the samples was expressed as the percentage of growth compared to the controls. The tests were carried out at least three times for each substance with 2 replicates per concentration and a minimum of 6 controls (no toxicant).
3 Results and discussion
3.1 Biodegradation and sludge respiration inhibition
Primary biodegradation experiments were conducted in order to screen the biodegradability of all 10 DILs as well as imidazole and 1-methyl-3-octyl-imidazolium chloride (IM18 Cl) as the reference compounds. The initial concentration of the cation and the concentration after 28 days were determined by ion chromatography (IC) and used to calculate the percentage of primary degradation. The results are displayed in Table S1 (see ESI†). Primary degradation of imidazole and IM18 Cl was complete within 7 and 14 days, respectively, indicating the general biological activity of the inoculum. The tested DILs, however, remained stable within the measurement uncertainty levels and showed less than 5% primary degradation within 28 days. Thus, they remain recalcitrant towards microbial degradation under the applied “ready biodegradability” test conditions.For DILs 4, 6 and 10 additional oxygen consumption tests with inoculum from a different domestic wastewater treatment plant (WWTP) were applied to enhance the variability of biological microorganisms. The compounds were chosen as the most appropriate candidates for biodegradation as they contain structural elements (a long alkyl or functionalized chain) that are known to increase the biodegradation potential.41 The results support the finding from the primary degradation study (Table S1†). Also in these experiments, less than 5% biodegradation was found for DILs 4 and 6. The 14% biodegradation observed for IL 10 can be attributed to the mineralization of the anionic moiety, which theoretically corresponds to 10% of the total oxygen demand of the DIL. Based on the low extent of biodegradation none of the structures can be classified as readily biodegradable, whereas the reference benzoic acid was completely mineralized within 3 days.
The results obtained in this study are in agreement with the observations made for monocationic ILs. For imidazolium-based monocationic ILs degradation of the side chain was observed if this consisted of eight or more carbon atoms.41 The longest substituted terminal alkyl side chain in our DILs was hexyl (6). Thus, the side chain might be too short for microbial degradation via ω-oxidation, which would explain the observed results. Although the set of DILs contained compounds with long spacer chains or ether links, this did not improve biodegradation of either the imidazolium- or the pyrrolidinium-based compounds. The linkage chain cannot be attacked via ω-oxidation and will only be accessible after head group removal. Tests with monocationic imidazolium-containing ILs had already shown that this core remains resistant to biodegradation.41,42 For pyrrolidinium-based substances degradation was complete only when these were substituted with long or alcohol-functionalized side chains.43 Therefore, the choice of longer terminal side chains (≥C8) or terminal hydroxyl groups, the introduction of ester groups in the linkage chain44 and the use of other biodegradation-susceptible head groups such as pyridinium45 might form DILs with enhanced biodegradability.
In the experiments discussed earlier, activated sludge from WWTPs was applied as inocula. If a DIL is toxic toward the microbial sludge community, the observed lack of biodegradation might be related to this. Therefore, a sludge inhibition test was performed and the O2 respiration rate of the sludge was studied in the presence of a synthetic sewage feed at different DIL concentrations. The results, expressed as the half maximal inhibition concentration (IC50), are shown in Table S1.† For IL 6 an IC50 value of 380 μM (187 mg L−1) was measured, which is higher than the concentration of the compound used in the tests (200 μM and 240 μM, respectively). However, residual inhibition of biodegradation cannot be excluded for this compound. No adverse effects on the microorganism's respiratory activity were found for any of the other compounds up to the highest tested concentration of 1 mM. Therefore, we can rule out inhibition of the microorganisms as a reason for the stability of these DILs. The experimentally found low toxicity of DILs to inocula is in good agreement with observations recently made for monocationic ILs substituted with short alkyl chains (≤-octyl).46
3.2 Toxicity
Test systems of different complexity were chosen for a first estimate of the toxicity of the DILs. The results, expressed as the IC50 or half maximal effective concentration (EC50), are displayed in Table 1. Additional literature data of monocationic homologs are shown for comparison. We chose a series of 1-alkyl-3-methyl-imidazolium based and 1-alkyl-1-methyl-pyrrolidinium based ILs with different substituted alkyl side chains (-ethyl, -butyl, -hexyl, -octyl and -decyl) to act as reference compounds for lower (such as -ethyl) and higher (e.g. -decyl) acute IL toxicity. | ||
| Fig. 1 Dose–response-curve for DILs with increasing linkage chain length and two monocationic ILs in acetylcholinesterase assay. | ||
An enhanced inhibition was also found for DILs with increasing terminal side chain length (ILs 2, 5 and 6), but less pronounced (IC50s within 1 decimal power) and even 2 terminally substituted -hexyl side chains (6) show a lower inhibition potential than short-chained monocationic compounds. Similar IC50 values were measured for DILs composed of different head groups connected via polyether groups (7–10). The values in the range from 27.9 to 52.6 μM indicated an inhibition potential that is comparable to that of the monocationic IL IM18 Cl (14).
For AchE it is known that the active center is located in a narrow gorge of about 20 Å. The decamethonium cation has already been found to fit very well into this cleft.48 When comparing the structures of decamethonium with DIL 4 the similarity is obvious. Thus, it can be assumed that this compound can be located in the same way, and prevents the substrate from reaching the enzymatic center, leading to the observed inhibition. The same might be true of DILs with similar or longer linkage chains (as for 7–10). On the other hand, the two head groups in the DILs 1–3, 5 and 6 might be too close to each other, and hence, the cation too bulky to fit into the gorge.
The sensitivity of D. magna to DILs is higher compared with the test using IPC-81 and algae. Again, IL 1 appears to be the most toxic DIL (EC50 = 10 μM), but tests with KI (EC50 = 17.6 μM) indicates once more the influence of iodide on the toxicity of 1. For the other compounds the EC50s are observed to be dependent on the terminal alkyl chain and the linkage chain length (IL 2–6). The introduction of a polyethylene glycol-containing spacer reduces the toxicity (7–10). In comparison to monocationic ILs the EC50s of DILs generally lay between 11 (-ethyl) and 12 (-butyl).
4 Conclusion
An initial evaluation of the hazard potential of 10 synthesized dicationic cations is presented. In comparison with standard dialkylimidazolium ILs, the levels of enzyme inhibition towards acetylcholinesterase and the acute toxicity towards IPC-81, S. vacuolatus and D. magna lie in many cases below the toxicity observed for monocationic ILs. Especially the use of short terminal side chains and head groups connected via polyethylene glycol or short alkyl chains could be identified as structural elements reducing the toxicity. The high thermal and electrochemical stability of DILs is reflected in their stability towards biodegradation processes. None of the DILs could be degraded during the biodegradation experiments. However, bearing in mind what is known about monocationic ILs it might be possible to design new DILs with an enhanced biodegradation potential. In particular, the use of other head groups such as pyridinium, known to be susceptible to biodegradation,45 appears promising. From a structural point of view multivalent ILs certainly have considerable potential that fulfill technical needs and pose a reduced hazard to humans and the environment.Acknowledgements
The authors gratefully thank the whole MINILUBES and UFT team for their advice and support. Thanks are due to Prof. Wolfgang Binder and his research group for synthesizing and providing bis-1,11-[(3-methyl-1H-imidazolium-1-yl)]-(3,6,9-trioxaundecane) dichloride and bis-1,11-[(1-methyl-pyrrolidinium-1-yl)]-(3,6,9-trioxaundecane) dichloride. The authors would like to acknowledge the financial support of the European project MINILUBES (FP7 Marie Curie ITN network 216011-2) by the European Commission.Notes and references
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- F. Shi, Y. Gu, Q. Zhang and Y. Deng, Catal. Surv. Asia, 2004, 8, 179–186 CrossRef CAS.
- S. Seki, Y. Kobayashi, H. Miyashiro, Y. Ohno, A. Usami, Y. Mita, N. Kihira, M. Watanabe and N. Terada, J. Phys. Chem. B, 2006, 110, 10228–10230 CrossRef CAS PubMed.
- R. Kawano, H. Matsui, C. Matsuyama, A. Sato, M. A. B. H. Susan, N. Tanabe and M. Watanabe, J. Photochem. Photobiol., A, 2004, 164, 87–92 CrossRef CAS PubMed.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- J. Ranke, S. Stolte, R. Störmann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183–2206 CrossRef CAS PubMed.
- M. Petkovic, K. R. Seddon, L. P. N. Rebelo and C. Silva Pereira, Chem. Soc. Rev., 2011, 40, 1383–1403 RSC.
- T. P. Thuy Pham, C.-W. Cho and Y.-S. Yun, Water Res., 2010, 44, 352–372 CrossRef PubMed.
- S. Stolte, S. Steudte, A. Igartua and P. Stepnowski, Curr. Org. Chem., 2011, 15, 1946–1973 CrossRef CAS.
- J. L. Anderson, R. Ding, A. Ellern and D. W. Armstrong, J. Am. Chem. Soc., 2005, 127, 593–604 CrossRef CAS PubMed.
- R. P. Singh and J. M. Shreeve, Chem. Commun., 2003, 1366–1367 RSC.
- H. Shirota, T. Mandai, H. Fukazawa and T. Kato, J. Chem. Eng. Data, 2011, 56, 2453–2459 CrossRef CAS.
- Y.-S. Ding, M. Zha, J. Zhang and S.-S. Wang, Colloids Surf., A, 2007, 298, 201–205 CrossRef CAS PubMed.
- C.-M. Jin, C. Ye, B. S. Phillips, J. S. Zabinski, X. Liu, W. Liu and J. M. Shreeve, J. Mater. Chem., 2006, 16, 1529–1535 RSC.
- F. Pagano, C. Gabler, P. Zare, M. Mahrova, N. Dörr, R. Bayon, X. Fernandez, W. H. Binder, M. Hernaiz, E. Tojo and A. Igartua, Proc. Inst. Mech. Eng., Part J, 2012, 226, 952–964 CrossRef CAS PubMed.
- G. Yu, S. Yan, F. Zhou, X. Liu, W. Liu and Y. Liang, Tribol. Lett., 2007, 25, 197–205 CrossRef CAS.
- J. L. Anderson and D. W. Armstrong, Anal. Chem., 2005, 77, 6453–6462 CrossRef CAS PubMed.
- K. Huang, X. Han, X. Zhang and D. Armstrong, Anal. Bioanal. Chem., 2007, 389, 2265–2275 CrossRef CAS PubMed.
- Z. Zhang, H. Zhou, L. Yang, K. Tachibana, K. Kamijima and J. Xu, Electrochim. Acta, 2008, 53, 4833–4838 CrossRef CAS PubMed.
- J. Y. Kim, T. H. Kim, D. Y. Kim, N.-G. Park and K.-D. Ahn, J. Power Sources, 2008, 175, 692–697 CrossRef CAS PubMed.
- C. Zafer, K. Ocakoglu, C. Ozsoy and S. Icli, Electrochim. Acta, 2009, 54, 5709–5714 CrossRef CAS PubMed.
- J.-C. Chang, W.-Y. Ho, I.-W. Sun, Y.-L. Tung, M.-C. Tsui, T.-Y. Wu and S.-S. Liang, Tetrahedron, 2010, 66, 6150–6155 CrossRef CAS PubMed.
- M. Lee, Z. Niu, C. Slebodnick and H. W. Gibson, J. Phys. Chem. B, 2010, 114, 7312–7319 CrossRef CAS PubMed.
- Q. Liu, F. van Rantwijk and R. A. Sheldon, J. Chem. Technol. Biotechnol., 2006, 81, 401–405 CrossRef CAS.
- M. Messali, Z. Moussa, A. Y. Alzahrani, M. Y. El-Naggar, A. S. ElDouhaibi, Z. M. a. Judeh and B. Hammouti, Chemosphere, 2013, 91, 1627–1634 CrossRef CAS PubMed.
- L. Ford, J. R. Harjani, F. Atefi, M. T. Garcia, R. D. Singer and P. J. Scammells, Green Chem., 2010, 12, 1783–1789 RSC.
- J. Arning, S. Stolte, A. Böschen, F. Stock, W.-R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2008, 10, 47–58 RSC.
- S. Stolte, J. Arning, U. Bottin-Weber, M. Matzke, F. Stock, K. Thiele, M. Uerdingen, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2006, 8, 621–629 RSC.
- S. Stolte, M. Matzke, J. Arning, A. Böschen, W.-R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2007, 9, 1170–1179 RSC.
- P. Zare, M. Mahrova, E. Tojo, A. Stojanovic and W. H. Binder, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 190–202 CrossRef CAS.
- Test no. 301: Ready Biodegradability, OECD Guidel. Test. Chem., 1992, no. 301, 1–62.
- F. Stock, J. Hoffmann, J. Ranke, R. Störmann, B. Ondruschka and B. Jastorff, Green Chem., 2004, 6, 286–290 RSC.
- N. Lacaze, G. Gombaud-Saintonge and M. Lanotte, Leuk. Res., 1983, 7, 145–154 CrossRef CAS.
- J. Ranke, K. Mölter, F. Stock, U. Bottin-Weber, J. Poczobutt, J. Hoffmann, B. Ondruschka, J. Filser and B. Jastorff, Ecotoxicol. Environ. Saf., 2004, 58, 396–404 CrossRef CAS.
- F. Stock, Dissertation, University of Bremen, 2004.
- M. Matzke, S. Stolte, K. Thiele, T. Juffernholz, J. Arning, J. Ranke, U. Welz-Biermann and B. Jastorff, Green Chem., 2007, 9, 1198–1207 RSC.
- M. Faust, R. Altenburger, W. Bodeker and L. H. Grimme, Schriftenr. Ver. Wasser-, Boden- Lufthyg., 1992, 89, 311–321 CAS.
- Test no. 202: Daphnia sp. Acute Immobilisation Test, OECD Guidel. Test. Chem., 2004, 1–12.
- Test no. 209: Activated Sludge Respiration Inhibition Test (Carbon and Ammonium Oxidation), OECD Guidel. Test. Chem., 2010, 1–18.
- R Core Team, R Foundation for Statistical Computing, Version 2.15.1, 2012 Search PubMed.
- S. Stolte, S. Abdulkarim, J. Arning, A.-K. Blomeyer-Nienstedt, U. Bottin-Weber, M. Matzke, J. Ranke, B. Jastorff and J. Thöming, Green Chem., 2008, 10, 214–224 RSC.
- E. Rorije, F. Germa and B. Philipp, SAR QSAR Environ Res, 2002, 13, 199–204 CrossRef CAS PubMed.
- J. Neumann, S. Steudte, C.-W. Cho, J. Thöming and S. Stolte, Green Chem., 2013 Search PubMed , submitted.
- N. Gathergood, P. J. Scammells and M. T. Garcia, Green Chem., 2006, 8, 156–160 RSC.
- K. M. Docherty, J. K. Dixon and C. F. Kulpa, Biodegradation, 2007, 18, 481–493 CrossRef CAS PubMed.
- M. Markiewicz, M. Piszora, N. Caicedo, C. Jungnickel and S. Stolte, Water Res., 2013, 47, 2921–2928 CrossRef CAS PubMed.
- http://www.il-eco.uft.uni-bremen.de.
- P. H. Axelsen, M. Harel, I. Silman and J. L. Sussman, Protein Sci., 1994, 3, 188–197 CrossRef CAS PubMed.
- S. Steudte, P. Stepnowski, C.-W. Cho, J. Thöming and S. Stolte, Chem. Commun., 2012, 48, 9382–9384 RSC.
- R. J. Bernot, M. A. Brueseke, M. A. Evans-White and G. A. Lamberti, Environ. Toxicol. Chem., 2005, 24, 87–92 CrossRef CAS.
- D. J. Couling, R. J. Bernot, K. M. Docherty, J. K. Dixon and E. J. Maginn, Green Chem., 2006, 8, 82–90 RSC.
- S. Stolte, J. Arning, U. Bottin-Weber, A. Muller, W.-R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2007, 9, 760 RSC.
- U. Kristen, Toxicol. In Vitro, 1997, 11, 181–191 CrossRef CAS.
- A. Castaño and M. J. Gómez-Lechón, Toxicol. In Vitro, 2005, 19, 695–705 CrossRef PubMed.
- M. J. Garle, J. H. Fentem and J. R. Fry, Toxicol. In Vitro, 1994, 8, 1303–1312 CrossRef CAS.
- J. Golstein and J. E. Dumont, J. Endocrinol. Invest., 1996, 19, 119–126 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c3ra45675g |
| This journal is © The Royal Society of Chemistry 2014 |
