Fullerene filling modulates carbon nanotube radial elasticity and resistance to high pressure
Cui-cui
Ling
ab,
Qing-zhong
Xue
*ab,
Dan
Xia
c,
Mei-xia
Shan
a and
Zhi-de
Han
a
aCollege of Science, China University of Petroleum, Qingdao 266580, Shandong, P. R. China. E-mail: xueqingzhong@tsinghua.org.cn; Fax: +86-532-86983366; Tel: +86-532-86983366
bState Key Laboratory of Heavy Oil Processing, China University of Petroleum, Qingdao 266580, Shandong, P.R. China
cInterdisciplinary Nanoscience Center (iNANO) and Department of Physics and Astronomy, University of Aarhus, DK-8000 Aarhus C, Denmark
First published on 14th November 2013
Abstract
The high pressure behavior of carbon nanotubes (CNTs) filled with fullerenes (C60@CNTs) is investigated systematically using molecular mechanics and molecular dynamics simulations. It is shown that the C60 filling can increase the transition pressure (Pc) of intrinsic CNTs and optimize the radial elasticity of CNTs. The C60 filling increases the Pc of CNT(17, 0) by a factor of ∼25, and the Pc of CNT(10, 10) by a factor of ∼5. An inelastic CNT(17, 0) can be transformed into a superelastic CNT(17, 0) by filling C60 into CNTs. Moreover, C60@CNTs with larger diameters (21.76 Å > d > 13.56 Å) show the better radial elasticity compared with intrinsic CNTs. These characteristics can make C60@CNTs possess potential applications in pressure sensors, electromechanical oscillators, nanotube memory etc. In addition, C60@CNTs with larger diameters (21.76 Å > d > 13.56 Å) undergo two structure transitions under high pressure, which is well in agreement with the experimental results. The Lennard–Jones potential can describe the interaction between C60 and CNT well and explain radial collapse and recovery properties of C60@CNT completely, which can provide theoretical guidance for experimental results.
I. Introduction
Since the discovery of carbon nanotubes (CNTs),1 people have found that CNTs possess remarkable electrical and mechanical properties, which enable them to be used for the development of superconductive devices for nanoelectromechanical system applications.Theoretical calculations and experimental studies have shown that CNTs can undergo significant plastic deformation under hydrostatic pressure.2–8 It is shown that the radial deformation of small CNTs whose diameters are smaller than ∼6.78 Å are reversible.9,10 Of particular interest is that the band-gap of CNTs can vary with radial deformation and eventually closes or opens at a critical deformation.11–13 This electronic-structure transformation has an important implication for device applications, such as pressure sensors, electromechanical oscillators, nanotube memory and nanosprings, etc. However, the deformation of larger tubes could be irreversible and a collapsed state is metastable or even absolutely stable.14 Our group found that the collapsed state of the armchair CNT was metastable and collapsed states of other chiral and modified CNTs were energetically favorable.15,16 The stable collapsed state of CNTs makes them lose radial elasticity, which will confine the development of CNT-based superconductive devices for nanoelectromechanical system applications. Therefore, improving the CNT's radial elasticity under hydrostatic pressure remains a major challenge.
The introduction of “foreign” materials can significantly alter the mechanical, electronic or conducting behavior of CNTs.17–25
For example, C60 filling not only can modulate the energy band gap and electronic properties of CNT,26 but also can change the CNT's mechanical properties such as resistance to high hydrostatic pressure.27–30 The experimental results show that the first transition pressure of CNT filled with C60 is lower than that of intrinsic CNT, but the second transition pressure is much higher than that of intrinsic CNT.29 It is expected that the experimental data will feed theoretical models that will in turn advance the understanding of the effect of C60 filling on the high-pressure effects on CNT.29 In addition, it is not clear enough if C60 filling can influence the radial elasticity of CNT. The radial elasticity upon unloading have much influence on the application of CNTs filled with C60 (C60@CNTs) nanocomposites in electromechanical system.
In this paper, using molecular mechanics (MM) and molecular dynamics (MD) simulations we investigate the radial collapse process of C60@CNTs and construct theoretical model based on Lennard–Jones potential to disclose the physical mechanism of collapse. It is shown that filling C60 can modulate the CNT's (d = 13.56 Å) radial elasticity and resistance to high pressure. An inelastic CNT(17, 0) can be transformed into a superelastic CNT(17, 0) by filling C60 into CNTs. In addition, the C60@CNTs with larger diameters (21.76 Å > d > 13.56 Å) undergo two structure transitions under high pressure, which are in very agreement with the experimental results. What's more, the C60 filling can improve the radial elasticity of CNTs. However, for CNT with d > 21.76 Å, C60 filling deteriorates the radial elasticity of CNT.
Both C60 filling26 and radial deformation11−13 can modulate the energy band gap of CNT, and further change the electronic properties of CNT. Therefore, our findings can provide a basis for further investigating the electromechanical properties of C60@CNTs and developing nanoelectronics devices using C60@CNTs such as pressure sensor, electromechanical oscillator, nanotube memory used in electromechanical system. In addition, the resistance to high pressure can also make C60@CNTs an ideal filler for nanocomposites for high load mechanical support.
II. Computational methods
The simulations have been carried out using a commercial software package called Materials Studio (MS) developed by Accelrys Software, Inc. The condensed-phase optimized molecular potential for atomistic simulation studies (COMPASS) force field method is chosen to model the atomic interaction.31 The COMPASS force-field is a parameterized, tested, and validated first ab initio force-field, which enables an accurate prediction of various gas-phase and condensed-phase properties of most of the common organic and inorganic materials.32,33 Moreover, it has been shown to be applicable in describing the mechanical properties of CNT.34,35The systems considered in this work are the C60@CNTs with the length of 73.5 Å. The CNT diameter ranges from 13.56 to 27.76 Å. In simulations, periodic boundary conditions are employed. MM and MD simulations are performed to simulate the filling of C60 into CNTs. In order to avoid the metastable phase of C60, the C60@CNTs systems relax 40 ps under 300 K firstly, and then cool to 1 K slowly. Finally, when the total energy of C60@CNTs reaches a minimum, the systems have stable structure.
In order to investigate the radial collapse of all systems, every system's structure is initially optimized at atmospheric pressure and subsequently subjected to step-wise monotonically hydrostatic pressure increments with FORCITE code. Then, after each pressure increment the unit cell volume is allowed to equilibrate for at least 30 ps at each step in the NPT ensemble, and a fixed time step size of 1 fs is used in all cases. The simulations are carried out at a temperature of 1 K to avoid the thermal effect, and the interactions are determined within a cut-off distance of 9.5 Å. The reduced volume (V/V0), where V and V0 are the unit cell volumes at an applied pressure and atmospheric pressure, respectively, was measured as a function of applied hydrostatic pressure.
III. Results and discussion
III.I. The filling of C60 into CNT (10, 10)
Fig. 1(a–g) show the longitudinal snapshots of C60-filled CNT(10, 10) (from no full to full).27 For full-filled CNT (7C60@CNT(10, 10)), as shown in Fig. 1(g), the investigation has revealed that C60 molecules align periodically along with the CNT axis. To investigate the structure of 7C60@CNT(10, 10), the concentration profiles of 7C60@CNT(10, 10) along z and y axes are shown in Fig. 2. From Fig. 2(a), we can find that the mean of a constant spacing between two C60 molecules was found to be 3.25 Å which is close to interlayer distance of graphite (3.4 Å), which shows that there exists a strong attractive van der Waals (vdW) interaction among C60 molecules along z axes. Herein, considering the diameter (7.10 Å) of C60, we can determine 10.35 Å as the average intermolecular distance center to center of C60 molecules. In addition, Fig. 1(h) shows the transverse snapshots of the 7C60@CNT(10, 10). We can observe that the inter wall spacing between C60 and CNT(10, 10) is homogeneous. This is verified according to the concentration profiles of 7C60@CNT(10, 10) along y axes, as shown in Fig. 2(b). One can observe that both left and right inter wall spacing between the C60 and CNT along y axes are 3.77 Å, which are close to interlayer distance of graphite (3.4 Å). The result shows that there exists the homogeneous attractive vdW interaction between C60 and CNT(10, 10). The result is in agreement with previous theoretical studies36,37 and experimental observations.38,39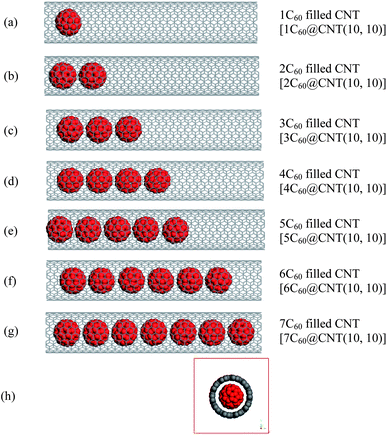 | ||
| Fig. 1 Longitudinal and transverse snapshots of C60@CNT(10, 10). (a–g) Longitudinal snapshots of C60(1−7)@CNT(10, 10), and (h) transverse snapshots of 7C60@CNT(10, 10). | ||
III.II. Effect of CNT chirality on the high pressure behavior of C60@CNTs
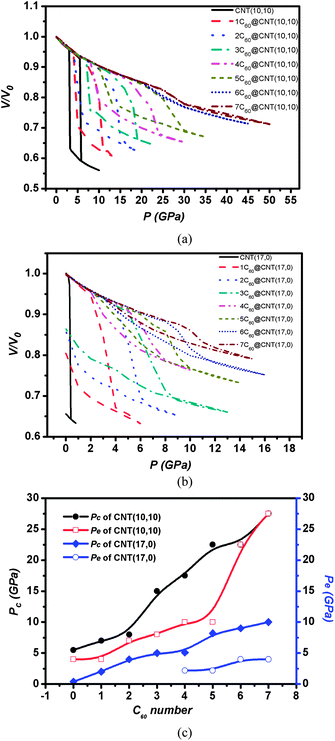
We investigated the high pressure behavior of 1C60@CNT(10, 10) firstly, as shown in Fig. 3(a). Herein, the applied pressure is hydrostatic pressure which is different from the pressure applied in one end of open tube.40 In addition, the periodic boundary conditions are employed in simulations. All the systems are infinitely extended along the axial direction. Therefore, C60 molecules can't be released by CNT.Fig. 3(a) shows the loading and unloading curves of CNT(10, 10) and 1C60@CNT(10, 10); the insets are transverse snapshots of CNT(10, 10) and longitudinal snapshots of 1C60@CNT(10, 10).
The simulations show that when a CNT(10, 10) deforms under pressure, it undergoes two shape transitions: (1) changing from a cycle to an oval (a continuous transition); and (2) changing from an oval to collapse (an abrupt transition).41,42 Here, we define two critical pressures, Pe and Pc, where Pe is the pressure at which the collapsed shape returns to the circle shape upon unloading, its value can characterize the radial elasticity of CNT; and Pc is the pressure to collapse CNT upon loading, its value can characterize the CNT's resistance to high pressure. From Fig. 3(a), it is found that the loading curve of 1C60@CNT(10, 10) is smooth while the loading curve of intrinsic CNT(10, 10) has a sudden change at 5.5 GPa. In other words, the 1C60@CNT(10, 10) shows the better resistance to high pressure compared with intrinsic CNT(10, 10).
Physically, the structural transformation of CNT is determined by the competition among the interaction forces experienced, the stress of CNT and the applied external force. Herein, we present a mechanical model for the specific 1C60@CNT(10, 10) based on Lennard–Jones potential.43Fig. 3(b) shows that the mechanical model and the force between C60 and CNT. Where R is the distance between an atom of CNT and the centre of the C60, F is the interaction force between the atom of CNT and the C60. When R is in the region of [0, R0], the value of F is positive which shows the interaction force is a repulsive force; when R is in the region of [R0, Rf], the value of F is negative which shows that the interaction force is an attractive force; when R is in the region of [Rf, +∞], the interaction force is extremely weak. We can find that the carbon atoms at the end of CNT near the C60 will endure an attractive force from the C60. When the CNT is subjected to an applied pressure, the carbon atoms at the end of CNT near the C60 are easier to be in the region of [0, R0] so that there exists a repulsive vdW force between the C60 and the carbon atoms of the end of CNT near the C60, which can prevent the end of CNT to deform and further influence the whole CNT's collapse gradually. Therefore, when the applied pressure is up to 5.5 GPa 1C60@CNT undergoes a gradual collapse and its the loading curve doesn't demonstrate a sudden change, as shown in Fig. 3(a).
In addition, we can observe that upon unloading the unloading curve of 1C60@CNT(10, 10) shows also a hysteresis but its Pe is larger than that of intrinsic CNT(10, 10), which indicates that the origin state of 1C60@CNT(10, 10) can be recovered more quickly and 1C60@CNT(10, 10) shows a better radial elasticity compared with intrinsic CNT(10, 10). From Fig. 3(b), we can find that after 1C60@CNT(10, 10) collapses the major CNT(10, 10) transforms into a linked double graphene layers paralleling to the plane like a ribbon, there exists a larger attractive vdW interaction between the double graphene layers. Upon unloading, the attractive vdW interaction can lead to the hysteresis of curve.44 However, upon unloading the repulsive force between the C60 and the carbon atoms of the end of CNT near the C60 can promote the system recover to the origin state quickly. Therefore the radial elasticity of 1C60@CNT(10, 10) is superior to that of intrinsic CNT(10, 10).
The effect of C60 number on the radial collapse and elasticity of CNT(10, 10) is investigated. Fig. 4(a) shows the loading and unloading curves of CNT(10, 10) and C60@CNT(10, 10). We can observe easily that both Pc and Pe of C60@CNT(10, 10) can increase as the number of C60 increases and both Pc and Pe of 7C60@CNT(10, 10) achieve 27.5 GPa, as shown in Fig. 4(c). It indicates that C60@CNT(10, 10) can support higher pressure than the corresponding individual CNTs and show super radial elasticity. In addition, we can find that the value of Pc and Pe of 6/7C60@CNT(10, 10) is consistent and their hysteresis in the unloading curves disappear, as shown in Fig. 4(a). After 6/7C60@CNT(10,10) collapse, little part of opposite walls of CNT(10, 10) can contact with each other, which indicates that there exists a very small attractive vdW interaction between the opposite walls. If there is little vdW interaction, the hysteresis will disappear and the loading and unloading curves are superposed.45 Therefore, the hysteresis in the unloading curve of 6/7C60@CNT(10, 10) nearly disappears. Herein, we can conclude that the C60 filling can increase the Pc of intrinsic CNT(10, 10) and optimize the radial elasticity of CNT(10, 10). What's more, the C60 filling number can modulate the Pc and radial elasticity of CNT(10, 10). The filling C60 increases the Pc of CNT(10, 10) by a factor of ∼5.
As shown in Fig. 4(a and b), compared with the curves of intrinsic CNT(10, 10) and (17, 0), we can observe that the Pc of CNT(17, 0) is always considerably lower than that of CNT(10, 10); the intrinsic CNT(17, 0) has a negligible pressure capacity, which has been investigated in our former research.15 The C60 filling can effectively improve the pressure capacity. Fig. 4(b) presents the loading and unloading curves of C60@CNT(17, 0), which show that C60 filling can increase the Pc of intrinsic CNT(17, 0) and improve its radial elasticity. As shown in Fig. 4(c), we can observe that C60 filling increases the Pc of CNT(17, 0) by a factor of ∼25, from 0.4 to 10.0 GPa; what's more, when C60 number is equal or greater than four, the CNT(17, 0) recovers the radial elasticity, and an inelastic CNT(17, 0) can be transformed into a superelastic CNT by filling C60 into CNT with Pe ranging from 0 to 4.8 GPa.
III.III. The high pressure behavior of 7C60@CNTs with different diameters
According to the experimental results, the inhomogeneous filling of C60 into CNTs can introduce inhomogeneity and consequently instability of CNTs toward collapse at lower pressure.28–30 From this section, we begin to investigate the filling of C60 into CNTs with different diameters and the radial collapse and elasticity of C60@CNTs.To assess the diameter dependence of high pressure behavior of 7C60@CNTs with different diameters, we investigated the high pressure behavior of the intrinsic CNTs with different diameters firstly, as shown in Fig. 5(a–d). Pc of the CNTs with different diameters is shown in Table 1. We can find that the values of Pc of CNT(12, 12), CNT(14, 14), CNT(16, 16) and CNT(20, 20) are 4.0, 2.8, 1.1 and 0.6 GPa, respectively. It indicates that the Pc decreases with increasing CNT diameter. Moreover, when the diameter is larger than a threshold, the loading and unloading curves of CNTs can't shape a hysteresis loop. In other words, their collapsed states are favorable. Herein, we can introduce the specific energy for different compression ratios of round tubes to explain the mechanism of high pressure behavior of the CNTs with different diameters. The relationship of specific energy correlated with the D/t ratio and compression ratio δ/D can be written as:46
 | (1) |
We investigate the effect of the diameter on the high pressure behavior of 7C60@CNTs, as shown in Fig. 5(a–d). We can find that the 7C60@CNTs with larger diameter undergoes two abrupt transitions under pressure. Herein, Pc1 is the first transition pressure; Pc2 is the second transition pressure. The Pc1 and Pc2 of 7C60@CNTs are shown in Table 1. We can find that Pc1 is lower than that of intrinsic CNT while Pc2 is higher than that of intrinsic CNT. We studied the relationship between the collapse pressures (Pc1 and Pc2) of filled CNT and diameter D, and find that Pc1 ∼ D−4.25, Pc2 ∼ D−2.39. Compared with the relationship between the collapse pressure Pc and diameter D of CNT (Pc ∼ D−3),47 we can draw a conclusion that the filled tubes behave the different relationship between the transition pressure (Pc1 or Pc2) and D, which may be caused by the heterogeneous interaction between the C60 and CNT wall. The experiments have yet shown two pressures induced transitions in the bundle CNT.28 Take the loading and unloading processes of the 7C60@CNT(16, 16) as an example. We show the concentration profiles of the structure 7C60@CNT(16, 16) along y axes, as shown in Fig. 5(e). We can observe that when 7C60 moleculars are filled in CNT(16, 16), the closest distance between the C60 and CNT is 3.056 Å, which indicates that there exists a strong attractive vdW interaction between C60 and one side of CNT(16, 16) wall. Therefore, the 7C60 moleculars are attracted to the side of CNT(16, 16) wall, and can lead to an inhomogeneous interaction between C60 and CNT(16, 16) wall, which can make CNT(16, 16) structure change at lower pressure. So the first transition of 7C60@CNT(16, 16) occurs at 0.7 GPa which is less than that of intrinsic CNT(16, 16). When the applied pressure continues to increase, the second transition takes place at 3.0 GPa which is larger than that of the intrinsic CNT(16, 16) due to the repulsive force between 7C60 and CNT which is stated in former section. In addition, we can observe that the unloading curve of 7C60@CNT(16, 16) shows the hysteresis due to the stronger attractive vdW interaction between opposite walls after 7C60@CNT(16, 16) collapses completely. Finally, we can find that the 7C60@CNT(16, 16) can return to its original state quickly compare with the intrinsic CNT(16, 16) due to the repulsive force between C60 and CNT under higher pressure.
| Armchair CNT | P c (GPa) | Filled-CNT | P c1 (GPa) | P c2 (GPa) |
|---|---|---|---|---|
| (12,12) | 4.0 | 7C60@(12,12) | 2.5 | 7.0 |
| (14,14) | 2.8 | 7C60@(14,14) | 1.5 | 4.0 |
| (16,16) | 1.1 | 7C60@(16,16) | 0.7 | 3.0 |
| (20,20) | 0.6 | 7C60@(20,20) | 0.3 | 2.0 |
Then, we investigate the high pressure behavior of 7C60@CNT(12, 12), CNT(14, 14), CNT(20, 20), as shown in Fig. 5(a, b and d). For 7C60@CNT(12, 12) and 7C60@CNT(14, 14), we can find that upon loading the 7C60@CNT(12, 12) and 7C60@CNT(14, 14) undergo two transitions under pressure, and upon unloading the hysteresises in unloading curves of two systems disappear and they return to their original state. After CNTs collapse, the partial opposite walls contact each other, which indicates that there exists a weak vdW interaction force between the opposite walls of collapsed CNT. Upon unloading, the weak vdW interaction force is not large enough to make the unloading curves of two systems show the hysteresis. For 7C60@CNT(20, 20), it undergoes two transitions under the external pressure clearly; the unloading curve of 7C60@CNT shows a tendency of returning to its original state compared to the intrinsic CNT(20, 20), as shown in Fig. 5(d). The results indicate that the 7C60@CNTs with larger diameters undergo two transitions under high pressure; Pc1 is lower than that of intrinsic CNT but the C60 filling improves the radial elasticity of the intrinsic CNTs.
III.IV. The mechanism for the high pressure behavior of 7C60@CNTs
Based on the above discussion, we can find that the 7C60@CNTs can't be sufficiently deformed by the vdW force between C60 and CNTs, but the vdW interaction force plays a major role in the high pressure behavior of 7C60@CNTs. In order to better understand the high pressure behavior of 7C60@CNTs with different diameters, we present a mechanical model for the specific 7C60@CNTs with different diameters based on Lennard−Jones potential,43 as shown in Fig. 6. As shown in Fig. 6(a), where dλ is the radial differential unit of CNT, S is the closest distance between 7C60 and dλ. F is the vdW interaction force between the 7C60 and dλ. Herein, we take the two differential units of CNT which are marked for dλ1 or dλ2, respectively; S1 and S2 are the closest distances between dλ1 or dλ2 and 7C60, respectively; F1 and F2 are the vdW interaction forces between dλ1 or dλ2 and 7C60, respectively. When S is in the region of [0, S0], the value of F is positive which shows the interaction force is the repulsive force; when S is in the region of [S0, Sf], the value of F is negative which shows that the interaction force is the attractive force; when S is in the region of [Sf, +∞], the interaction force is extremely weak. We roughly obtain the trend in the variation of F vs. S, which is illustrated in Fig. 6(b) with a plot of the force distribution at different points around the 7C60. The force distribution indicates that when CNT and 7C60 are next to each other, they will approach each other until the interaction reaches minimum (F = 0). We assume that M is the bending modulus, a threshold that can just start the deformation of CNT. When the difference of forces (dF = F1 – F2) in the adjacent different unit dλ is larger than M, the CNTs will deform. The mechanical relationship can be described as follows:| |dF/ds| > M | (2) |
 | ||
| Fig. 6 (a) The scheme of the mechanical model. (b) The variation of the interaction force between the differential unit of CNT and 7C60 with their distance. | ||
According to former section's discussion, the closest distance between the C60 and CNT is 3.056 Å, which indicates that there exists a strong attractive vdW interaction between C60 and dλ closest to 7C60. Due to the attractive vdW interaction a lower pressure can make the partial CNT near 7C60 be easily in the region of [S0, Sf], undergo deformation and coat the surface of 7C60, and the first collapse of CNT occurs, as shown in Fig. 5(a–d). Therefore, the Pc1 is lower than that of the intrinsic CNT. In other words, the inhomogeneous filling can introduce inhomogeneity and consequently instability toward collapse. When the applied pressure continues to increase, the carbon atoms coated surface of 7C60 approach to the 7C60 sequentially so that there exists a larger repulsive vdW interaction between the 7C60 and CNT, which can prevent the CNT further collapse and the Pc2 is higher than Pc of the intrinsic CNT. Upon unloading, the repulsive force between 7C60 and CNT can promote the CNT to return to its origin state quickly, so the radial elasticity of 7C60@CNTs is superior to that of intrinsic CNTs.
III.V. Effect of CNT diameter on the high pressure behavior of C60@CNTs
Fig. 7 shows the longitudinal and transverse snapshots of different diameter CNTs fully filled with C60. With increasing CNT diameter different ordered phases of C60 molecules inside the CNT were observed. The arrangement pattern of C60 in CNT(10, 10) is linear, which has been demonstrated; the arrangement patterns of C60 in CNT(12, 12) and (14, 14) are zigzag; the arrangement pattern of C60 in CNT(16, 16) is double helix. The results are in agreement with the reference results.48 | ||
| Fig. 7 Longitudinal and transverse snapshots of C60@CNTs with different diameters. (a–d) 7C60@CNT(10, 10), 8C60@CNT(12, 12), 10C60@CNT(14, 14) and 19C60@CNT(16, 16). | ||
The high pressure behaviors of these systems are investigated. Fig. 8 shows that the loading and unloading curves of 8C60@CNT(12, 12), 10C60@CNT(14, 14) and 19C60@CNT(16, 16). For 8C60@CNT(12, 12) and 10C60@CNT(14, 14), we can observe that the systems take place two structure transitions. Table 2 summarizes the Pc values of CNTs as a function of the filler C60. The Pc1 of 8C60@CNT(12, 12) is not easy to be observed, but at 2.0 GPa V/V0 has been lower than that of intrinsic CNT(12, 12). Therefore, we considered that the Pc1 of 8C60@CNT(12, 12) is 2.0 GPa, and Pc1 of 10C60@CNT(14, 14) is 0.8 GPa; Pc2 of 8C60@CNT(12, 12) and 10C60@CNT(14, 14) are 12.0 and 13.0 GPa respectively. Obviously, it is shown that the Pc1 of 8C60@CNT(12, 12) and 10C60@CNT(14, 14) is lower than that of intrinsic CNT(12, 12) and (14, 14) and Pc2 are much higher than that of intrinsic CNT(12, 12) and (14, 14).
| Filler | P CNTc (GPa) | P filledc1 (GPa) | P filledc2 (GPa) | ΔPc = Pfilledc1 − PCNTc (GPa) | ΔPc = Pfilledc2 − PCNTc (GPa) | |
|---|---|---|---|---|---|---|
| CNT(10,10) | C60 | 5.5 | 25.0 | +19.5 | +19.5 | |
| CNT(12,12) | C60 | 4.0 | 2.0 | 12.0 | −2.0 | +8.0 |
| CNT(14,14) | C60 | 2.8 | 0.8 | 13.0 | −2.0 | +10.2 |
| CNT(16,16) | C60 | 1.1 | 7.5 | 17.5 | +6.4 | +16.4 |
The experimental results demonstrate that the first structure transition of CNTs bundle filled with C60 (mean diameter 1.35 ± 0.1 nm) takes place at a lower pressure of 2–2.5 GPa; the second transition takes place at a higher pressure of 10–30 GPa. In our simulation, the diameters of CNT(12, 12) and CNT(14, 14) are a little larger than 1.35 ± 0.1 nm; therefore, the Pc1 and Pc2 is a little lower than that of experimental results. Simulation results are in the range of experimental results28,29 and confirm the validity of simulation method used here.
For CNT(12, 12) and CNT(14, 14), the zigzag layout of C60 inside CNTs, which is different from the linear layout of C60 inside CNT(10, 10), provide inhomogeneous filling. According to the mechanical model based on Lennard–Jones potential, upon loading inhomogeneous filling induces the inhomogeneous attractive vdW interaction between C60 and CNT and further leads to CNT mechanical instability at lower pressure. Further loading, the repulsive vdW interaction between C60 and CNT reinforces the resistance to high pressure. Therefore, 8C60@CNT(12, 12) and 10C60@CNT(14, 14) take place two structure transitions. The mechanical model can well explain the experimental results and confirm the validity of simulation method used here again.
For CNT(16, 16), the double helix-filling of 19C60 into CNT(16, 16) can be considered to provide an approximate homogeneous tube filling consolidating mechanical stability of CNT(16, 16) approximately. Therefore, both Pc1 and Pc2 of the 19C60@CNT(16, 16) can increase compared with that of CNT(16, 16).
The radial elasticity of C60@CNTs is a significant issue to be investigated. As shown in Fig. 8(a and b), upon unloading 8C60@CNT(12, 12) and 10C60@CNT(14, 14) return to origin state quickly compared with intrinsic CNT. It shows that the radial elasticity of 8C60@CNT(12, 12), 10C60@CNT(14, 14) is superior to that of intrinsic CNTs. The repulsive vdW attraction between C60 and CNT makes 8C60@CNT(12, 12) and 10C60@CNT(14, 14) return to their origin state quickly and optimize the radial elasticity of CNTs.
However, after 19C60@CNT(16, 16) collapses, C60 molecules occur the relative slippage and the all C60 molecules realign in the plane, as shown in Fig. 8(c). Upon unloading the attractive vdW interaction among C60 molecules make all C60 be constrained in the plane and the attractive interaction between C60 molecules and CNT can prevent the CNT return to the original state, so the 19C60@CNT(16, 16) shows the worse radial elasticity compared with intrinsic CNT(16, 16).
We can conclude that filling C60 into CNTs with larger diameters (21.76 Å > d > 13.56 Å) can introduce inhomogeneity and consequently instability of CNTs toward collapse at lower pressure compared with intrinsic CNTs, but the Pc2 increases and the radial elasticity of the system became better compared with intrinsic CNTs. However, the filling C60 into CNTs with much larger diameters (d > 21.76 Å) can make Pc of CNTs increase but the radial elasticity of CNTs becomes worse compared with intrinsic CNTs.
Conclusions
In summary, we have shown that the C60 filling can increase Pc of intrinsic CNTs and optimize the radial elasticity of intrinsic CNTs while making the CNTs recover quickly and maintaining the structural integrity of CNTs upon unloading. The filling increases Pc of CNT(17, 0) by a factor of ∼25, and Pc of CNT(10, 10) by a factor of ∼5. The C60 filling number can modulate CNT's radial elasticity. An inelastic CNT(17, 0) can be transformed into superelastic CNTs by filling C60 into CNT(17, 0). Moreover, C60@CNTs with larger diameters (21.76 Å > d > 13.56 Å) show better radial elasticity compared with intrinsic CNTs. It is well known that both radial deformation and filling can change the electrical properties of CNT. In view of the recoverability of C60@CNT upon unloading, C60@CNT can be used to develop nanoelectronic devices such as pressure sensors, electromechanical oscillators, nanotube memory used in the nanoelectromechanical systems.In addition, C60@CNTs with larger diameters (21.76 Å > d > 13.56 Å) undergo two structure transitions under pressure; Pc2 of C60@CNTs is higher than that of the intrinsic CNTs. The Lennard–Jones potential can describe the interaction between C60 and the CNT wall and explain radial collapse of C60@CNT completely, which can provide theoretical guidance for experimental results.
However, for CNT with diameter d > 21.76 Å, upon unloading the vdW interaction among C60 molecules influences the radial elasticity of CNT and makes the radial elasticity of CNT become worse. The irreversibility of C60@CNT will make it lose application in electromechanical systems.
Acknowledgements
This work is supported by the Natural Science Foundation of China (11374372, 41330313), Taishan Scholar Foundation (ts20130929), the Fundamental Research Funds forthe Central Universities (13CX06004A,14CX02018A and 14CX02025A), and the Qingdao Science&Technology Program (12-1-4-7-(1)-jch).References
- S. Lijima, Nature, 1991, 354, 56–58 CrossRef.
- P. Tangney, R. B. Capaz, C. D. Spataru, M. L. Cohen and S. G. Louie, Nano Lett., 2005, 5, 2268–2273 CrossRef CAS PubMed.
- N. M. Pugno, J. Mech. Phys. Solids, 2010, 58, 1397–1410 CrossRef CAS.
- J. A. Elliott, J. K. W. Sandler, A. H. Windle, R. J. Young and M. S. P. Shaffer, Phys. Rev. Lett., 2004, 92, 095501 CrossRef PubMed.
- X. Yang, G. Wu and J. Dong, Appl. Phys. Lett., 2006, 89, 113101 CrossRef.
- V. Gadagkar, P. K. Maiti, Y. Lansac, A. Jagota and A. K. Sood, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 085402 CrossRef.
- P. Puech, E. Flahaut, A. Sapelkin, H. Hubel, D. Dunstan, G. Landa and W. S. Bacsa, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 233408 CrossRef.
- C. Caillier, A. Ayari, V. Gouttenoire, A. S. Miguel, V. Jourdain, M. Picher and J. L. Sauvajol, Appl. Phys. Lett., 2010, 97, 173111 CrossRef.
- P. E. Lammert, P. Zhang and V. H. Crespi, Phys. Rev. Lett., 2000, 84, 2453–2456 CrossRef CAS PubMed.
- M. Hasegawa and K. Nishidate, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 115401 CrossRef.
- M. S. C. Mazzoni and H. Chacham, Appl. Phys. Lett., 2000, 76, 1561–1563 CrossRef CAS.
- O. Gulseren, T. Yildirim, S. Ciraci and C. Kilic, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 155410 CrossRef.
- J. Q. Lu, J. Wu, W. Duan, F. Liu, B. F. Zhu and B. L. Gu, Phys. Rev. Lett., 2003, 90, 156601 CrossRef PubMed.
- L. X. Benedict, V. H. Crespi, N. G. Chopra, A. Zettl, M. L. Cohen and S. G. Louie, Chem. Phys. Lett., 1998, 286, 490–496 CrossRef CAS.
- C. C. Ling, Q. Z. Xue, L. Y. Chu, N. N. Jing and X. Y. Zhou, RSC Adv., 2012, 2, 12182–12189 RSC.
- C. C. Ling, Q. Z. Xue, N. N. Jing and D. Xia, Nanoscale, 2012, 4, 3894–3900 RSC.
- E. Borowiak-Palen, E. Mendoza, A. Bachmatiuk, M. H. Rummeli, T. Gemming, J. Nogues, V. Skumryev, R. J. Kalenczuk, T. Pichler and S. R. P. Silva, Chem. Phys. Lett., 2006, 421, 129–133 CrossRef CAS.
- K. Svensson, H. Olin and E. Olsson, Phys. Rev. Lett., 2004, 93, 145901 CrossRef CAS PubMed.
- X. P. Gao, Y. Zhang, X. Chen, G. L. Pan, J. Yan, F. Wu, H. T. Yuan and D. Y. Song, Carbon, 2004, 42, 47–52 CrossRef CAS.
- Q. Wang, K. M. Liew and V. K. Varadan, Appl. Phys. Lett., 2008, 92, 043120 CrossRef.
- L. Wang, H. W. Zhang, Z. Q. Zhang, Y. G. Zheng and J. B. Wang, Appl. Phys. Lett., 2007, 91, 051122 CrossRef.
- B. Ni, S. B. Sinnott, P. T. Mikulski and J. A. Harrison, Phys. Rev. Lett., 2002, 88, 205505 CrossRef PubMed.
- Q. Wang, Carbon, 2011, 49, 729–732 CrossRef CAS.
- F. W. Sun and H. Li, Carbon, 2011, 49, 1408–1415 CrossRef CAS.
- D. J. Hornbaker, S. J. Kahng, S. Misra, B. W. Smith, A. T. Johnson, E. J. Mele, D. E. Luzzi and A. Yazdan, Science, 2002, 295, 828–831 CrossRef CAS PubMed.
- S. Okada, S. Saito and A. Oshiyama, Phys. Rev. Lett., 2001, 86, 3835–3838 CrossRef CAS PubMed.
- N. M. Pugno and J. A. Elliott, Physica E, 2012, 944–948 CrossRef CAS.
- Ch. Caillier, D. Machon, A. S. Miguel, R. Arenal, G. Montagnac, H. Cardon, M. Kalbac, M. Zukalova and L. Kavan, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 125418 CrossRef.
- A. L. Aguiar, A. San-Miguel, E. B. Barros, M. Kalb, D. Machon, Y. A. Kim, H. Muramatsu, M. Endo and A. G. Souza Filho, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 195410 CrossRef.
- C. A. Kuntscher, A. Abouelsayed, A. Botos, A. Pekker and K. Kamaras, Phys. Status Solidi B, 2011, 248, 2732–2735 CrossRef CAS.
- D. Rigby, H. Sun and B. E. Eichinger, Polym. Int., 1997, 44, 311–330 CrossRef CAS.
- H. Sun, P. Ren and J. R. Fried, Comput. Theor. Polym. Sci., 1998, 8, 229–246 CrossRef CAS.
- H. Sun, J. Phys. Chem. B, 1998, 102, 7338–7364 CrossRef CAS.
- Q. Wang, W. H. Duan, K. M. Liew and X. Q. He, Appl. Phys. Lett., 2007, 90, 033110 CrossRef.
- Q. B. Zheng, Y. Geng, S. J. Wang, Z. G. Li and J. K. Kim, Carbon, 2010, 48, 4315–4322 CrossRef CAS.
- S. Okada, S. Saito and A. Oshiyama, Phys. Rev. Lett., 2001, 86, 3835–3838 CrossRef CAS PubMed.
- M. Hodak and L. A. Girifalco, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 075419 CrossRef.
- B. W. Smith, M. Monthioux and D. E. Luzzi, Nature, 1998, 396, 323–324 CrossRef CAS.
- K. Hirahara, S. Bandow, K. Suenaga, H. Kato, T. Okazaki, H. Shinohara and S. Lijima, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 115420 CrossRef.
- M. Chen, J. Zang, D. Q. Xiao, C. Zhang and F. Liu, Nano Res., 2009, 2, 938–944 CrossRef CAS.
- J. Zang, A. Treibergs, Y. Han and F. Liu, Phys. Rev. Lett., 2004, 92, 105501 CrossRef PubMed.
- D. Y. Sun, D. J. Shu, M. Ji, F. Liu, M. Wang and X. G. Gong, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 165417 CrossRef.
- J. E. Lennard-Jones, Proc. Phys. Soc., 1931, 43, 461–482 CrossRef CAS.
- P. Tangney, R. B. Capaz, C. D. Spataru, M. L. Cohen and S. G. Louie, Nano Lett., 2005, 5, 2268–2273 CrossRef CAS PubMed.
- S. Lebedkin, K. Arnold, O. Kiowski, F. Hennrich and M. M. Kappes, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 094109 CrossRef.
- N. K. Gupta, G. S. Sekhon and P. K. Gupta, Thin Wall Struct., 2005, 43, 895 CrossRef.
- V. Gadagkar, S. Saha, D. V. S. Muthu, P. K. Maiti, Y. Lansac, A. Jagota, A. Moravsky, R. O. Loutfy and A. K. Sood, J. Nanosci. Nanotechnol., 2007, 7, 1753 CrossRef CAS PubMed.
- M. Hodak and L. A. Girifalco, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 075419 CrossRef.
| This journal is © The Royal Society of Chemistry 2014 |