Influence of substrate bias and post-deposition Cl treatment on CdTe film grown by RF magnetron sputtering for solar cells†
Hui Lia,
Xiangxin Liu*a,
Biao Yanga and
Pingjian Wangb
aThe Key Laboratory of Solar Thermal Energy and Photovoltaic System, Institute of Electrical Engineering, CAS, Beijing 100190, China. E-mail: shinelu@mail.iee.ac.cn; lihui@mail.iee.ac.cn
bAll-China Environment Federation (ACEF), Beijing, 100013, China
First published on 9th December 2013
Abstract
CdTe thin films were grown by RF magnetron sputtering at deposition pressures of 1.5–4 Pa and substrate potentials of 8.8, 0, −8.2, −18.9, −28.3, −38.1, −48.0, and −98.7 V. All as-grown CdTe thin films had undergone CdCl2 treatment in dry air. The deposition pressure had a large influence on the crystalline quality, morphology, and grain size. At 1.5–3 Pa, CdTe thin films with good crystalline quality and column morphology were successfully obtained. The substrate potential also influenced the phase composition, grain size, morphology, microstress, and roughness of the obtained CdTe thin films. The phase composition, morphology, grain size, microstress, and crystalline quality changed significantly after CdCl2 annealing treatment. For CdTe grown at 3 Pa, the grain size reached a maximum when the Cl treatment time was 20 min; for CdTe thin films deposited at 2 Pa, grain size was the greatest when the Cl treatment time was 43 min. The maximum grain size corresponded to the best performance of the CdTe solar cell. CdTe thin films grown at different substrate potentials were subjected to Cl treatment at 400 °C for 43 min. The internal structure was observed using scanning electron microscopy of sample cross-sections sliced by a focused ion beam. After Cl treatment, voids were seen at the CdTe grain boundaries, and at the interfaces CdS/F:SnO2 and CdS/CdTe. The CdTe film grown at a substrate potential of −18.9 V produced the best solar cell, with device parameters of η = 12.78%, Voc = 779 mV, Jsc = 22.91 mA cm−2, and FF = 71.62%.
1. Introduction
Cadmium telluride (CdTe), a direct energy band gap (Eg) semiconductor with Eg of 1.45 eV at room temperature, is a good solar cell material because its band gap closely matches the peak of the solar spectrum. CdTe is quite stable because only the stoichiometric CdTe compound is formed at temperatures higher than 300 °C. Its optical absorption coefficient is in the range of 104 to 105 cm−1, which means that a CdTe layer 2 μm thick will absorb nearly 100% of the incident solar radiation, greatly decreasing the amount of material needed for a solar cell.Many techniques can be applied to grow CdTe thin films, including sputtering, close spaced sublimation (CSS), and vapor transport deposition (VTD).1,2 The best energy conversion efficiency (η) obtained for a small-area CdTe thin film solar cell is 19.6%,3 which is still much lower than its theoretical efficiency of 29%.4 Many groups have made efforts for improving the efficiency of CdTe solar cells. A CdTe cell deposited by radio-frequency (RF) magnetron sputtering with a 2.3 μm thick absorber layer achieved an efficiency of 14% with aluminum doped zinc oxide (AZO) as front electrodes in 2003.5 RF sputtering is still considered as a promising deposition method for high efficiency CdTe based solar cell, due to its standardized equipment, low temperature process and best demonstrated ultra-thin device performance. To obtain device-quality CdTe films, it is typical to modify the deposition parameters such as deposition pressure, substrate temperature, gas flow, distribution of magnetic field, growth pressure, RF power.6 However, the influence of substrate bias on CdTe thin films has rarely been studied, though it is well known that the application of a substrate bias can modify the results of physical vapor deposition, where film properties such as morphology, grain size, and crystallinity are sensitive to the impinging of energy and ions on the substrate.7–10 The surface mobility of sputtered atoms or ions arriving at the substrate can be adjusted by not only the system deposition pressure but also the substrate bias applied during the growth process. The substrate potential is known to change the surface morphology and grain size of CdTe thin films.30
Ultra-thin CdTe solar cells are low in cost and require small amounts of material, facilitating the rapid development of CdTe solar cells. The efficiency and open-current voltage (Voc) of ultra-thin CdTe solar cells can reach up to 20% and 1 V, respectively.11 Using ultra-thin CdTe films as the absorbing layer greatly reduces the amount of material required.12 The key barrier to realizing such a device is to first realize an optimal ultra-thin CdTe layer. This is an advantage of low-temperature RF sputtering deposition, which among all deposition methods was able to produce the highest-efficiency solar cell with a CdTe layer less than 1 μm thick.13 Efficiencies of as high as 8% and 11% have been obtained with CdTe layers 0.25 μm and 0.5 μm thick, respectively, grown by RF magnetron sputtering.13 However, decreasing the CdTe thickness also leads to several issues in the device physics. For example, pinholes and layer nonuniformity can generate severe weak diode effects that can reduce the open circuit voltage (Voc) of the device, such as insufficient depletion width or the presence of overlap between the PN junction and the back Schottky junction. Thus, in the present study, the conventional thickness of 2–2.3 μm, easily obtained according to literature procedures, is used to characterize the influence of substrate bias; the insights thus obtained will aid the synthesis of ultra-thin CdTe thin films for high-efficiency, low-cost solar cells.
A critical procedure in the fabrication of high-efficiency CdTe solar cells is “Cl treatment,” that is, annealing at high temperature in an atmosphere of CdCl2.14–16 During Cl treatment, CdTe recrystallizes, resulting in grains growth with as-grown grains size smaller than 1 μm.17 The increased grain size improves device properties by decreasing the number and length of grain boundaries and the occurrence of deep energy levels within the band gap, which can serve as electrical recombination centers that prevent current transport and provide shunting paths.18 Cl treatment also improves the performances of solar cells even in samples without obvious grain increase.19
RF magnetron sputtering usually yields grains smaller than 1 μm. Cl treatment of these films changes their morphology and crystallographic properties, and passivates grain boundaries to produce a smooth interface between CdS and CdTe, where the chemical composition of the interface can be written as CdTexS1−x/CdSyTe1−y. The alloyed CdTexS1−x/CdSyTe1−y interface avoids the lattice mismatch, interfacial defects, and inhomogeneous microstresses that would typically arise from the interface of CdS (hexagonal) and CdTe (cubic).20 The CdTexS1−x/CdSyTe1−y layer adjacent to the CdS/CdTe interface is crucial to increasing Voc and diode quality because it decreases the nonradioactive recombination rate at and near the CdS/CdTe interface and/or increases the built-in electric field.21,22 Cl treatment reduces the density of deep levels inside the band gap and changes the defect structure, leading to better devices. Furthermore, Cl treatment changes the dominant current transport mechanism, from interface recombination and tunneling to depletion region recombination, indicating a decrease in the density and dominance of interface states.19 Cl treatment thus improves the optical response of solar cells.23
The photoconductivity response as well as optical transmission of CdS in the 500–600 nm range was observed after Cl treatment.24 Cl treatment improved carrier collection from the bulk as well as across the hetero-interface.19 Moreover, Cl treatment improved the p-doping of CdTe, which is quite important for obtaining a high-quality device. Thus, it is clear that Cl treatment improves Voc, increases short circuit density (Jsc), and reduces shunting, leading to a high-quality device.19,25 However, excessive Cl treatment results in adhension-loss problems and decreases Voc.25 Cl treatment furthermore does not yield exactly identical results for CdTe thin films obtained by different growth techniques, or even on different films formed by the same technique. Therefore, it is desirable to study Cl treatment of CdTe thin films grown by RF magnetron sputtering at different deposition pressures, with and without substrate bias.
To understand the effects of Cl treatment and the mechanisms that limit device performance, it is necessary to observe the internal structure and interfacial properties of as-grown and Cl-treated CdTe solar cells. Such an investigation is typically conducted by high-resolution scanning electron microscopy (SEM) of samples prepared by direct manually cleaving. However, direct manual cleavage may introduce damage to the thin film, causing loss of information about the sample and the device. Focused ion beam (FIB) milling is a good high-resolution tool for nanofabrication, including sample sectioning, that causes almost no damage.26–28 FIB milling combined with high-resolution SEM analysis was conducted to observe as-grown and Cl-treated CdTe solar cells grown by the CSS technique.29 However, this technique has not yet been used for sputtered CdTe solar cells.
In this study, CdS and CdTe polycrystalline thin films were deposited in the same vacuum chamber by RF magnetron sputtering. The influence of deposition pressure and substrate bias on crystalline quality, morphology, phase composition, and grain size was studied. Different Cl treatments were performed on CdTe films grown with different parameters. CdTe thin films and device properties were characterized after Cl treatment. We used FIB milling to give reproducible, clean, and high-quality CdTe/CdS solar cell cross-sections that were then examined by high-resolution field emission gun scanning electron microscopy (FEG-SEM). A CdTe solar cell with an efficiency of 12.78% was obtained via substrate bias on soda-lime glass without a high-resistance transparent (HRT) layer.
2. Experiments
The CdS and CdTe films reported here were all grown in a home-made double-chamber RF magnetron sputtering system, as described by Li et al.30 CdS and CdTe thin films were grown on 10 × 10 cm2 commercial SnO2:F-coated soda-lime glass substrates (TEC15) from Pilkington North America Inc. The growth parameters for CdS and CdTe can be found elsewhere.30 The thickness of CdS and CdTe was measured by an optical transmission in situ thickness apparatus (Fig. S1†). We measured the plasma potential (Vp) at 1.32 W cm−2 RF power and 2 Pa Ar pressure, by using a piece of 10 × 10 cm2 TEC15 substrate as a planar Langmuir probe. Two pairs of choking coils with resonant frequencies of 13.56 and 27.12 MHz, respectively, were connected in series to impede the RF signal and its double frequency harmonic. A Keithley 2400 source V meter was applied to conduct the current–voltage (I–V) measurement. The obtained I–V curve was corrected by subtracting the DC potential drop from the RF choking. The nominal substrate potentials of 0, −10, −20, −30, −40, −50, and −100 V corresponded to the actual substrate potentials (Vs) of 8.8, 0, −8.2, −18.9, −28.3, −38.1, −48.0, and −98.7 V, respectively, as illustrated in Table 1. The actual substrate potentials are used in the rest of this paper. The procedures for Cl treatment and back contact deposition have been previously described.30| Nominal substrate potential | Floating | 0 | −10 | −20 | −30 | −40 | −50 | −100 |
| Actual substrate potential (Vs) | 8.8 | 0 | −8.2 | −18.9 | −28.3 | −38.1 | −48.0 | −98.7 |
| Plasma potential (Vp) | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 |
| ΔV (Vp − Vs) | 3.4 | 12.2 | 20.4 | 31.1 | 40.5 | 50.3 | 60.2 | 110.9 |
As-grown and Cl-treated CdTe films were characterized by Zeiss SIGMA scanning electron microscope and a Bruker D8 focus X-ray diffraction (XRD) apparatus. The SnO2 (200) peaks in all XRD spectra were used for internal angle calibration. FIB cross-section-milled samples were prepared using a FEI Helio Nano Lab Nova200 Dual Beam system (FEI Company, Hillsboro, OR USA), equipped with a focused Ga+ liquid metal ion source. Ion beam thinning was carried out at normal incidence with respect to all sample surfaces. Secondary electron imaging was performed in the same system and captured by using a low-kV immersion lens. The surface morphology and roughness of CdTe thin films were observed by atomic force microscopy (AFM) using a dimension Icon atomic force microscope (Bruker, USA). I–V curves of these cells were measured using a Keithley 2400 source meter under standard test conditions (STC) of 1000 W m−2 AM 1.5 irradiance, generated by a Newport Oriel 92193A-1000 solar simulator. External quantum efficiency (EQE) was measured in a solar cell spectral response/QE/IPCE measurement system, Model QEX7. During our EQE measurement, an optical lens was applied to focus the optical area to about 0.04 cm2.
3. Results and discussion
3.1. RF magnetron deposition of CdTe thin film
The system pressure had a strong impact on the grain size, morphology, and crystallinity of CdTe films. Fig. 1 shows plane-view SEM images of CdTe films deposited at 1.5, 2, 3, and 4 Pa at floating potentials. The CdTe films deposited at 1.5, 2, and 3 Pa exhibit grains that are regular in shape (Fig. 1a–c), while the grains of CdTe deposited at 4 Pa are spherical (Fig. 1d). The grain size of CdTe films deposited at 1.5–3 Pa is about 200–600 nm, while the grain size in CdTe films deposited at 4 Pa is only about 200 nm. CdTe films deposited at 1.5–3 Pa have good crystalline quality, whereas CdTe thin films deposited at 4 Pa show only an amorphous structure, as determined by XRD and SEM (Fig. 1d and 2). The crystalline structure is closely related to the mean free path (MFP) of various atoms and ions, given by eqn (1),31 where λ denotes the MFP in units of m; and Ms and Mg are the molecular masses of the sputtered atom and the background gas, respectively. rs and rg are the atomic radii of the sputtered atoms and the background gas, respectively. P is the gas pressure in units of Pa.| λ−1 = 7.58 × 1020 × (rs + rg)2 × (1 + Ms/Mg)1/2P | (1) |
Table S1† lists the MFP of the different atoms and ions during deposition. The MFP for all particles is much smaller than the target–substrate distance of 118 mm (Table S1†). The average probability of collision (APC) for a sputtered atom or ion is calculated according to eqn (2).
| APC = (target–substrate distance)/λ, | (2) |
The calculated APC for different atoms and ions is presented in Table S1.†
The relationship among MFP, APC, and deposition pressure is clearly seen in Fig. 3. Increasing the deposition pressure decreases the MFP and thus increases the APC. When the deposition pressure is 1.5–4 Pa, all atoms and ions have an MFP smaller than the target–substrate distance of 118 mm and an APC larger than 3 (Table S1†). Therefore, diffusion is the primary mechanism by which sputtered atoms and ions arrive at the surface of the substrate. At high deposition pressure, large values of APC decrease the momentum and kinetic energy for sputtered atoms and ions, lowering their mobility on the substrate and leading to poor crystalline quality and small grain size. At low deposition pressure, large values of MFP and small values of APC result in high mobility of the sputtered atoms or ions on the substrate, yielding a high growth rate and large gaps between CdTe grains. However, at low deposition pressures, the sputtering rate is quite low and the plasma is unstable owing to low plasma concentration. Thus, carefully tuning the deposition pressure is critical to obtaining CdTe thin films with a high growth rate, high crystalline quality, and large grain size. We therefore chose deposition pressures of 2 Pa and 3 Pa to obtain the CdTe thin films.
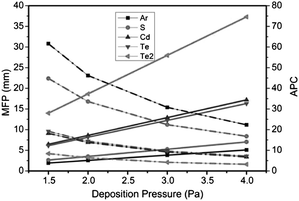 | ||
| Fig. 3 Influence of deposition pressure on mean free path (MFP) and average probability of collision (APC) (118 mm/MFP) for sputtered atoms and ions. | ||
Plane-view SEM images for CdTe thin films obtained with substrate potentials of 8.8, 0, −8.2, −18.9, −28.3, −38.1, −48.0, and −98.7 V have been reported in our previous paper.30 The samples grown at different substrate potentials were further investigated by cross-sectional SEM (Fig. 4a–h). No pinholes or voids are observed within the films or at SnO2:F/CdS and CdS/CdTe interfaces. CdTe displays a columnar growth mode with clearly visible grain boundaries under substrate potentials of 8.8 to −38.1 V (Fig. 4a–f), while for CdTe thin films deposited with substrate potentials between −48.0 and −98.7 V, the grain boundaries cannot be easily distinguished (Fig. 4g and h).
The main reason for the morphology change is related to the kinetic energy of sputtered atoms or ions arriving at the substrate. The potential difference between the substrate surface and the plasma induces the acceleration of Ar+ and sputtered atoms or ions, providing kinetic energy for surface atoms or ions on growing films; the higher their kinetic energy, the higher is their mobility.32 However, when the kinetic energy of species arriving at the substrate is too high, they will be re-sputtered onto the deposited film. This damages the thin film, reduces the growth rate, exerts microstresses, and increases the density of dislocations and interface defects, all of which degrade the device performance. However, very few atoms or ions impinge upon the substrate surface with their full bias voltage. A complicated charge-exchange process usually occurs near the growing film surface, where the densities of various atoms, molecules, and ions are high. Most of the kinetic energy of impinging species is lost through high-energy photon radiation as products of the charge-exchange process, leading to a broad low-energy distribution of ions and neutrals bombarding the surface.33
Fig. 5a shows the I–V curve measured by a Langmuir probe after subtracting the contribution of the choke coil. The floating potential (Vf) obtained from the I–V curve is 8.8 V when the current is zero (Fig. 5a). A straight line, approximating the ion current, is subtracted from the raw data (Fig. 5a). The background-substracted data are then plotted on a semi-logarithmic scale and the two distinct regions (Fig. 5b) are again approximated with straight lines, where the green dashed line represents the contribution from the high-energy tail electrons and the red dotted line represents the retardation region where the electron current increases rapidly.34 The Vp value is determined from the intersection of the two lines. In our deposition chamber, Vp is 12.2 V (Table 1) at Ar pressure of 2 Pa and RF power density of 1.32 W cm−2. The plasma current can be calculated by eqn (3), obtained by fitting the straight line approximating the ion current, where V denotes the substrate potential during the measurement process and I denotes the plasma current.
| I (mA) = A × V (V) − B | (3) |
Using the values of A = 0.04 ± 0.003 and B = −20.50 ± 0.003, eqn (3) yields plasma currents of −20.1, −20.5, −20.9, −21.4, −21.8, −22.3, −22.8, and −25.1 mA at substrate potentials of 8.8, 0, −8.2, −18.9, −28.3, −38.1, −48.0, and −98.7 V, respectively.
When the sputtering power is within a range of values, the deposition ion energy (Uk) is correlated with the potential difference (ΔV = Vp − Vs) between the plasma (Vp) and the substrate (Vs). The ΔV value is 3.4, 12.2, 20.4, 31.1, 40.5, 50.3, 60.2, and 110.9 V, as listed in Table 1. Higher-substrate potential means that the substrate will be bombarded with ions that have higher energy. The deposition energy greatly changes the morphology of the growing film surface. When ΔV is small, the average energy of the impinging ions is low. When ΔV is high, the deposition energy is also high. This means that the depositing atoms not only impinge upon the grains but also impart energy to the ions already on the surface, increasing their mobility. This increases the grain size and causes more ions to move to the grain gaps, leading to closely compacted grains. When ΔV further increases beyond the sputtering threshold, the high energy not only causes atoms to migrate to the surface of the deposited films but also starts to cause re-sputtering of the growing films. This creates many lattice defects and amorphous structures, consistent with the indistinguishable grain boundaries of highly biased CdTe films.
The substrate potential greatly impacts the roughness of CdTe films. The three-dimensional AFM images in Fig. 6 show the roughness of CdTe thin films deposited at different substrate potentials. For substrate potentials between 8.8 V and −18.9 V, the relatively small grains decrease in size as the substrate potential is increased. The roughness of CdTe thin films obtained with substrate potentials of 8.8, 0, −8.2, and −18.9 V is 37.0, 22.2, 12.3, 9.48 nm, respectively. The decrease in roughness with the increase in substrate potential of 8.8, 0, −8.2, and −18.9 V may be attributed to the bombarding the highest points of particles and higher surface mobility. For the floating voltages and substrate potentials higher than −28.3 V, CdTe thin films are mainly composed of large polydisperse particles that resemble hillocks and islands distributed randomly on the surface. For CdTe films grown at the −28.3 V substrate potential, the roughness is 24.8 nm; as the substrate potential is increased to −38.1, −48.0, and −98.7 V, the roughness decreases to 17.3, 14.8, and 29.5 nm, respectively, owing to the enhanced diffusion and accumulation effects that accompany an increase in the bias voltage.35
The substrate potential affects not only the morphology and roughness but also the phase composition, crystalline quality, and microstress of the films, as reported in ref. 30. We have previously reported that CdTe thin films deposited with various substrate potentials had high crystalline quality and mainly showed a zinc blende (cubic) structure with a preferred growth orientation of (111). For CdTe films grown at substrate potentials of +8.8, 0, −8.2, and −18.9 V, a quasi-stable wurtzite (hexagonal) phase coexisted with the cubic phase. Substrate potentials more negative than −28.3 V helped CdTe convert to a more stable cubic phase, preventing the formation of the quasi-stable hexagonal phase. We had inferred that when the substrate potential increased, the energy of the sputtering target atoms also increased, yielding the phase composition that corresponds to the stable cubic structure of CdTe. We have reported that an increase in ion bombardment leads to an increase in residual stress. No evident in-plane strain was found in CdTe thin films grown at substrate potentials of +8.8, 0, −8.2, or −18.9 V. However, the (111) diffraction peaks for CdTe films grown at substrate potentials of −28.3, −38.1, −48.0, and −98.7 V showed a large shift in angle toward smaller degrees as the substrate potential became more negative. We understood this as being an indication of enhanced in situ annealing due to the energy from constant ion flux, obtained across the cathode (substrate) dark space. When the substrate potential is less negative than −28.3 V, the velocity of positive ions gained from this bias field is low, and charge-exchange processes probably occur owing to a low acceleration field. This bombards the growing films with a broad low-energy distribution of ions and neutral species, rather than ions that strike the film directly with full bias voltage. However, when the substrate potential is more negative than −28.3 V, positive ions gain enough energy to strike the growing film and transferred their lateral momentum to surface atoms.
3.2. CdTe polycrystalline thin films after Cl treatment
The morphologies and grain sizes of CdTe films change greatly depending on the duration of Cl treatment. Fig. 7 shows plane-view SEM images of Cl-treated CdTe films grown at 3 Pa under floating potential. When the Cl treatment time is 10 min, the film maintains an average grain size of about 500 nm (Fig. 7a) and exhibits a slight breakdown in the crystalline morphology. When the Cl treatment time is increased to 15 min, the morphology changes remarkably (Fig. 7b), and the grain size increases owing to recrystallization. As the Cl treatment time is prolonged, the grain size of CdTe continues to increase. After 20 min of Cl treatment, the surface morphology changes from columnar to planar, with grains larger than 1 μm (Fig. 7c). Increasing the Cl treatment time further causes the grain size to decrease instead of increase (Fig. 7d and e). The result indicates that the optimal Cl treatment time is 20 min for CdTe grown at 3 Pa. | ||
| Fig. 7 SEM images of CdTe films grown at 3 Pa, after CdCl2 treatment at 400 °C for varying durations. (a) 10 min. (b) 15 min. (c) 20 min. (d) 25 min. (e) 27 min. (f) 30 min. The scale bar is 400 nm. | ||
The CdTe film grown at 2 Pa responds quite differently to Cl treatment, compared to the CdTe film grown at 3 Pa. Fig. 8 shows SEM images of CdTe grown at 2 Pa under floating potential after Cl treatment. It is easily seen from Fig. 8 that the morphology and grain size for CdTe polycrystalline thin films change remarkably, from columnar to planar, with the duration of Cl treatment. The grain size of the CdTe thin film continues to grow during 10 min to 25 min of treatment (Fig. 8a–d). The grain size increases to more than 1 μm when the Cl processing time is 25 min (Fig. 8d), and changes little when the Cl annealing time is further increased to 55 min (Fig. 8e–l). The grain size reaches a maximum when the Cl treatment time is 43 min (Fig. 8h and i). Increasing the Cl treatment time beyond 50 min causes further increases in grain size and morphology that require additional study. These optimal Cl treatment time is 43 min for CdTe grown at 2 Pa.
The morphology for all CdTe grown with different substrate potentials subjected to Cl treatment at 400 °C for 43 min has been reported in ref. 30. Fig. 9a–h show cross-sectional SEM images of Cl-treated CdTe thin films grown with different substrate potentials, treated with Cl at 400 °C for 43 min and sliced via FIB. In all of the Cl-treated films, voids are clearly visible at CdS/CdTe interfaces, and mainly at grain boundaries within CdTe thin films (Fig. 9a–h). Lattice mismatch between CdS and CdTe, as high as 10%, may be the reason for the occurrence of voids at the interface between these materials. The voids at grain boundaries in CdTe films may result from CdTe recrystallization or the release of internal stress in CdTe during Cl treatment. Voids in CdTe thin films obtained with substrate potentials of 8.8, 0, −8.2, −18.9, and −28.3 V are significantly smaller than the voids in the CdTe films synthesized at substrate potentials of −38.1, −48.0, −98.7 V, after both sets of films had undergone the same Cl treatment. Our results indicate that to obtain high-quality CdTe films after Cl treatment, the substrate potential should be no higher than −28.3 V. The void density and size are greatest at a substrate potential of −38.1 V.
3.3. Quality of CdTe solar cells
We fabricated solar cells from Cl-treated CdTe films and measured their I–V curves at 1000 W m−2 AM 1.5 irradiance. Fig. 10 shows box charts of η, Voc, Js, and the fill factor (FF) for solar cells fabricated on a Cl-treated CdTe film grown at 3 Pa under floating potential. The values for η, Voc, Jsc and FF are quite low when the Cl treatment time is 10 min, mainly due to the low level of recrystallization. As the Cl treatment time increases, η, Voc, Jsc, and FF gradually increase as well. This is because prolongation of the Cl treatment time increases the amount of CdTe that recrystallizes, thus increasing the grain size. When the Cl treatment time is 20 min, η, Voc, Jsc, and FF reach their maximum values of 11.66%, 749 mV, 23.60 mA cm−2, and 69.60%, respectively, based on measurements from several solar cell samples (Fig. 10). This improvement in performance corresponds to our morphological studies on the CdTe films, which indicate a shift from rough films with small columnar grains, to smoother films with large planar grains whose size is greater than 1 μm. Cl treatment for 20 min is optimal; the device quality decreases when the Cl treatment time is prolonged further.We also fabricated solar cells from CdTe polycrystalline thin films grown at 2 Pa under floating potential after Cl treatment at 400 °C for 10–55 min, and measured the I–V curves of these cells in the same manner as for the films grown at 3 Pa. Fig. 11 shows box charts of η, Voc, Jsc, and FF for CdTe solar cell devices. As with the films grown at 3 Pa, the η, Voc, Jsc, and FF values of the solar cells are poor when the Cl treatment time is only 10 min (Fig. 11). This is mainly because 10 min of Cl treatment does not significantly change the grain size of the CdTe films. With an increase in Cl annealing time, obvious grain growth CdTe occurs. When the Cl annealing time is 43 min, the grain size reaches a maximum, and η, Voc, Jsc, and FF also reach their maximum values of 12.00%, 790 mV, 23.01 mA cm−2, and 69.87%, respectively. For this experiment, the solar cell parameters are obtained from measurements on the same CdTe solar cell device. The performance of this solar cell is better than that of the solar cell fabricated on CdTe grown at 3 Pa, though its correlation with morphology is the same; when the Cl treatment time is 43 min, the rough CdTe film with small columnar grains becomes a smoother film with large planar grains greater than 1 μm. The relationship between film morphology and device performance means that the grain size could be used as an indicator for device performance optimization.
The solar cells fabricated on the CdTe films grown at 2 Pa show the best properties with Cl treatment at 400 °C for 43 min in dry air. Therefore, this Cl treatment condition was employed to treat CdTe polycrystalline thin films grown at 2 Pa with substrate potentials of 8.8, 0, −8.2, −18.9, −28.3, −38.1, −48.0, and −98.7 V. CdTe solar cell devices were fabricated by evaporating Cu and Au buffer layers and back contact layers, without etching, onto the Cl-treated films. Under standard experimental conditions, the primary performance parameters η, Voc, Jsc, and FF show dependence on substrate potential (Fig. 12). When Vs increases from floating potential to −8.2 V, the performance degrades gradually. When Vs is −18.9 V, the performance surprisingly recovers and attains a maximum. When Vs negatively increases from −18.9 V to −38.1 V, the performance drops remarkably, and attains the minimum when the substrate potential is −38.1 V. As the substrate potential becomes more negative, the performance recovers again. The device shows the best performance when the substrate potential is −18.9 V. The Eff, Voc, Jsc, and FF values of the best-performing solar cell in this case are 12.78%, 779 mV, 22.91 mA cm−2, and 71.62%, respectively, which has been certified by the Photovoltaic and Wind Power Systems Quality Test Center, Chinese Academy of Sciences. The typical I–V curve of this cell is shown in Fig. 13a.
 | ||
| Fig. 13 (a) Light I–V curve for CdTe solar cell with Eff of 12.78%, (b) EQE of CdTe solar cell (red line) and transmission curves of TEC15 glass (green line). EQE: external quantum efficiency. | ||
CdTe thin films obtained at the −18.9 V substrate potential have the largest grain size; XRD indicates no stress in these films after Cl treatment.30 On the other hand, CdTe thin films obtained at a substrate potential of −38.1 V have the largest void density and exhibit obvious stress after Cl treatment. The void density, grain size, and stress directly influence the performance of CdTe solar cells. Combining plane-view SEM, cross-sectional SEM, and XRD results for CdTe with and without Cl treatment reveals that although substrate-biased growth forms films with closely packed grains, such as in the −28.3 V biased sample, the morphological changes that occur when substrate bias is applied during deposition are not significant to the performance of the final CdTe solar cell. However, because strain release and recrystallization occur during the post-treatment, altering the grain size and void density, the treatment time has a much larger influence. Optimizing the Cl treatment remains a key issue for obtaining high-performance CdTe solar cells.
Fig. 13b shows the EQE (red line) of the best cell and the transmission curve (green line) of TEC15 glass. The TEC15 substrate transmission is about 74–82% in the spectral range of 400–820 nm owing to glass and SnO2:F absorption (Fig. 13b). The EQE of the solar cell is about 68–80% in the spectra range of 600–820 nm (Fig. 13b). The EQE greatly decreases in the blue region of 360–560 nm, mainly because of CdS absorption. Thus, it is quite obvious that the electrical current loss in the CdTe solar cell now is mainly due to the absorption of TEC15 and the CdS window layer. Therefore, the EQE and Jsc values of CdTe solar cells may be further improved using glass and TCO with higher transmission, and by reducing the CdS thickness, in addition to tuning the properties of the CdTe layer. Voc may be increased by improving the quality of the CdS film and its interfaces with TCO and CdTe. These works are currently underway.
4. Conclusions
Our study shows that the deposition pressure and substrate potential, as well as post-deposition treatment with Cl, greatly influence the morphology, grain size, crystalline quality, phase composition, and microstress of CdTe polycrystalline thin films grown by RF magnetron sputtering. The optimum Cl treatment time was 20 min for CdTe films that had been deposited at 3 Pa, and 43 min for CdTe films deposited at 2 Pa. The best solar cell performance was obtained from films that were grown at 2 Pa with a substrate potential of −18.9 V. The highest η, Voc, Jsc, and FF that we obtained for a CdTe solar cell on soda-lime glass were 12.78%, 779 mV, 22.91 mA cm−2, and 71.62%, respectively. We also show that CdTe solar cells can be further improved by reducing the thickness of the CdS layer and optimizing its interface with CdTe and TCO, work that is currently underway in our group.Acknowledgements
The authors thank Prof. Yue Zhang and Dr Weiguang Chi for their kind support of sputtering target compression, as well as their constructive suggestions for sputtering deposition. The authors also thank NSG-Pilkington for supplying the TCO-coated soda-lime glass used in this study, and the Photovoltaic and Wind Power Systems Quality Test Center of the Chinese Academy of Sciences for certifying the performance of solar cell samples. This work was financially supported by the Chinese Academy of Sciences (CAS), the 100 Talents Program of the CAS, and the 100 Talents Preferred Support Plan of the CAS. (ARP no. Y010411C41 and Y210431C41).References
- A. Luque and S. Hegedus, Handbook of Photovoltaic Science and Engineering, John Wiley & Sons Ltd, Chichester, 2003, p. 623 Search PubMed.
- S. S. C. Ting and L. Chu, Prog. Photovoltaics, 1993, 1, 31–42 Search PubMed.
- P. r. o. F. S. website, 2013.
- M. A. Green, K. Emery, Y. Hishikawa, W. Warta and E. D. Dunlop, Prog. Photovoltaics, 2013, 21, 1–11 Search PubMed.
- A. Gupta and A. D. Compaan, Compound Semiconductor Photovoltaics, 2003, vol. 763, pp. 161–166 Search PubMed.
- M. Shao, Doctor's thesis, 1995.
- A. Anders, N. Pasaja, S. H. N. Lim, T. C. Petersen and V. J. Keast, Surf. Coat. Technol., 2007, 201, 4628–4632 CrossRef CAS PubMed.
- O. B. Postel and J. V. R. Heberlein, Diamond Relat. Mater., 1999, 8, 1878–1884 CrossRef CAS.
- J. Geng, A. Schuler, P. Oelhafen, P. Gantenbein, M. Duggelin, D. Mathys and R. Guggenheim, J. Appl. Phys., 1999, 86, 3460–3462 CrossRef CAS PubMed.
- A. Sonnenfeld and P. R. von Rohr, Plasma Processes Polym., 2009, 6, S722–S726 CrossRef CAS.
- K. J. Hsiao and J. R. Sites, Prog. Photovoltaics, 2012, 20, 486–489 CAS.
- K. Zweibel, Science, 2010, 328, 699–701 CrossRef CAS PubMed.
- K. A. Wieland, N. R. Paudel and A. D. Compaan, Sol. Energy Mater. Sol. Cells, 2012, 105, 109–112 CrossRef PubMed.
- J. Fritsche, A. Klein and W. Jaegermann, Adv. Eng. Mater., 2005, 7, 914–920 CrossRef CAS.
- K. M. AbuEl-Rub, S. R. Hahn, S. Tari and M. A. K. L. Dissanayake, Appl. Surf. Sci., 2012, 258, 6142–6147 CrossRef CAS PubMed.
- W. K. Metzger, D. Albin, M. J. Romero, P. Dippo and M. Young, J. Appl. Phys., 2006, 99, 103703 CrossRef PubMed.
- J. P. Enriquez and X. Mathew, J. Mater. Sci.: Mater. Electron., 2005, 16, 617–621 CrossRef CAS PubMed.
- H. Infante and G. Gordillo, Surf. Rev. Lett., 2002, 9, 1681–1685 CrossRef CAS.
- S. A. Ringel, A. W. Smith, M. H. Macdougal and A. Rohatgi, J. Appl. Phys., 1991, 70, 881–889 CrossRef CAS PubMed.
- B. E. McCandless, I. Youm and R. W. Birkmire, Prog. Photovoltaics, 1999, 7, 21–30 CAS.
- K. Nakamura, T. Fujihara, T. Toyama and H. Okamoto, Jpn. J. Appl. Phys., Part 1, 2002, 41, 4474–4480 CrossRef CAS.
- W. Song, D. Mao, J. U. Trefny, R. K. Ahrenkiel, D. H. Levi and S. Johnston, NCPV Photovoltaics Program Review, 1999, 462, 188–193 CAS.
- H. R. Moutinho, M. M. Al-Jassim, D. H. Levi, P. C. Dippo and L. L. Kazmerski, J. Vac. Sci. Technol., A, 1998, 16, 1251–1257 CAS.
- S. Lalitha, R. Sathyamoorthy, S. Senthilarasu and A. Subbarayan, Sol. Energy Mater. Sol. Cells, 2006, 90, 694–703 CrossRef CAS PubMed.
- X. Z. Wu, High-Resolution Focused Ion-Beams, Sol. Energy, 2004, 77, 803–814 CrossRef CAS PubMed.
- J. Orloff, Rev. Sci. Instrum., 1993, 64, 1105–1130 CrossRef CAS PubMed.
- S. Reyntjens and R. Puers, J. Micromech. Microeng., 2001, 11, 287–300 CrossRef CAS.
- I. Utke, P. Hoffmann and J. Melngailis, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.–Process., Meas., Phenom., 2008, 26, 1197–1276 CrossRef CAS.
- J. D. Major, L. Bowen and K. Durose, Prog. Photovoltaics, 2012, 20, 892–898 CAS.
- X. Liu, H. Li and F. Huang, Photovoltaic Specialists Conference (PVSC), 38th IEEE, 2012.
- K. V. S. Mahieu and D. Depla, Reactive Sputter Deposition, 2008 Search PubMed.
- M.-H. Tsai, W.-J. Shen, Y.-S. Chang and J.-W. Yeh, Thin Solid Films, 2012, 520, 6 Search PubMed.
- M. A. Liberman and A. J. Lichtenberg, Principle of Plasma Discharges and Materials Processing,section 11.6, John Wiley & Sons, Inc., Hoboken, New Jersey , 2005, 2nd edn Search PubMed.
- M. Shao, The Doctor Dissertation of the University of Toledo, 1995.
- Y. H. Lv, L. Ji, X. H. Liu, H. X. Li, H. D. Zhou and J. M. Chen, Appl. Surf. Sci., 2012, 258, 3864–3870 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra44831b |
| This journal is © The Royal Society of Chemistry 2014 |










