Correlation between electrochromism and electronic structures of tungsten oxide films†
Mukta V. Limayea,
J. S. Chena,
Shashi B. Singha,
Y. C. Shaoa,
Y. F. Wanga,
C. W. Paob,
H. M. Tsaib,
J. F. Leeb,
H. J. Linb,
J. W. Chiouc,
M. C. Yangd,
W. T. Wue,
J. S. Chene,
J. J. Wud,
M. H. Tsaif and
W. F. Pong*a
aDepartment of Physics, Tamkang University, Tamsui 251, Taiwan. E-mail: wfpong@mail.tku.edu.tw
bNational Synchrotron Radiation Research Center, Hsinchu 300, Taiwan
cDepartment of Applied Physics, National University of Kaohsiung, Kaohsiung 811, Taiwan
dDepartment of Chemical Engineering, National Cheng Kung University, Tainan 701, Taiwan
eDepartment of Materials Science and Engineering, National Cheng Kung University, Tainan 701, Taiwan
fDepartment of Physics, National Sun Yat-Sen University, Kaohsiung 804, Taiwan
First published on 2nd December 2013
Abstract
The in-situ X-ray absorption spectroscopy of three tungsten oxide films was performed to study the electronic and atomic structures following repeated cycles of coloration and bleaching processes. The transparent tungsten oxide films become deep blue upon intercalation of Li+ ions in the WO6 octahedra when an external electrical bias was applied. These films reverted to transparent when a reverse external electrical bias was applied. W L3-edge X-ray absorption near-edge structure (XANES) measurements of the nanocrystalline and crystalline tungsten oxide films revealed that the intensity of the white-line feature decreases after coloration and recoverably increases after bleaching owing to the filling and unfilling of the W 5d–O 2p conduction band states. The second derivative of the W L3-edge XANES spectra indicated an increase in structural disordering following repeated cycles of coloration and bleaching. However, the extended X-ray absorption fine structure analysis showed that the nearest-neighbor W–O bond distances in the samples overall remain unchanged by coloration and bleaching. The nanocrystalline tungsten oxide film exhibited more effective recovery (∼97% after first cycle) of the electronic structures than the other two crystalline samples in terms of the filling and unfilling of the W 5d–O 2p conduction band states after repeated coloration and bleaching. These results show that the nanocrystalline tungsten oxide sample has superior electrochromic properties to the crystalline samples.
1. Introduction
Electrochromic (EC) materials are of great interest because their optical properties can be changed reversibly by the application of an electrical bias.1–3 These materials have a wide range of applications in smart windows, driving mirrors, displays, automobile glazing, modulation of the transmittance of light, solar radiation and others.1–6 The coloration process is well known to occur in such materials upon the intercalation of ions (H+, Li+, Na+ and others) when a suitable external electrical bias is applied. The ions can be deintercalated by reversing the polarity of the external bias, causing the material to revert back to being transparent in a process that is known as bleaching. Therefore, the intercalation and deintercalation of ions and the charge balancing of accompanying electrons are responsible for EC switching. Among various EC materials such as WO3, MoO3, NiO and Nb2O5, WO3 is one of the most extensively studied because of its useful EC properties.7–11 The evaluation of EC materials is typically based on their coloration efficiency (CE), reliability, response time and durability (which measures how long the EC properties of the material can be sustained when cations are intercalated/deintercalated under a cyclically applied electrical bias).12,13 Previous studies have shown that the crystal structure (crystalline or amorphous) and microstructure of tungsten oxide greatly influence its EC properties.12–14 The CE of amorphous tungsten oxide is higher than that of crystalline tungsten oxide,15,16 but its durability is poorer.12 Accordingly, nanocrystalline tungsten oxide has been proposed to have better EC properties owing to its large surface area and low density.12,13,17–20In general, the EC properties of the tungsten oxide films can be explained by two mechanisms, namely the valence state transition between W6+ and W5+ during the insertion of Li+ ions and polaron formation.21–26 In the intervalence transition, the intercalated Li+ ions that are trapped in the lattice alter the electronic structures, whereas additional Li+ ions also cause local distortions and form polarons. Specifically, Kuzmin et al.23 investigated the electronic and atomic structures of amorphous WO3 thin films by ex-situ X-ray absorption spectroscopy (XAS). They observed a relatively large average W–O bond distance in the first coordination shell of tungsten after coloration, which could be due to the formation of small-radius polarons based on the results of an extended X-ray absorption fine structure (EXAFS) study. This effect was associated with a strong deformation of the lattice around the tungsten with trapped electrons and the formation of the W5+ state. However, according to ex-situ EXAFS measurement Yang et al.27 observed contraction of the W–O bond distance following coloration, which could be due to the electrostatic attraction between oxygen and Li+ ions. These contradictory findings suggested that ex-situ EXAFS investigations of the relationship between the electrochromism and electronic/atomic structures of the tungsten oxide films might not be conclusive.
Pauporté et al.28 performed in-situ EXAFS studies of the amorphous WO3 thin films with electroinserted Li+ ions. They observed an increase of the W–O bond distance upon Li+ insertion, which was attributed to the formation of the W–O–Li bond. To the best of our knowledge, there has been no report of the in-situ XAS study of the nanocrystalline tungsten oxide films subjected to repeated cycles of coloration and bleaching. The observed electronic/atomic structures will be used to elucidate the mechanism that determines the EC properties. Besides that it is interesting to study the electronic structure of the films when its EC performance deteriorates. We have injected ∼63 mC cm−2 of Li+ charges in the films to achieve a deteriorated state within a few cycles of coloration and bleaching and studied electronic properties of both recoverable and deteriorated EC states.
This work investigates the electronic structures of three Li+ intercalated tungsten oxide thin films using in-situ W L3-edge X-ray absorption near-edge structure (XANES) measurements following repeated cycles of coloration and bleaching processes. They were (1) crystalline tungsten oxide formed without adding thiourea (400T-0), (2) thiourea-assisted nanocrystalline tungsten oxide embedded in an amorphous matrix of tungsten oxide (400T-045) and (3) thiourea-assisted crystalline tungsten oxide (450T-045). The EC properties like transmittance modulation, CE, response time and durability were demonstrated elsewhere13 which revealed that the nanostructured tungsten oxide film (400T-045) had better EC properties than the other two crystalline tungsten oxide films (400T-0 and 450T-045). In this study the local atomic structures of the samples under coloration and bleaching were also examined using the in-situ EXAFS technique. The nature of EC switching upon repeated cycles of coloration and bleaching in the tungsten oxide films can be explained in terms of changes in the electronic states by the filling and unfilling of the W 5d–O 2p hybridized conduction band states. The enhancement of the EC properties of the 400T-045 sample is attributed to the nanocrystalline structure tungsten oxide embedded in an amorphous tungsten oxide matrix which provides a large surface area for EC reactions. It facilitates the intercalation of Li+ ions into the tungsten oxide lattices owing to a high Li+ ion diffusion coefficient as well as rapid charge transfer.
2. Experimental section
The three samples, namely the crystalline tungsten oxide (400T-0), thiourea-assisted nanocrystalline tungsten oxide embedded in the amorphous matrix (400T-045) and thiourea-assisted crystalline tungsten oxide (450T-045), were synthesized using peroxopolytungstic acid (PTA). The samples were annealed at 400 °C (400T-0), underwent PTA with thiourea at 400 °C (400T-045) and underwent PTA with thiourea at 450 °C (450T-045), respectively. The preparation procedures and EC properties like transmittance modulation, CE, response time, reliability and Li ion diffusion coefficient for all three samples have been discussed in detail elsewhere.13 In this study, the tungsten oxide films (∼330 nm thick) were deposited on fluorine-doped tin oxide (FTO) coated on the SiO2 substrate via spin coating. As-deposited tungsten oxide thin film samples were described as bare samples.XAS experiments were carried out at the National Synchrotron Radiation Research Center (NSRRC), Hsinchu, Taiwan. The room temperature in-situ W L3-edge XANES and EXAFS of the tungsten oxide films were measured at the wiggler-17C and 01C beamlines. Tungsten powder was used as a reference to calibrate all spectra at the W L3-edge. The XAS spectra of the tungsten oxide films were obtained using a Lytle detector in the fluorescence mode. To avoid the possible self-absorption effect observed in concentrated sample, the XAS spectra of reference WO3 were obtained using the transmission mode. An electrolytic cell was used to conduct in-situ W L3-edge XANES and EXAFS measurements. The direction of the external bias (applied current +0.1 mA coloration/−0.1 mA bleaching for 3 min. or current density of 0.35 mA cm−2) applied to the tungsten oxide films was changed and the spectra were recorded. The area of the tungsten oxide films available for Li ions intercalation/deintercalation was ∼0.28 cm2. To evaluate durability, a current was applied cyclically, and each XANES and EXAFS data were recorded for about 20 and 35 min. With the pre-edge background being subtracted, all spectra were normalized to an edge jump of unity. The normalization was performed by matching the absorption coefficients from the pre-edge region to ∼40 eV above the W L3-edge. One cycle comprises one coloration and one bleaching processes. The XANES spectrum of the 400T-045 sample was obtained for three cycles. The XANES spectra of the 400T-0 and 450T-045 samples were recorded for two and one cycle, respectively, because clear recovery of color could not be obtained after these cycles for these samples.
3. Results and discussion
Fig. 1(a) schematically depicts the intercalation of Li+ ions in the tungsten oxide lattice. Tungsten and oxygen atoms are arranged as WO6 octahedra (tungsten atom surrounded by six oxygen atoms).29 Fig. 1(b) presents X-ray diffraction (XRD) patterns of the tungsten oxide films deposited on the FTO, which is coated on the SiO2 substrate. The diffraction peaks associated with the FTO substrate are marked with symbol *.30 In addition to those peaks, the other diffraction peaks in the patterns of the 400T-0 and 450T-045 films are indexed as those of monoclinic tungsten oxide phase according to the ICDD-PDF no. 01-072-0677. Sample 400T-045 yields a broad feature at 2θ = 22.5–25°, indicated by an arrow, suggesting the nanocrystalline nature of the sample. Raman analysis showed that the tungsten oxide samples have the monoclinic structure.13 Wu et al. performed microstructural analysis using high-resolution transmission electron microscopy (HRTEM) and found that the 400T-045 film is composed of porous nanocrystalline tungsten oxide embedded within an amorphous tungsten oxide matrix, however, samples 400T-0 and 450T-045 are highly porous and crystalline.13 The EC mechanism of tungsten oxide in Li+ electrolytes is well known to be5,8,20
 | (1) |
The transparent WO3 convert into deep blue color LixWO3 upon injection of Li+ ion and electron. The process of coloration and bleaching is known to be reversible. Fig. 1(c) and (d) schematically depict an electrolytic cell when Li+ ions are intercalated (∼63 mC cm−2) and deintercalated (−63 mC cm−2), respectively, by changing the polarity of an externally applied bias or current (0.35 mA cm−2 or 0.1 mA). The photographs of the 400T-045 tungsten oxide thin film (taken outside the electrolytic cell) with coloration and bleaching are shown in Fig. 1(c) and (d), respectively. The photographs of the other two films 400T-0 and 450T-045 are shown in Fig. S1 of ESI† (ref. 31). In the coloration process, electrons are injected into the tungsten oxide film through an external circuit with a current of +0.1 mA for 3 min. Excess negative charge in the form of electrons is compensated by the simultaneous intercalation of Li+ ions from the electrolyte into the tungsten oxide (i.e., intercalation of ∼63 mC cm−2 of Li+ ions). The Li+ intercalation causes the transfer of excess electrons into the conduction band, giving rise to optical absorption and the deep blue coloration to the transparent tungsten oxide film as shown in Fig. 1(c). In the bleaching process, a reverse current of −0.1 mA applied for again 3 min. and Li+ ions are deintercalated/extracted from the tungsten oxide (i.e., extraction of ∼63 mC cm−2 of Li+ ions). The blue films again become transparent, as presented in Fig. 1(d).
The charge injected (∼63 mC cm−2) in the samples are high compared to usual (∼20 mC cm−2). Our previous study of these samples using ∼20 mC cm−2 of Li+ charges showed that up to 30–50 cycles of coloration and bleaching the EC performance is reversible.13 In this study, the reason behind the selected little high charge (∼63 mC cm−2) injection is the limitation of synchrotron radiation X-ray beam time. Each XAS measurement (coloration and bleaching) requires at least 2 hours. It was practically not possible to perform measurements for 30–50 cycles to study the electronic structure of films under recovered (reversible EC performance) and deteriorated condition (irreversible EC performance). Therefore we have injected high charge density in the films to study the electronic structure of the films under recovered and deteriorated conditions in a few cycles of coloration and bleaching. However, the selected charge injection value is not too high, as the recoverable of EC performance was observed upto few cycles. We have performed initial experiments and optimized the parameters, shown in the ESI† (Table S1, ref. 31). The study of the electronic structure of deteriorated film condition is equally important. If we have not selected the higher charge injection (∼63 mC cm−2) condition, then we would may not be able to study the electronic structures of deteriorated film condition, which is more interesting and gives information of complete electronic structures during different (recoverable and deteriorate) states of samples.
Fig. 2(a)–(c) display normalized W L3-edge XANES spectra of the 400T-0, 400T-045 and 450T-045 samples, respectively, under repeated cycles of coloration and bleaching and the spectrum of the reference WO3 powder. All W L3-edge XANES spectra exhibit a broad white-line (WL) feature, which is attributed primarily to electron transitions from W 2p3/2 orbitals to unoccupied W 5d–O 2p hybridized conduction band states. Clearly, the general line-shapes and WL-widths in the W L3-edge XANES spectra of the 400T-0, 400T-045 and 450T-045 samples under repeated cycles of coloration and bleaching are close to those of the reference WO3. The first coloration (+0.1 mA) of the 400T-0 sample shown in Fig. 2(a) involved changing the color into deep blue as the Li+ ions were intercalated; the decrease in the WL intensity was attributed to the electrons injected from an external circuit to fill the W 5d–O 2p states. Upon first bleaching (−0.1 mA), the film became transparent owing to the deintercalation of Li+ ions. The WL intensity increases relative to that of the first coloration (+0.1 mA), suggesting the unfilling of the W 5d–O 2p states. The observed decrease (at +0.1 mA, coloration) and recoverable increase (at −0.1 mA, bleaching) of the WL intensity relative to that of the bare 400T-0 sample indicated a decrease and an increase of the W 5d–O 2p density of states (DOSs) in the conduction band during coloration and bleaching, respectively. Fig. 2(a) also reveals that the recovery of the WL intensity (to that of bare 400T-0 WL), particularly the WL intensity following the bleaching process, does not reach the exact original value of the bare sample. The poor recovery of the WL intensity following repetitive cycling could be due to the increase in structural disordering in the sample. A similar decrease and increase of the WL intensity was observed at the W L3-edge upon coloration and bleaching of the other two 400T-045 and 450T-045 samples [Fig. 2(b) and (c)]. The WL intensity after the first cycle of the 400T-045 sample recovered better than those of the 400T-0 and 450T-045 samples.
To determine the durability of the samples during repeated cycles of coloration and bleaching, Fig. 2(d) plots the integrated WL intensity in the W L3-edge spectra (between 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 and 10
190 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 eV) after subtraction of the background, indicated by dashed lines, for all three samples. The oscillations in the plot related to changes in the electronic structures associated with the filling and unfilling of the W 5d–O 2p conduction band states in the coloration and bleaching processes. Fig. 2(d) demonstrates that coloration is associated with a decrease in the WL intensity upon Li+ intercalation, which causes a transfer of electrons between Li+ ions and the WO6 octahedra and the filling of the W 5d–O 2p states. In the bleaching process, the WL intensity increases as Li+ are deintercalated. This leads to unfilling of the W 5d–O 2p conduction band states. In the first cycle, the difference between the WL intensity of coloration differs greatly from that of bleaching and this difference decreases gradually as the number of cycles increases. The recovery under repeated cycles of coloration and bleaching shown in Table 1 was determined from the intensities of the WL feature at W L3-edge relative to those of the bare samples. The integrated intensity (between 10
230 eV) after subtraction of the background, indicated by dashed lines, for all three samples. The oscillations in the plot related to changes in the electronic structures associated with the filling and unfilling of the W 5d–O 2p conduction band states in the coloration and bleaching processes. Fig. 2(d) demonstrates that coloration is associated with a decrease in the WL intensity upon Li+ intercalation, which causes a transfer of electrons between Li+ ions and the WO6 octahedra and the filling of the W 5d–O 2p states. In the bleaching process, the WL intensity increases as Li+ are deintercalated. This leads to unfilling of the W 5d–O 2p conduction band states. In the first cycle, the difference between the WL intensity of coloration differs greatly from that of bleaching and this difference decreases gradually as the number of cycles increases. The recovery under repeated cycles of coloration and bleaching shown in Table 1 was determined from the intensities of the WL feature at W L3-edge relative to those of the bare samples. The integrated intensity (between 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 and 10
190 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 eV) for the bare samples is set to 100 and the proportional change in this intensity for the samples after each cycle (of coloration and bleaching) was calculated.
230 eV) for the bare samples is set to 100 and the proportional change in this intensity for the samples after each cycle (of coloration and bleaching) was calculated.
| Sample | 1st Bleaching | 2nd Bleaching | 3rd Bleaching |
|---|---|---|---|
| 400T-0 | 65% | 47% | — |
| 400T-045 | 97% | 61% | 31% |
| 450T-045 | 62% | — | — |
After the first cycle, the sample 400T-0 exhibited a recovery of almost 65% (relative to the WL intensity of bare 400T-0); this value becomes ∼47% after the second cycle. The 400T-045 sample exhibited ∼97% (relative to the WL intensity of bare 400T-045) recovery following the first cycle and ∼61% and ∼31% after the second and third cycles, respectively. The 450T-045 sample exhibited ∼62% (relative to the WL intensity of bare 450T-045) recovery after the first cycle. Table 1 clearly reveals that the deintercalation of Li+ ions from tungsten oxide after a few cycles is slow. Notably, Fig. 2(d) further indicates that the 400T-045 sample exhibits greater recovery of the electronic structures than the 400T-0 or 450T-045 sample, as it exhibits a ∼97% recovery of the WL intensity after the first cycle relative to the corresponding bare sample. The changes in the electronic structures are associated with the filling and unfilling of the W 5d–O 2p conduction band states, which related to the EC performance of the samples.
As stated previously, it was observed that the EC properties of the 400T-045 sample were better than those of the other two (400T-0 and 450T-045) samples when ∼20 mC cm−2 Li+ ions were injected.13 The EC properties (coloration efficiency, transmittance modulation at wavelength 632 nm after 30 cycles and diffusion coefficient) of the samples are tabulated in Table S2 of ESI† (ref. 31). The 400T-045 sample exhibited ∼40% transmittance modulation at a wavelength of 632 nm after 30 cycles of coloration/bleaching, however the other two samples (400T-0 and 450T-045) exhibit ∼10% of transmittance modulation after a few cycles. It indicates superior durability of 400T-045 sample than other two. The samples 400T-0, 400T-045 and 450T-045 exhibited CE of 7, 37 and 6 cm2 C−1 respectively, suggesting high CE of 400T-045 compared to other two samples. Also, it was observed that 400T-045 sample possesses a fast response time and sufficient reliability.13 The Li+ ion diffusion coefficient obtained in the 400T-045 sample is ∼10−11–10−10 cm2 S−1 and those in the 400T-0 and 450T-045 samples are ∼10−14–10−12 cm2 S−1, revealing that the Li+ ion diffusion coefficient is two to three orders of magnitude greater in an amorphous matrix embedded with nanocrystalline tungsten oxide than in the other two crystalline samples.13 Hence, the superior EC properties of the 400T-045 sample were attributed to the high Li+ ion diffusivity. Our W L3-edge XANES results clearly demonstrate coloration and bleaching induced changes in the electronic structures associated with intercalation/deintercalation of Li+ ions or their diffusivity in the WO6 octahedra. The better recovery of the electronic structures following repeated cycling in the nanocrystalline tungsten oxide (400T-045 sample) can be due to the embedded nanocrystalline tungsten oxide in an amorphous tungsten oxide matrix which provides a large reaction surface area.
Fig. 3(a)–(c) plot the second derivative of the W L3-edge XANES spectra of the 400T-0, 400T-045 and 450T-045 samples under repeated cycles of coloration and bleaching and reference WO3 to elucidate structural disordering after repeated cycling. The second derivatives of the spectra of all three bare samples show two valley-shaped features (indicated by two solid bars), whose energy separation represents the splitting of the W 5d orbitals into t2g and eg by the ligand field of the surrounding oxygen atoms.32 The t2g orbitals have lower energies than those of eg orbitals. The splitting of W 5d orbitals is greater in an octahedral field than in a tetrahedral or disordered octahedral field.33 Yamazoe et al.32 experimentally (from W L3-edge XANES) and theoretically demonstrated that ordered octahedral WO6 exhibits a large splitting between t2g and eg orbitals (∼4.9–5.6 eV), whereas a distorted octahedral structure like WO3 exhibits a splitting of only ∼4.0 eV. As presented in Fig. 3(a)–(c), the energy splitting between t2g and eg for bare 400T-0, 400T-045, 450T-045 and reference WO3 samples are 3.8 ± 0.2 eV, 3.8 ± 0.2 eV, 3.8 ± 0.2 eV and 4.5 ± 0.2 eV, respectively. The observed relatively small energy splitting suggests a distorted WO6 octahedral symmetry, which is consistent with the higher W L3-edge WL intensity of the bare samples than reference WO3 shown in Fig. 2(a)–(c).
In Fig. 3(a)–(c), the second derivatives of the W L3-edge XANES spectra of the samples with coloration (+0.1 mA) reveal that both t2g and eg bands are broadened relative to those of the bare sample. The bands tended to return to their normal state (split features) with bleaching (−0.1 mA). Fig. 3(d) plots the energy splitting between t2g and eg orbitals versus the number of cycles of coloration and bleaching. The 400T-045 sample exhibited the best recovery of splitting after the first cycle. Importantly, the figure demonstrates that as the number of cycles increases, the energy splitting between t2g and eg decreases and the bands become broad such that the eg feature is almost smeared out, especially after the second and third cycles, as displayed in Fig. 3(a)–(c). The decrease in energy splitting and the broadening of the eg feature with the increased number of cycling are strongly associated with the increased structural disorder in the WO6 octahedra. Tsai et al.34 also observed the broadening of Mo 4d t2g and eg bands by inserted K-atom induced distortion of the MoO6 octahedra. During repeated cycles, some intercalated Li+ ions were trapped in the WO6 octahedra and not all them could be extracted by bleaching. The injected Li+ ions slowly adapted into the WO6 octahedra and gave rise to structural disorder in the samples. The results in Fig. 3(d) reveal that structural distortion of the WO6 octahedra is a major contributor to the observed poor recovery of electronic properties following repeated cycling. The nanocrystalline 400T-045 sample exhibited better recovery of energy splitting in the first cycle (of coloration and bleaching) than the other two (400T-0 and 450T-045) crystalline samples. Further structural distortion of the WO6 octahedral units in the samples by coloration and bleaching was examined using in-situ W L3-edge EXAFS.
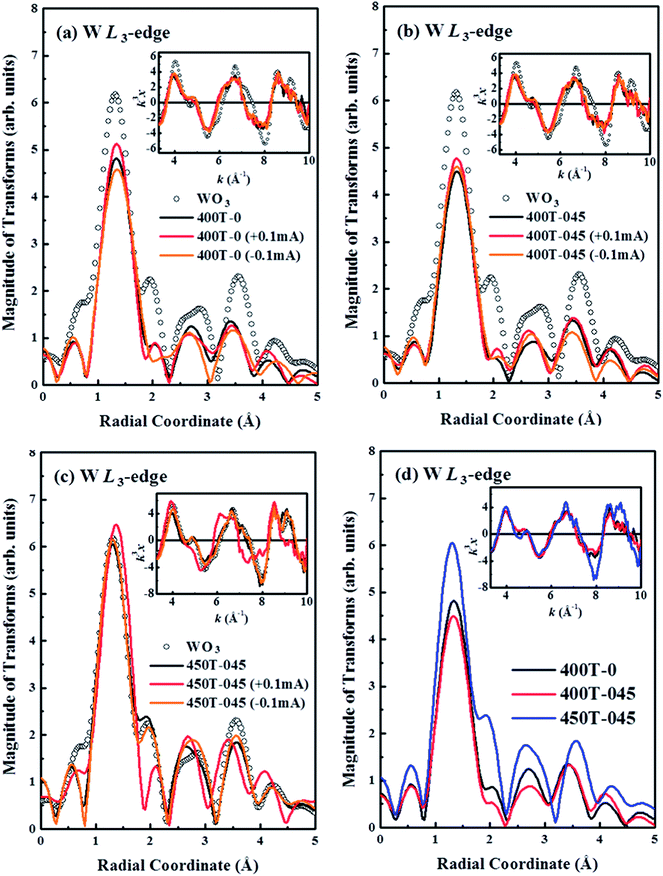
Fig. 4(a)–(c) display the Fourier transform (FT) and the corresponding EXAFS k3χ data at W L3-edge for the 400T-0, 400T-045 and 450T-045 samples and the reference WO3, respectively, in the first cycle of coloration and bleaching process. The main feature at ∼1.33 Å (without phase correction) corresponds to the first W–O shell. The main features of the four samples indicated two different nearest-neighbor W–O bond distances.35 The general line-shapes and radial distribution of the FT spectra of the tungsten oxide samples following the first cycle of coloration and bleaching are similar to those of the corresponding bare samples and the reference WO3. These results reveal that the nearest-neighbor W–O bond distances of the tungsten oxide samples following the first cycle are close to those of the bare samples and reference WO3. Nevertheless, the intensity of the main W–O feature in the FT spectra of the 400T-0 sample, presented in Fig. 4(a), increases with coloration, because the intercalated Li+ ions in the WO6 octahedra increase the coordination number around the W atoms in LixWO3, as specified by eqn (1). Upon bleaching, the Li+ ions deintercalated and the intensity of the main W–O feature in the FT spectra decreased and becomes smaller than that of the bare sample, indicating that bleaching depletes the Li+ ions. Meanwhile, the bleached sample still has a relatively large structural disordering or Debye–Waller factors as evidenced by a lower W–O feature in the FT spectra than the bare sample. This result is consistent with that of the second derivative of W L3-edge XANES [Fig. 3(d)], which showed that the 400T-0 sample did not have a full recovery of structural ordering after the first cycle. The FT of the EXAFS spectra of the bare 400T-045 sample with coloration and bleaching as shown in Fig. 4(b) is similar to that of the 400T-0 sample presented in Fig. 4(a). Coloration slightly increases the intensity of the main W–O feature in the FT spectra and bleaching caused it to return to nearly the original value of the bare 400T-045 sample, suggesting effective recovery of structural ordering of the 400T-045 sample after the first cycle in consistence with the result of the second derivative of W L3-edge XANES [Fig. 3(d)].
Fig. 4(c) presents the FT of the EXAFS spectra of the 450T-045 sample with coloration and bleaching. Coloration of the 450T-045 sample caused the main W–O feature in the FT spectra at ∼1.33 Å to shift slightly to ∼1.40 Å and its intensity also increases. As shown in Fig. 4(d), the observed higher intensity of the FT spectrum of the bare 450T-045 sample than those of the other two samples (400T-0 and 400T-045) can be associated with a variation in the annealing temperatures of the samples. Sample 450T-045 was annealed at a higher temperature (450 °C) than the other two samples (400 °C), producing more crystalline phases, which were also observed in the XRD and HRTEM measurements.13 However, bleaching of the 450T-045 sample caused the main W–O feature in its FT spectra to shift back to its original position at 1.33 Å. Its intensity also decreased, returning to the original value as the bare 450T-045 sample. Other groups have also observed elongation of the bond distances with cation intercalation in tungsten oxides.23,28,36 The intensity of the main W–O feature in the FT of the spectrum of the nanocrystalline 400T-045 sample as shown in Fig. 4(d) is much smaller than those of the two (400T-0 and 450T-045) crystalline samples. This property indicates that the WO6 octahedra in the nanocrystalline sample (i.e. high surface to volume ratio) was much more structurally distorted than those of other two crystalline samples. Clearly, the superior enhancement of the EC properties or coloration efficiency in the 400T-045 sample was due to embedded nanocrystallites in the amorphous tungsten oxide matrix, which results in a large reaction surface area for the intercalation of enough Li+ ions into WO3 that improves Li+–tungsten oxide interaction.
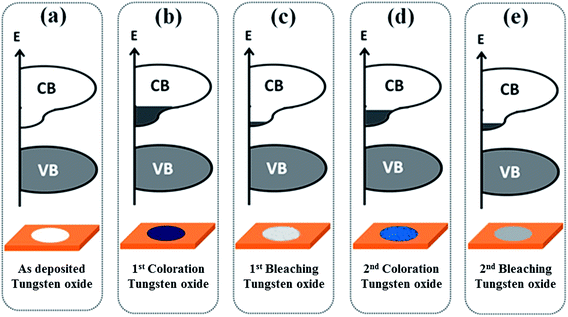
Fig. 5 schematically depicts the mechanism of EC switching and the changes in the electronic structures during coloration and bleaching of the tungsten oxide films. Fig. 5(a) presents the electronic structures of a bare tungsten oxide film; the conduction band minimum (CBM) is primarily composed of the W 5d–O 2p DOSs and the valence band maximum (VBM) near the Fermi level is dominated by O 2p states.2,37 Tungsten oxide has a band gap of 2.6 eV.38 In the first coloration process with current density of 0.35 mA cm−2 as shown in Fig. 5(b), the filling of the states near CBM is observed, which is consistent with the decrease of the WL intensity in the W L3-edge spectra (Fig. 2). The changes of DOSs in the conduction band of the tungsten oxide during coloration substantially affect the optical absorption and cause the transparent tungsten oxide film to become deep blue. In this process, electrons are injected into the tungsten oxide film via an external circuit. The simultaneous intercalation of Li+ ions from the electrolyte into the tungsten oxide compensates for the excess negative charges of the electrons, which causes transfer of excess electrons into the conduction band, giving rise to changes in the optical properties and converts the transparent tungsten oxide film into the deep blue as also shown in Fig. 5(b) and 1(c). However, coloration overall does not change the nearest-neighbor W–O bond distances in the samples. There were several reports that explained the EC properties on the basis of an intervalence transition between W6+ and W5+ or the formation of polarons.21–26 However, the present study mainly shows that the electronic structural changes associated with the filling of the W 5d–O 2p conduction band states causes coloration in the tungsten oxide films. Hashimoto et al.24 also observed changes in the conduction band states in a colored tungsten oxide film using electron energy-loss spectroscopy. The unfilling of the W 5d–O 2p conduction band states (supported by the increase of the W L3-edge WL intensity) has also been observed during the deintercalation of Li+ ions, making the film again transparent, as shown in Fig. 5(c) and 1(d).
Additionally, the first bleaching process did not empty the conduction band completely as revealed by the W L3-edge WL intensity [the WL intensity of the bleached sample is less than that of the bare sample as shown in Fig. 2(d)]. Electrons partially filled the W 5d–O 2p conduction band states during the second coloration [Fig. 5(d)], but to a lesser extent than in the first coloration process [Fig. 5(b)]. This finding is consistent with the smaller decrease in the WL intensity after the second coloration than the first coloration as presented in Fig. 2(d). Furthermore, the second coloration converts the film to blue color, though it was not a deep blue as in the first coloration, because repeated coloration and bleaching enhanced structural disordering as revealed by the second derivative of W L3-edge XANES (Fig. 3(d)). In the second bleaching [Fig. 5(e)], unfilling of the conduction band reappeared, though to a lesser extent than in the first bleaching process [Fig. 5(c)]. This trend is consistent with the observation that the fluctuation of the WL intensities at the W L3-edge between coloration and bleaching decreases gradually with the number of coloration-bleaching cycles. After the second bleaching, the film is clearly less transparent than the one after the first bleaching. An increase in the structural disorder of the WO6 octahedra is responsible for the observed poor recovery of the filling and unfilling of the conduction band after repeated cycles. Structural disorder could be due to that the application of a reverse electrical bias during bleaching did not remove Li+ ions completely from the WO6 octahedra after intercalation during coloration.34 The number of trapped Li+ ions in the WO6 octahedral lattices gradually increases and degrades the EC properties and CE.
The present study obtained a relationship between the EC switching and electronic structures. The filling of the W 5d–O 2p conduction band states makes the tungsten oxide film deep blue and the unfilling of these states reverses the effect and makes the film transparent. The recovery of the electronic structures of the nanocrystalline sample (400T-045) after the first cycle of coloration and bleaching was better than that of the other two (400T-0 and 450T-045) crystalline samples, and its EC properties were superior. Earlier investigations have clearly shown that the nanocrystalline film is highly porous with large active surface area and have microstructures that result in short diffusion paths and allow enough ions to be intercalated into WO3 to promote ion–tungsten oxide interactions.12,13,39,40 Nanostructure is the origin for a high coloration efficiency and high rate of transfer (removal) of electrons to (from) the conduction band during coloration (bleaching) for the 400T-045 sample. Therefore, a better EC performance for the 400T-045 samples is attributed to its unique nanostructure.
4. Conclusions
In summary, using in-situ W L3-edge XAS measurements of nanocrystalline and crystalline tungsten oxide films with repeated cycles of coloration and bleaching, this study obtained a correlation between the EC switching and electronic structures of these films. W L3-edge XANES investigations revealed that Li+ intercalation and the filling of the W 5d–O 2p conduction band states associated with coloration which turns the transparent film to deep blue. In the bleaching process, unfilling of the W 5d–O 2p conduction band states, which reverses the films to be transparent, is caused by deintercalation/extraction of Li+ ions. Changes in the electronic structures by filling and unfilling of the W 5d–O 2p conduction band states is concluded to be responsible for the EC switching in the tungsten oxide films. The 400T-045 sample, which contains nanocrystalline tungsten oxide embedded in the amorphous tungsten oxide matrix, exhibited greater recovery (∼97%) of its electronic structure after the first cycle than the other two crystalline samples (400T-0 and 450T-045). This result is attributed to a large surface area that was available for electrochemical reactions and the fact that the amorphous matrix facilitates the intercalation of Li+ ions into the WO6 octahedral lattices, which gives rise to a high Li+ ion diffusion coefficient and rapid charge transfer. The poor recovery of the filling and unfilling of the W 5d–O 2p conduction band states after repeated cycles attributed to an increase in the structural disorder of the WO6 octahedra. This study has provided better understanding of the EC properties of the tungsten oxides and their potential usage as an energy-saving smart window and other devices.Acknowledgements
The author (WFP) is grateful to the National Science Council of Taiwan for financial support this research under Grant no. NSC 99-2119-M-032-004-MY3.References
- M. Grätzel, Nature, 2001, 409, 575 CrossRef PubMed.
- G. Niklasson and C. Granqvist, J. Mater. Chem., 2007, 17, 127 RSC.
- K. Bange, Sol. Energy Mater. Sol. Cells, 1999, 58, 1 CrossRef CAS.
- D. R. Rosseinsky and R. J. Mortimer, Adv. Mater., 2001, 13, 783 CrossRef CAS.
- D. T. Gillaspie, R. C. Tenent and A. C. Dillon, J. Mater. Chem., 2010, 20, 9585 RSC.
- X. Chang, L. Dong, Y. Yin and S. Sun, RSC Adv., 2013, 3, 15005 RSC.
- S. K. Deb, Sol. Energy Mater. Sol. Cells, 2008, 92, 245 CrossRef CAS PubMed.
- C. G. Granqvist, Sol. Energy Mater. Sol. Cells, 2000, 60, 201 CrossRef CAS.
- R. Sydam, M. Deepa and A. K. Srivastava, RSC Adv., 2012, 2, 9011 RSC.
- G. Bräuer, Surf. Coat. Technol., 1999, 112, 358 CrossRef.
- G.-f. Cai, X.-l. Wang, D. Zhou, J.-h. Zhang, Q.-q. Xiong, C.-d. Gu and J.-p. Tu, RSC Adv., 2013, 3, 6896 RSC.
- S.-H. Lee, R. Deshpande, P. A. Parilla, K. M. Jones, B. To, A. H. Mahan and A. C. Dillon, Adv. Mater., 2006, 18, 763 CrossRef CAS.
- W.-T. Wu, W.-P. Liao, J.-S. Chen and J.-J. Wu, ChemPhysChem, 2010, 11, 3306 CrossRef CAS PubMed.
- P. J. Wojcik, A. S. Cruz, L. Santos, L. Pereira, R. Martins and E. Fortunato, J. Mater. Chem., 2012, 22, 13268 RSC.
- J. Scarminio, A. Urbano and B. Gardes, Mater. Chem. Phys., 1999, 61, 143 CrossRef CAS.
- E. Ozkan, S.-H. Lee, C. E. Tracy, J. R. Pitts and S. K. Deb, Sol. Energy Mater. Sol. Cells, 2003, 79, 439 CrossRef CAS.
- A. S. Aricò, P. Bruce, B. Scrosati, J.-M. Tarascon and W. V. Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef PubMed.
- J. Wang, E. Khoo, P. S. Lee and J. Ma, J. Phys. Chem. C, 2009, 113, 9655 CAS.
- Y. Zhang, J. Yuan, J. Le, L. Song and X. Hu, Sol. Energy Mater. Sol. Cells, 2009, 93, 1338 CrossRef CAS PubMed.
- D. Ma, G. Shi, H. Wang, Q. Zhang and Y. Li, J. Mater. Chem. A, 2013, 1, 684 CAS.
- T. Yoshimura, J. Appl. Phys., 1985, 57, 911 CrossRef CAS PubMed.
- R. S. Crandall and B. W. Faughnan, Phys. Rev. Lett., 1977, 39, 232 CrossRef CAS.
- A. Kuzmin and J. Purans, J. Phys.: Condens. Matter, 1993, 5, 2333 CrossRef CAS.
- S. Hashimoto and H. Matsuoka, J. Appl. Phys., 1991, 69, 933 CrossRef CAS PubMed.
- E. Cazzanelli, G. Mariotto, C. Vinegoni, A. Kuzmin, J. Purans, D. Fisica, U. Calabria and R. Cosenza, Ionics, 1999, 5, 335 CrossRef CAS.
- M. Saenger, T. Höing, B. Robertson, R. Billa, T. Hofmann, E. Schubert and M. Schubert, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 245205 CrossRef.
- Y. Yang and J. Yao, J. Phys. Chem. Solids, 2000, 61, 647 CrossRef CAS.
- T. Pauporté, Y. Soldo-Olivier and R. Faure, J. Electroanal. Chem., 2004, 562, 111 CrossRef PubMed.
- H. Zheng, J. Z. Ou, M. S. Strano, R. B. Kaner, A. Mitchell and K. Kalantar-zadeh, Adv. Funct. Mater., 2011, 21, 2175 CrossRef CAS.
- Y. Masuda, T. Ohji and K. Kato, ACS Appl. Mater. Interfaces, 2012, 4, 1666 CAS.
- See ESI.†.
- S. Yamazoe, Y. Hitomi, T. Shishido and T. Tanaka, J. Phys. Chem. C, 2008, 112, 6869 CAS.
- S. Bare, G. Mitchell, J. J. Maj, G. E. Vrieland and J. L. Gland, J. Phys. Chem., 1993, 97, 6048 CrossRef CAS.
- H. M. Tsai, K. Asokan, C. W. Pao, J. W. Chiou, C. H. Du, W. F. Pong, M.-H. Tsai and L. Y. Jang, Appl. Phys. Lett., 2007, 91, 022109 CrossRef PubMed.
- T. Pauporté, Y. Soldo-Olivier and R. Faure, J. Phys. Chem. B, 2003, 107, 8861 CrossRef.
- C.-K. Wang, D. R. Sahu, S.-C. Wang, C.-K. Lin and J.-L. Huang, J. Phys. D: Appl. Phys., 2012, 45, 225303 CrossRef.
- A. Hjelm, C. Granqvist and J. Wills, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 2436 CrossRef CAS.
- G. Granqvist, Handbook of Inorganic Electrochromic Materials, Elsevier, The Netherlands, 2002 Search PubMed.
- D. Ma, H. Wang, Q. Zhang and Y. Li, J. Mater. Chem., 2012, 22, 16633 RSC.
- F. Lin, J. Cheng, C. Engtrakul, A. C. Dillon, D. Nordlund, R. G. Moore, T.-C. Weng, S. K. R. Williams and R. M. Richards, J. Mater. Chem., 2012, 22, 16817 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45421e |
| This journal is © The Royal Society of Chemistry 2014 |